Published online May 4, 2017. doi: 10.5492/wjccm.v6.i2.107
Peer-review started: July 1, 2016
First decision: August 5, 2016
Revised: December 20, 2016
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: May 4, 2017
Processing time: 306 Days and 7.3 Hours
To address the hypothesis that young, gonad-intact female mice have improved long-term recovery associated with decreased neuroinflammation compared to male mice.
Eight to ten week-old male, female, and ovariectomized (OVX) mice underwent closed cranial impact. Gonad-intact female mice were injured only in estrus state. After injury, between group differences were assessed using complementary immunohistochemical staining for microglial cells at 1 h, mRNA polymerase chain reaction for inflammatory markers at 1 h after injury, Rotarod over days 1-7, and water maze on days 28-31 after injury.
Male mice had a greater area of injury (P = 0.0063), F4/80-positive cells (P = 0.032), and up regulation of inflammatory genes compared to female mice. Male and OVX mice had higher mortality after injury when compared to female mice (P = 0.043). No group differences were demonstrated in Rotarod latencies (P = 0.62). OVX mice demonstrated decreased water maze latencies compared to other groups (P = 0.049).
Differences in mortality, long-term neurological recovery, and markers of neuroinflammation exist between female and male mice after moderate traumatic brain injury (MTBI). Unexpectedly, OVX mice have decreased long term neurological function after MTBI when compared to gonad intact male and female mice. As such, it can be concluded that the presence of female gonadal hormones may influence behavioural outcomes after MTBI, though mechanisms involved are unclear.
Core tip: Differences in mortality, long-term neurological recovery, and markers of neuroinflammation exist between female and male mice after moderate traumatic brain injury (MTBI). Unexpectedly, ovariectomized mice have decreased long term neurological function after MTBI when compared to gonad intact male and female mice.
- Citation: Umeano O, Wang H, Dawson H, Lei B, Umeano A, Kernagis D, James ML. Female gonadal hormone effects on microglial activation and functional outcomes in a mouse model of moderate traumatic brain injury. World J Crit Care Med 2017; 6(2): 107-115
- URL: https://www.wjgnet.com/2220-3141/full/v6/i2/107.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v6.i2.107
In the United States, up to 6 million people sustain head injury annually[1-3]. Traumatic brain injury (TBI) is often graded as mild, moderate, or severe based on the patient’s initial level of consciousness and presenting Glasgow coma score (GCS)[4]. Mild to moderate TBI (MTBI) is the most common type and occurs from injury of minimal duration and severity[5,6]. However, physiological manifestations such as diminished cerebral blood flow, neuroinflammation, impaired neurotransmission, cerebral edema, and abnormal glucose metabolism may occur after MTBI[2,7,8]. Further, MTBI is most common in young adults, resulting in significant long-term comorbidities, such as depression, substance abuse, chronic pain, unemployment, and post-traumatic stress disorder[6,7]. Currently, no proven therapy exists for patients with MTBI.
Sex differences in recovery after TBI are largely due to female gonadal hormones decreasing acute neuroinflammation[9,10] and increasing neuronal survival[11,12]. This has resulted in progesterone moving into clinical trial for patients with moderate to severe TBI[13]. While prior work has largely focused on models of severe TBI where neuroinflammation is most pronounced, these sex effects have not been adequately assessed in MTBI.
Thus, this study sought to assess sex differences in modifying recovery after MTBI. Specifically, the hypothesis was that young, gonad-intact female mice have improved long-term recovery associated with decreased neuroinflammation compared to male mice. Additionally, ovariectomized (OVX) mice were included in neurobehavioral outcomes to begin to model potential sex effects in “menopausal” states.
All experiments were approved by the Duke University Institutional Animal Care and Use Committee and were designed to minimize suffering and numbers of animals. Experimental cohorts consisted of 8-10 wk old C57BL/6J male, gonad-intact female mice, and OVX mice (Jackson Laboratories, Bar Harbor, ME). OVX surgeries were performed at Jackson Laboratories with injury performed 4-6 wk after ovariectomy. All mice were housed in groups of 5 mice/cage in a 12-h day/night light cycle for 5-7 d prior to injury. Prior to and immediately after injury, all animals were provided free access to standard laboratory rodent chow and filtered water. All observers were blinded to grouping during injury and throughout all outcomes measurements.
The following experimental groups were utilized after injury with MTBI: Group 1 - Microglial activation/macrophage recruitment - Stereology for F4/80+ cells was performed in the hippocampus of 16 mice (5 male, 5 female, 3 sham female, 3 sham male) at 1 h after injury; Group 2 - Blood-brain barrier (BBB) permeability - Immunoglobulin G (IgG) staining was performed in the cerebral cortex of 16 mice (5 male, 5 female, 3 sham female, 3 sham male) at 1 h after injury; Group 3 - Inflammatory gene regulation - Reverse transcriptase polymerase chain reactions (RT-PCR) was performed on whole brain samples of 20 mice (5 sham female, 5 sham male, 5 female, 5 male) at 1 h after injury; Group 4 - Neurobehavioral Recovery - Experimental - 59 mice (22 female, 20 male, 17 OVX) were subjected to Rotarod (RR) testing over Days 1-7 and water maze (WM) testing at Days 28-31 after injury. Mortality was assessed during the 31 d of testing; Group 5 - Neurobehavioral Recovery - Sham - 25 mice (10 female, 10 male, 5 OVX) were subjected to Rotarod (RR) testing over Days 1-7 and water maze (WM) testing at Days 28-31 after injury.
Prior to injury, female mice underwent morning vaginal smears in order to determine reproductive state[14]. Mice in the estrus stage, characterized by having a cluster of irregularly shaped, cornified squamous epithelia cells that lacked nuclei, were used in experiments. Mice in other stages were subsequently smeared on consecutive days until they could be classified as estrus.
This murine TBI model was adapted from a previously described model of closed cranial trauma for the rat[15-17]. After anesthesia induction with 4.6% isoflurane, the trachea was intubated and the lungs were mechanically ventilated with 1.6% isoflurane in 30% O2/70% N2. Rectal temperature was maintained at 37 °C. Mice were positioned in a stereotactic device, scalp incised, and the skull exposed. A concave 3-mm metallic disc was glued to the skull immediately caudal to bregma. A 2.0-mm-diameter pneumatic impactor (Air-Power, Inc. High Point, NC) was used to deliver a single midline impact to the disc surface. The impactor was discharged at 6.8 ± 0.2 m/s with head displacement of 3 mm. After impact, anesthesia was discontinued, the animals were allowed to recover spontaneous ventilation, and trachea was extubated. Following recovery, mice were allowed free access to food and water. Adequate MTBI was arbitrarily defined as having day 1 RR latency between 50% and 90% of baseline. Day 1 RR latency greater than 90% of baseline is indicative of inadequate injury, while Day 1 RR latency less than 50% of baseline is indicative of severe TBI. Sham mice underwent comparable anesthesia and surgical manipulation but received no cortical impact.
One hour after injury, the mice were anesthetized with 4.6% isoflurane in 30% O2/70% N2 and euthanized using intracardiac perfusion with normal saline. Whole brain samples were removed, placed in formalin and stored at 4 °C. Axial sections (40 μm) were cut on a vibratome in 20-μm intervals over the rostral-caudal extent of the lesion and collected in cryoprotectant solution containing ethylene glycol, sucrose, and sodium phosphate.
To assess microglia activation/macrophage recruitment staining, F4/80 immunohistochemistry was performed. Free-floating sections were incubated in 1% H2O2 for 5 min and transferred to 0.1% Saponin for 1 h. Next, sections were incubated for 30 min in 10% goat serum followed by two successive blocking steps with Avidin and Biotin for 15 min each. Monoclonal rat anti-mouse F4/80 (MCA497R; Serotec, Raleigh, NC, United States) was applied overnight at 40 °C at a dilution of 1:20000. After washing with Phosphate Buffered Saline, biotinylated goat anti-rat IgG secondary antibody (Vector Laboratories, Inc.; 1:3000) was applied for 20 min followed by avidin-biotin-peroxidase complex treatment for 12 min (ABC kit, Vector Laboratories, Inc., Burlingame, CA, United States).
For determining BBB permeability, we stained for presence of IgG, which is usually not present with an intact BBB[18]. For IgG determination, no primary antibody was used; brain slices were incubated using 1:3000 anti-mouse IgG antibody. Staining was visualized with diaminobenzidine (Vector Laboratories, Inc. Burlingame, CA, United States). Sections were mounted onto slides and allowed to dry overnight. Following immunostaining, all sections were counterstained with hematoxylin (Fisher Scientific, Fair Lawn, NJ, United States) for 4 min. After dehydration, sections were cover slipped using Di-N-Butyle Phthalate in Xylene resin. Determination of IgG presence was determined by the area of fluorescence within the tissue samples.
Cell counting was conducted using a Nikon 218912 light microscope interfaced with the StereoInvestigator software package (MicroBrightField, Williston, VT, United States). The number of stained cells per cubic millimeter of hippocampus was estimated by the optical fractionator method. The optical fractionator is an unbiased counting method, which is independent of the size, shape, and orientation of the cells to be counted. The parameters of the fractionator sampling scheme were established in a pilot experiment and uniformly applied to all animals. Before counting, all the slides were coded to avoid experimenter bias. As determined by Stereo Investigator, we chose six sagittal sections (40 μm thick) spaced eight sections apart along the dorsal hippocampal formation by systematic random sampling. This number of sections proved sufficient to provide a coefficient of error between 0.09 and 0.11. On each section, the whole hippocampal area was delineated. For microglial quantification, the sampling grid was 399.027 (X) μm × 367.92 (Y) μm and cells were counted within a probe volume defined by the counting frame (50 μm × 50 μm) and the dissector height (11 μm). Only cells within the counting frame or overlapping the right or superior border of the counting frame, and for which nuclei came into focus while focusing down the dissector height, were counted. The total number of F4/80 cells was calculated per hippocampal volume of 1920 μm thickness[19,20].
Frozen, pulverized whole brain tissue was processed for ribonucleic acid (RNA) extraction at 1 h after injury. RNA quantity and quality were assessed with the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE) and by agarose gel electrophoresis. Only samples with a 260/280 ratio between 1.9-2.1, and a 260/230 ratio greater than 2.0, were further processed. First strand complementary deoxyribonucleic acid (cDNA) was generated from 2 μg total RNA using the RT2 First strand kit (SABiosciences, Frederick, MD, United States) according to the manufacturer’s instructions. Gene expression was measured using the Mouse Nuclear Factor kappa B (NFκB) targeted PCR Array (SABiosciences), which profiles the expression of 84 genes related to the NFκB pathway. RT-PCR was performed according to manufacturer’s instructions using the 384-well plate format (4 samples, 96 wells per sample). One sample from each experimental group was run per plate to minimize potential batch effect between RT-PCR runs. Quality of the cDNA and PCR efficiency was verified by housekeeping genes and RT-PCR controls included in the PCR Array.
Raw RT-PCR data was analyzed using the Web-Based PCR Array Data Analysis software (SABiosciences) and Microsoft Excel (Microsoft, Redmond, WA). The difference between the sequences of interest and the reference sequence (ΔCt) values, the differences between the experimental and control sequences of interest (ΔΔCt), and the based fold-change were calculated from raw threshold cycle data, using beta-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as internal standards for normalization.
An automated RR (Ugo Basile, Comerio, Italy) was used to assess vestibulomotor function[15,21]. On the day prior to injury, mice underwent a conditioning trial at a set rotational speed for 60 s and then three additional trials with an accelerating rotational speed. The average time to fall from the rotating cylinder in the latter three trials was recorded as baseline latency. On days 1, 3, 5, and 7 post-MTBI, the mice had three consecutive trials with accelerating rotational speed (inter-trial interval = 15 min). The average latency to fall from the rod was recorded. Mice unable to grasp the rotating rod were given a latency value of 0 s. Test was automatically suspended after 600 s of running.
Based on prior data[15,22], at 28 d after injury an aluminium pool (105 cm in diameter, 60 cm in depth) was painted black and filled to 17 cm with water (25 °C-27 °C, opacified with powdered milk). The maze was kept in a room dedicated to behavioural testing with light, sound, and visual cues held constant. The goal was a hidden plastic platform, 7.5 cm in diameter, submerged 1 cm below the water surface. Mice were tested for 4 consecutive days with 4 trials per day (inter-trial interval = 1 h). Mice were placed in one of four different quadrants for each trial. Starting quadrants were randomly defined each day. Mice were allowed to search for the platform for a maximum of 90 s at which time the mice were guided to the platform. Mice remained on the platform for 5 s. The mice were kept in heated cages between trials. Our predefined surrogate marker of behavioural learning was water maze latency times, i.e., the time to find the platform and swimming speed were recorded by a computerized video tracking system (Ethovision 2.2.14, Noldus Information Technology, Leesburg, VA).
Parametric analyses were performed on all data sets. Mortality was analysed using the χ2 test. RR and WM latencies were compared with repeated measures analysis of variance (ANOVA) with time as the repeated variable and using Dunnet’s method to correct for multiple comparisons. Stereological analysis results, quantitative PCR results, F4/80 stains and BBB permeability were analysed with student’s t-test. Statistical significance was assumed with P < 0.05. All values were expressed as mean ± (standard error of the mean) SEM and were performed on JMP (v7.0.1, SAS, Cary, NC).
Tissue sections of injured area were collected in a standardized fashion. The entire Area of IgG stained brain was greater in male compared to female mice at 1 h after MTBI (male vs female: 32.09 mm2 + 26.82 vs 26.64 mm2 + 26.04, P = 0.006). Further, IgG stained brain area was greater in MTBI male compared to sham male mice (32.09 mm2 + 26.82 vs 3.20 mm2 + 1.70; P = 0.004). F4/80-positive cells were greatest in MTBI male mice compared to MTBI female, sham male, and sham female mice at 1 h after injury (respectively: 0.036 cells/mm3 + 0.02, vs 0.021 cells/mm3 + 0.01, vs 0.006 cells/mm3 + 0.001, vs 0.011 cells/mm3 + 0.001, P = 0.032).
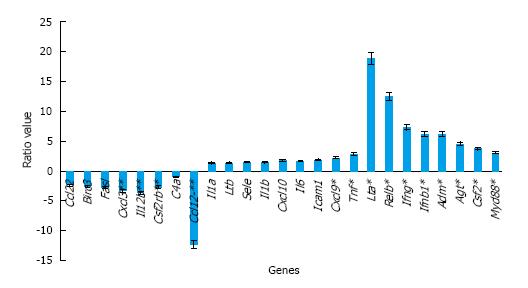
Using focused arrays, multiple genes were differentially expressed between sham male, sham female, male, and female mice at 1 h after MTBI (Table 1). Using relative expression of gene activation [fold change (FC)] of injured vs sham animals, MTBI in male mice was associated with greater activation of inflammatory genes tumor necrosis factor (TNF-α; FC = 30.257, SD = 0.287), interleukin (IL)-1a (FC = 11.356, SD = 0.252), IL-6 (FC = 4.769. SD = 0.379), and C-X-C motif chemokine (CXCL)-10 (FC = 9.327, SD = 0.172). Compared to female shams, MTBI in female mice was also associated with greater activation of inflammatory genes TNF-α (FC = 10.856, SD = 0.082), IL-6 (FC = 2.952, SD = 0.177), IL-1a (FC = 8.807, SD = 0.109), and CXCL-10 (FC = 5.369, SD = 0.091). When comparing male to female mice after MTBI, inflammatory genes TNF, CXCL-10, and IL-6 had greater expression in male mice (R = 2.786, 1.737, and 1.614, respectively, Figure 1).
| Gene | Fold change males | Stdev males | Fold change females | Stdev females |
| Ccl22 | -4.379926621 | 0.219833970 | -1.808791666 | 0.07209482 |
| Birc3 | 2.424699902 | 0.347280537 | 6.393699028 | 0.04457375 |
| Fasl | 1.282942934 | 0.586876307 | 3.595274272 | 0.15614853 |
| Cxcl3 | 1.423783611 | 0.443555855 | 4.738673464 | 0.13768048 |
| Il12b | 1.887247846 | 0.153962747 | 6.969172751 | 0.07141293 |
| Csf2rb | -2.029836886 | 0.504576222 | 2.002641941 | 0.04827339 |
| C4a | -5.138319145 | 0.111529531 | 2.068435781 | 0.09152752 |
| Ccl12 | 1.004666934 | 0.407715962 | 12.36078104 | 0.06841648 |
| Il1a | 11.35623576 | 0.252021657 | 8.806597114 | 0.10903830 |
| Ltb | 1.883908897 | 0.112197741 | 1.410932285 | 0.03827274 |
| Sele | 2.645681512 | 0.175734401 | 1.81041664 | 0.07499996 |
| Il1b | 15.62412184 | 0.259189696 | 10.81446519 | 0.08663391 |
| Cxcl10 | 9.327227458 | 0.172197097 | 5.36922489 | 0.09101903 |
| Il6 | 4.768742556 | 0.379381266 | 2.952867421 | 0.17661706 |
| Icam1 | 3.304520395 | 0.364423780 | 1.778613038 | 0.12022752 |
| Cxcl9 | 1.25441829 | 0.250388088 | -1.750912312 | 0.07922075 |
| Tnf | 30.25742899 | 0.286872446 | 10.85969992 | 0.08157591 |
| Lta | -1.18052165 | 0.435317336 | -22.22152490 | 0.08186537 |
| Relb | 2.632882795 | 0.110186834 | -4.717581418 | 0.11019118 |
| Ifng | 2.706432675 | 0.341618642 | -2.698000440 | 0.12030042 |
| Ifnb1 | -1.300071257 | 0.147446990 | -8.018307062 | 0.12328928 |
| Adm | 4.645463022 | 0.411030849 | -1.324109025 | 0.10189112 |
| Agt | 1.850525351 | 0.353829239 | -2.447168033 | 0.06028075 |
| Csf2 | 3.379073426 | 0.166397911 | -1.105541488 | 0.07037648 |
| Myd88 | 3.113516447 | 0.407823225 | 1.013600401 | 0.05586930 |
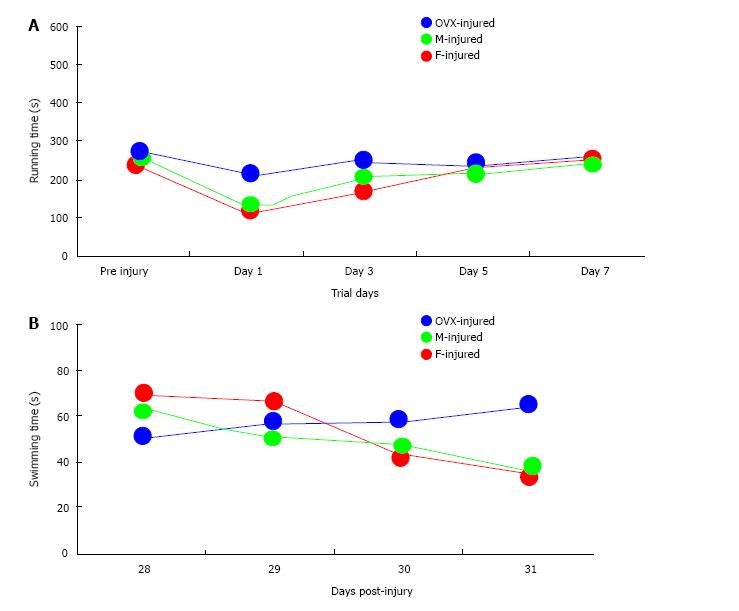
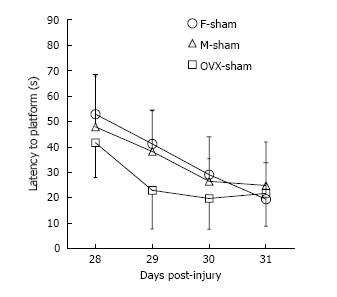
Of the total 59 mice that were injured, 49 survived [20 of 22 (91%) female, 17 of 20 (85%) male, and 12 of 17 (71%) OVX; P = 0.043]. Of the 49 surviving mice, 46 were adequately injured to fulfill the definition of MTBI (19 of 20 female mice, 17 of 17 male mice, and 10 of 12 OVX mice). All surviving animals meeting criteria for MTBI were analyzed (Figure 2). No differences in RR latencies between groups over the first 7 d after injury were found (P = 0.62). OVX mice demonstrated longer WM latencies over 28-31 d after injury (P = 0.049). While overall latencies were not different between male and female mice after MTBI, the difference in WM latencies between the first day and last day of testing was greater for female mice (M = 32.69 s) than male mice (M = 26.15 s; P = 0.034), which may serve as a corollary marker for learning. Sham data for male, female and OVX mice (Figure 3) show no sex interaction between the groups for water maze latencies.
At one hour after MTBI, female mice demonstrate decreased BBB permeability (IgG), microglial activation/macrophage recruitment (F4/80), and inflammatory gene expression (RT-PCR) compared to male mice. Concurrently, male mice demonstrate greater mortality than female mice after MTBI. Absence of female gonadal hormones (OVX) results in greater mortality and worse long-term neurobehavioral recovery.
While published data from preclinical models of severe TBI are ubiquitous, modeling of MTBI may be under-represented. In the United states, MTBI represents 75% of the total TBI cases[23]. Further, MTBI may result in long-term disability in 21% of cases[24,25]. In light of clinical trials with progesterone for moderate to severe TBI, potential effects of sex and female gonadal hormones in modifying outcome after MTBI should be investigated.
MTBI has been previously reproduced through preclinical injury modeling[26]. In the current model, MTBI was defined as an injury producing Day 1 RR latency between 50% and 90% of the baseline. While somewhat arbitrary, this definition allows noticeable neurobehavioral impairment but falls short of severe injury and associated high mortality. Further, post-injury Day 1 RR latencies were similar across all groups. Thus, the present definition of MTBI is an acceptable approximation of MTBI outcome in patients while at the same time allowing for between groups comparisons.
Data presented here demonstrate that the pathophysiology of MTBI may be quite different than that involved in severe TBI. A strong sex effect on recovery has been previously demonstrated in preclinical models of severe TBI[27]. In addition, gonadal hormones, particularly progesterone, have repeatedly demonstrated improvement in outcome in models of severe TBI[12,28,29]. Recently, progesterone has been used in combination with nicotinamide and interest has increased for estrogen as neuroprotecants against severe TBI[30,31]. However, in our model, sex may not be a major modifier of neurobehavioral outcome after MTBI. This finding may represent the lack of sustained injury after MTBI and the sensitivities of the RR and WM tests for uncovering mild to moderate injury. Perhaps new behavioral tests should be defined for MTBI. An excellent review of the development of various TBI models was recently published[32], including preclinical models that may represent MTBI. In fact, MTBI clearly differs from other more severe injury models of TBI in that immediate neurological deficits are not present while profound histopathological evidence exists for brain injury[33].
Interestingly, the removal of female gonadal hormones, as seen in OVX, worsened long-term neurobehavioral outcomes in this model of MTBI more so than the complete absence of female sex hormones as seen with male mice. Underlying mechanisms for this finding remain unclear in MTBI, buy this theme has been echoed in other neural injury models[30,31]. Thus, future investigation should include exogenous administration of female gonadal hormones (both estrogen and progesterone) in an attempt to reverse these detrimental effects after MTBI.
Sex differences in BBB permeability after MTBI suggest that vascularity and vasculogenesis may be influenced by gonadal hormones[32,33]. Moreover, estrogens have been shown to be vasculogenic inducers through associations with endothelial progenitor cells[32,34,35]. Thus, it can be postulated that gonad-intact female mice may demonstrate increased vasculogenesis after MTBI due to the presence of female gonadal hormones, when compared to male and OVX mice.
There are several limitations to this study that should be addressed. First, neither microglial activation nor BBB permeability was determined for OVX-female mice. The primary hypothesis was that young, gonad-intact female mice have improved long-term recovery associated with decreased neuroinflammation compared to male mice[36-39]. The addition of (OVX) mice occurred to begin to model potential sex effects in “menopausal” states. It is now clear that future studies should compare OVX, male, and gonad-intact female mice when assessing the role of gonadal hormones and sex differences after MTBI. Second, the effects of TBI-induced pituitary dysfunction, could be a potential confounding factor. Clinical and experimental studies clearly demonstrated that significant proportion of patients develop hypopituitarism after TBI with gonadotropin deficiency being one of the more common deficiences[40]. Resultin follicular stimulating hormone and luteinizing hormone deficiences result in decreased circulating estrogen and progesterone. Thus, pituitary effects alone may explain the heterogeneity in the outcome after TBI in young female rats. In addition, mice excluded due to insufficient injury were dissimilar across groups [2 of 17 OVX (11%), 1 of 22 males (5%), and 0 females (0%) were excluded]. While this may be the result of model variability, future work should examine the effects of gonadal hormones across the spectrum of TBI severity. Also, the interaction of age and sex was not examined in this study. Further, this study did not evaluate multiple isolated MTBI injuries, which may be more applicable to MTBI in humans with accumulation of injury over time. Finally, as previously mentioned, RR and WM may not be the most sensitive assessments for subtle neurocognitive impairment seen after MTBI. However, because these metrics were validated in previous studies[15,41-45], we consider them appropriate for use in the present study.
While these limitations exist, there remain compelling clinical implications for the current findings. First, associations between loss of female gonadal hormones and worse long-term neurobehavioral outcomes have potential ramifications for risk stratification in postmenopausal women that sustain MTBI. Second, demonstration that sex affects recovery after MTBI differently than in severe TBI may influence future investigations of potential targets for therapeutic development and require thoughtful consideration during clinical trial design. Finally, lack of sex differences in recovery after MTBI may demonstrate the resiliency of the young brain to mild/moderate injury. Exploration of the mechanisms, especially when contrasted with the aged brain, may bring translatable ideas into clinical research.
In conclusion, female sex is associated with decreased BBB permeability, markers of neuroinflammation, and mortality. Further, loss of female gonadal hormones is associated with worse long-term neurobehavioral recovery after MTBI. Future research should define sex-specific mechanisms of neuroinflammation after MTBI and the role of female gonadal hormones in potentiating recovery.
Sex differences in mild traumatic brain injury (TBI) are understudied, but prior studies in other forms of brain injury have found distinct sex differences. Further, pathophysiologic mechanisms for sex differences are not completely understood.
Understanding relevant pathophysiology of sex differences after TBI will alter treatment choices and development for patients.
A new model of mild TBI is clinically relevant in that it mimics the form of injury that the majority of patients experience. Further, increased understanding of sex differences in this form of TBI begins to alter the way clinicians approach these patients.
Future research should define sex-specific mechanisms of neuroinflammation after MTBI and the role of female gonadal hormones in potentiating recovery.
The manuscript is interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Critical care medicine
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Soriano-Ursua MA, Tanriverdi F S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Heegaard W, Biros M. Traumatic brain injury. Emerg Med Clin North Am. 2007;25:655-678, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Ghajar J. Traumatic brain injury. Lancet. 2000;356:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 608] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 3. | Töro K, Szilvia F, György D, Pauliukevicius A, Caplinskiene M, Raudys R, Lepik D, Tuusov J, Vali M. Fatal traffic injuries among children and adolescents in three cities (capital Budapest, Vilnius, and Tallinn). J Forensic Sci. 2011;56:617-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Foreman BP, Caesar RR, Parks J, Madden C, Gentilello LM, Shafi S, Carlile MC, Harper CR, Diaz-Arrastia RR. Usefulness of the abbreviated injury score and the injury severity score in comparison to the Glasgow Coma Scale in predicting outcome after traumatic brain injury. J Trauma. 2007;62:946-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Powell JW, Barber-Foss KD. Traumatic brain injury in high school athletes. JAMA. 1999;282:958-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 351] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1919] [Cited by in RCA: 1850] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 7. | Iverson GL. Outcome from mild traumatic brain injury. Curr Opin Psychiatry. 2005;18:301-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 431] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 8. | Vos PE. Biomarkers of focal and diffuse traumatic brain injury. Crit Care. 2011;15:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Nilsson BO. Modulation of the inflammatory response by estrogens with focus on the endothelium and its interactions with leukocytes. Inflamm Res. 2007;56:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Dimayuga FO, Reed JL, Carnero GA, Wang C, Dimayuga ER, Dimayuga VM, Perger A, Wilson ME, Keller JN, Bruce-Keller AJ. Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. J Neuroimmunol. 2005;161:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391-402, 402.e1-2. [PubMed] |
| 14. | Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;Appendix 4:Appendix 4I. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 419] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 15. | Lynch JR, Wang H, Mace B, Leinenweber S, Warner DS, Bennett ER, Vitek MP, McKenna S, Laskowitz DT. A novel therapeutic derived from apolipoprotein E reduces brain inflammation and improves outcome after closed head injury. Exp Neurol. 2005;192:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Lynch JR, Wang H, McGirt MJ, Floyd J, Friedman AH, Coon AL, Blessing R, Alexander MJ, Graffagnino C, Warner DS. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke. 2005;36:2024-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 1994;80:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 848] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 18. | Triguero D, Buciak JB, Yang J, Pardridge WM. Blood-brain barrier transport of cationized immunoglobulin G: enhanced delivery compared to native protein. Proc Natl Acad Sci USA. 1989;86:4761-4765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 146] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Indraswari F, Wang H, Lei B, James ML, Kernagis D, Warner DS, Dawson HN, Laskowitz DT. Statins improve outcome in murine models of intracranial hemorrhage and traumatic brain injury: a translational approach. J Neurotrauma. 2012;29:1388-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Lei B, Dawson HN, Roulhac-Wilson B, Wang H, Laskowitz DT, James ML. Tumor necrosis factor α antagonism improves neurological recovery in murine intracerebral hemorrhage. J Neuroinflammation. 2013;10:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 515] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 22. | Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5022] [Cited by in RCA: 5260] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 23. | Bruns JJ, Jagoda AS. Mild traumatic brain injury. Mt Sinai J Med. 2009;76:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Thornhill S, Teasdale GM, Murray GD, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: prospective cohort study. BMJ. 2000;320:1631-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 377] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, Paniak C, Pépin M. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;36:84-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 841] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 26. | Foda MA, Marmarou A. A new model of diffuse brain injury in rats. Part II: Morphological characterization. J Neurosurg. 1994;80:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 399] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 27. | Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001;24:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 245] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 28. | Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2008;57:386-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 29. | He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Veiga S, Melcangi RC, Doncarlos LL, Garcia-Segura LM, Azcoitia I. Sex hormones and brain aging. Exp Gerontol. 2004;39:1623-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Peterson TC, Hoane MR, McConomy KS, Farin FM, Bammler TK, MacDonald JW, Kantor ED, Anderson GD. A Combination Therapy of Nicotinamide and Progesterone Improves Functional Recovery following Traumatic Brain Injury. J Neurotrauma. 2015;32:765-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Day NL, Floyd CL, D’Alessandro TL, Hubbard WJ, Chaudry IH. 17β-estradiol confers protection after traumatic brain injury in the rat and involves activation of G protein-coupled estrogen receptor 1. J Neurotrauma. 2013;30:1531-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Povlishock J. The History and Evolution of Experimental Traumatic Brain Injury Models. Methods Mol Biol. 2016;1462:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Turner RC, VanGilder RL, Naser ZJ, Lucke-Wold BP, Bailes JE, Matsumoto RR, Huber JD, Rosen CL. Elucidating the severity of preclinical traumatic brain injury models: a role for functional assessment? Neurosurgery. 2014;74:382-394; discussion 394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159-165; discussion 166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 552] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 36. | Fadini GP, de Kreutzenberg S, Albiero M, Coracina A, Pagnin E, Baesso I, Cignarella A, Bolego C, Plebani M, Nardelli GB. Gender differences in endothelial progenitor cells and cardiovascular risk profile: the role of female estrogens. Arterioscler Thromb Vasc Biol. 2008;28:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45:530-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Hoetzer GL, MacEneaney OJ, Irmiger HM, Keith R, Van Guilder GP, Stauffer BL, DeSouza CA. Gender differences in circulating endothelial progenitor cell colony-forming capacity and migratory activity in middle-aged adults. Am J Cardiol. 2007;99:46-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115-3121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 318] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 40. | Tanriverdi F, Schneider HJ, Aimaretti G, Masel BE, Casanueva FF, Kelestimur F. Pituitary dysfunction after traumatic brain injury: a clinical and pathophysiological approach. Endocr Rev. 2015;36:305-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 41. | James ML, Wang H, Venkatraman T, Song P, Lascola CD, Laskowitz DT. Brain natriuretic peptide improves long-term functional recovery after acute CNS injury in mice. J Neurotrauma. 2010;27:217-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Laskowitz DT, McKenna SE, Song P, Wang H, Durham L, Yeung N, Christensen D, Vitek MP. COG1410, a novel apolipoprotein E-based peptide, improves functional recovery in a murine model of traumatic brain injury. J Neurotrauma. 2007;24:1093-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | James ML, Sullivan PM, Lascola CD, Vitek MP, Laskowitz DT. Pharmacogenomic effects of apolipoprotein e on intracerebral hemorrhage. Stroke. 2009;40:632-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Wang H, Lynch JR, Song P, Yang HJ, Yates RB, Mace B, Warner DS, Guyton JR, Laskowitz DT. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol. 2007;206:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Wang H, Gao J, Lassiter TF, McDonagh DL, Sheng H, Warner DS, Lynch JR, Laskowitz DT. Levetiracetam is neuroprotective in murine models of closed head injury and subarachnoid hemorrhage. Neurocrit Care. 2006;5:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |