Copyright
©The Author(s) 2025.
World J Exp Med. Sep 20, 2025; 15(3): 106677
Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.106677
Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.106677
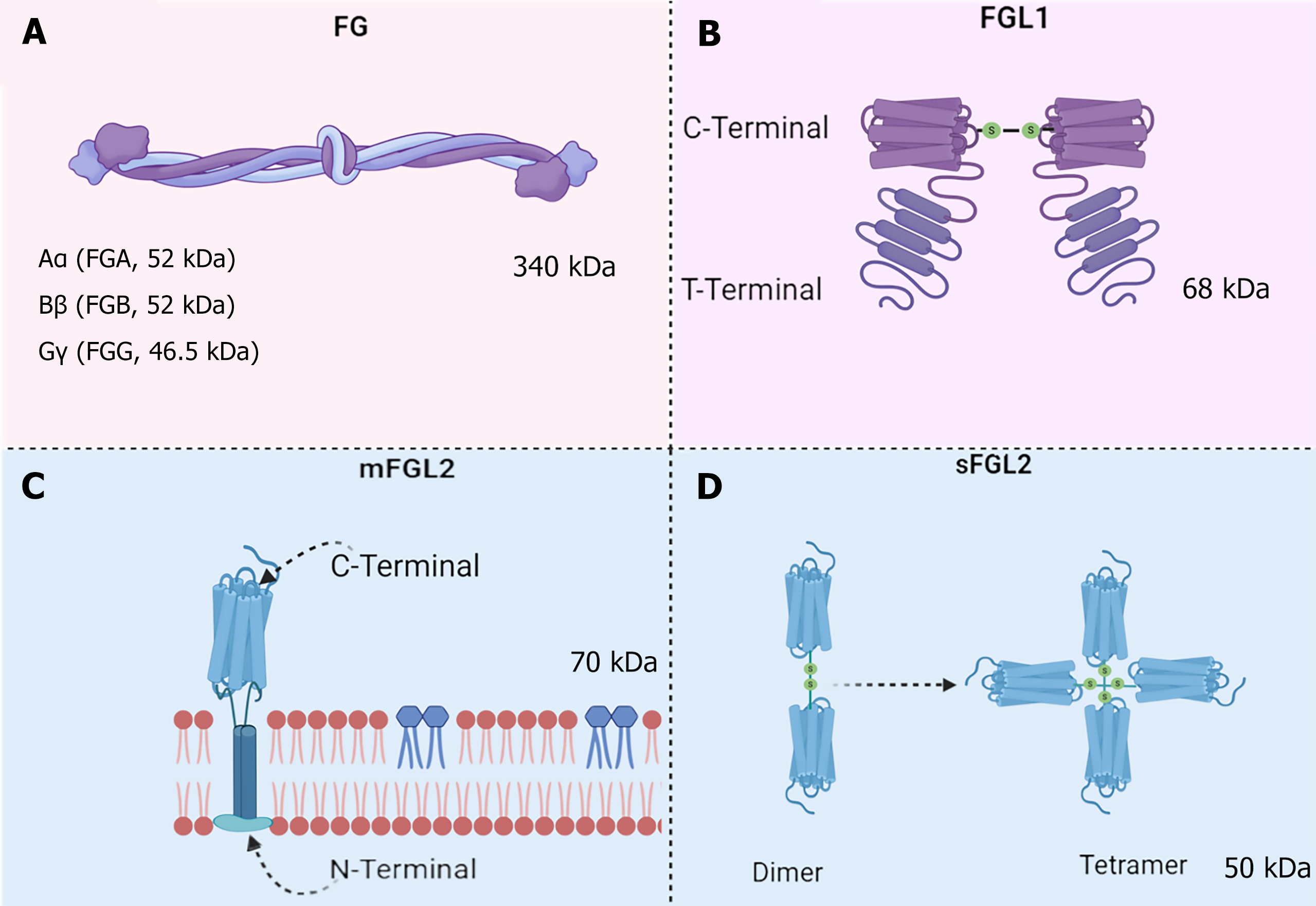
Figure 1 Structure of fibrinogen superfamily proteins.
A: Fibrinogen (FG) is a soluble glycoprotein with a molecular weight of 340 kDa, comprising three distinct subunits: Aα, Bβ, and Gγ chains; B: The monomeric form of FG-like protein (FGL) 1 contains an N-terminal C-terminal FG-like domain and a C-terminal FG-related domain. Two identical 34 kDa subunits assemble into a functional dimer via disulfide linkages; C: Membrane-bound FGL2 is classified as a type II transmembrane glycoprotein, featuring an intracellular domain at its N-terminus, a transmembrane segment, and an extracellular region at the C-terminus; D: Soluble FGL2 exhibits structural polymorphism, existing as both monomeric and tetrameric forms. Initially, monomers dimerize through disulfide bonds, followed by further association into tetramers via additional covalent bridges. FG: Fibrinogen; FGL1: Fibrinogen-like protein 1; mFGL2: Membrane-bound fibrinogen-like protein 2; sFGL2: Soluble Fibrinogen-like protein 2.
- Citation: Qu RH, Rong Y, Ni WZ, Huang XL, Chen YZ, Li HF. Fibrinogen superfamily proteins: Key regulators in hepatic disorders. World J Exp Med 2025; 15(3): 106677
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/106677.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.106677