Published online Nov 4, 2013. doi: 10.5492/wjccm.v2.i4.48
Revised: June 5, 2013
Accepted: July 4, 2013
Published online: November 4, 2013
AIM: To clarify the significance of vascular endothelial growth factor (VEGF) in peritoneal metastasis from gastric cancer, using the gastric cancer cell line MKN-45 compared with the high potential peritoneal dissemination gastric cancer cell line MKN-45P.
METHODS: The supernatant of culture medium of MKN-45 cells or MKN-45P cells was collected and the concentrations were measured of various cytokines, matrix metalloproteinases, growth factor and angiogenic factors, including VEGF. We performed an initial pilot study to explore whether bevacizumab, a humanized monoclonal antibody against VEGF, had any suppressive effect on the peritoneal dissemination from gastric cancer in an experimental nude mouse model of peritoneal metastasis.
RESULTS: The concentrations of interleukin-6 (IL-6), IL-8, VEGF and matrix metalloproteinase-2 protein in the culture supernatant were each significantly higher than each of those for MKN-45. In the in vivo study, the volume of ascites and the mitotic index were significantly lower in the therapy group than in the non-therapy group. The survival curve of the therapy group was significantly higher than that of the non-therapy group. These results suggested that VEGF was correlated with peritoneal metastasis from gastric cancer.
CONCLUSION: Findings suggested that bevacizumab for inhibiting VEGF could suppress peritoneal dissemination from gastric cancer.
- Citation: Aoyagi K, Kouhuji K, Miyagi M, Kizaki J, Isobe T, Hashimoto K, Shirouzu K. Molecular targeting therapy using bevacizumab for peritoneal metastasis from gastric cancer. World J Crit Care Med 2013; 2(4): 48-55
- URL: https://www.wjgnet.com/2220-3141/full/v2/i4/48.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v2.i4.48
Peritoneal metastasis is the most common form of recurrence from gastric cancer and is associated with a poor prognosis. Therefore, the management of any dissemination in the peritoneal cavity is important in the treatment of gastric cancer. However, there is as yet no effective treatment against peritoneal metastasis from gastric cancer. The development of peritoneal metastasis is a multistep process, beginning with the detachment of cancer cells from the primary tumor, their attachment to peritoneal mesothelial cells, retraction of the mesothelial cells, and exposure of the basement membrane. After attachment to the basement membrane, the cancer cells degrade in the extracellular matrix and then proliferate[1-3]. Finally, the cancer cells induce angiogenesis and lymphangiogenesis. Many cytokines, growth factors, matrix metalloproteinases and angiogenic factors play important roles in these steps. Tumor growth requires new vessel formation and this is driven predominantly by vascular endothelial growth factor (VEGF), the most potent angiogenic molecule known and the principle target for antiangiogenic therapy. VEGF levels in malignant ascites are remarkably elevated[4]. VEGF has been reported to enhance vascular permeability and angiogenesis in the abdominal wall and contributes to the establishment of peritoneal dissemination with malignant ascites[4,5]. In ovarian cancer, three pathological events are thought to cause malignant ascites: obstruction of the lymphatic vessels by tumor cells inhibiting lymphatic drainage from the peritoneal cavity; hyperpermeability of microvessels lining the peritoneal cavity; and angiogenesis[6]. In gastric cancer, there was a tendency for the tumor/normal ratio of VEGF mRNA to be correlated with distant metastasis[7] and positive expression of tissue VEGF, circulating VEGF, VEGF-C and VEGF-D were each associated with poor prognosis in resected gastric cancer[8]. We have previously reported that tissue VEGF was a useful indicator of peritoneal recurrence of gastric cancer[9]. The aim of the present study was to clarify the significance of VEGF in peritoneal metastasis from gastric cancer. We compared cytokines, matrix metalloproteinases (MMPs) and VEGF in the gastric cancer cell line MKN-45 and in the high potential peritoneal dissemination gastric cancer cell line MKN-45P, using an enzyme-linked immunosorbent assay (ELISA) method. Furthermore, we investigated whether administration of VEGF antibody could prevent peritoneal metastasis from gastric cancer. Bevacizumab is a humanized monoclonal antibody against VEGF and was the first commercially available angiogenesis inhibitor. We investigated whether bevacizumab had a suppressive effect on peritoneal dissemination from gastric cancer, experimentally, using a mouse peritoneal metastasis model.
We used the high potential peritoneal dissemination cell line MKN-45P, established from the human gastric cancer cell line MKN-45 (derived from a poorly differentiated adenocarcinoma in a 62 year old woman; Health Science Research Resources Bank, Tokyo, Japan) in our institute, as described previously[10]. Briefly, nude mice (BALB/c nu/nu) were subcutaneously inoculated with MKN-45 cells and the subcutaneous nodules were removed and injected into other nude mice intraperitoneally. The cancer cells from the peritoneal nodules were injected into the abdominal cavity of other mice. The process was continued through to a seventh generation. The resulting high potential peritoneal dissemination cell line was named MKN-45P. MKN-45 and MKN-45P cells were each maintained in RPMI-1640 medium (Nihon Seiyaku Co., Komaki, Aichi, Japan) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Gibco Uxbridge, Middlesex, United Kingdom), 2 mmol/L-glutamine and penicillin-streptomycin (50 IU/mL and 50 μg/mL, respectively) at 37.0 °C in humidified air with 5% CO2.
For measurement of cytokines in conditioned medium, the MKN-45 cells (1 × 106 cells/10 mL) or MKN-45P cells (1 × 106 cells/10 mL) were placed in 100 mm tissue culture dishes (IWAKI Co., Funabashi, Chiba, Japan) and cultured for 72 h in medium containing 10% FBS at 37.0 °C in humidified air with 5% CO2. The number of cells in each cell line was evaluated visually at 12, 24, 48 and 72 h (values: mean of three fields). The supernatant was then collected and the concentrations of interleukin-1β (IL-1β), IL-6, IL-8, IL-10, hepatocyte growth factor (HGF), transforming growth factor-β1 (TGF-β1), VEGF, MMP-2, MMP-9 and tissue inhibitor of metalloproteinases-1 (TIMP-1) proteins were each measured using the ELISA method (IL-1β, IL-8 and IL-10: Bio Source Europe S. A., Nivelles, Belgium; IL-6: Fujirebio Inc., Tokyo, Japan; HGF: Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan; TGF-β1 and VEGF: RD System Inc., Minneapolis, MN, United States; MMP-2, MMP-9 and TIMP-1: Daiichi Fine Chemical Co. Ltd., Takaoka, Toyama, Japan). Each cytokine was measured in 5 samples and the means of these were compared between the MKN-45 cells and the MKN-45P cells.
4 wk old athymic male BALB/c nu/nu nude mice, each weighing 18 g, were obtained from CLEA (Tokyo, Japan). The mice were housed in cages under specific pathogen-free conditions and provided with sterilized food and water ad libitum.
The humanized murine monoclonal antibody against human VEGF (bevacizumab, Avastin) was purchased from Genetech (San Francisco, CA, United States).
The experimental group consisted of 5 wk old male mice (n = 10). We determined a working concentration of bevacizumab according to Wildiers et al[11]. On day 0, we injected 1 × 107 MKN-45P cells into the abdominal cavity of each mouse, followed by a single intraperitoneal (ip) injection of 200 μg bevacizumab in 1 mL saline on day 0 and day 4. On day 21, five mice were sacrificed under ether anesthesia; these were weighed and then we calculated the mean number of tumor nodules in a 1 cm2 area in three fields on the mesentery and calculated the volume of ascites. We also extracted retroperitoneal tissues for histological examination. Another five mice were monitored until they died and the survival rate was calculated using the Kaplan-Meier method. A matching number of control mice were given 1 mL of drug-free saline.
After extraction, the retroperitoneal tissues were fixed for 12 h in 10% neutral buffered formaldehyde, then cut every 5 mm horizontally and embedded in paraffin. Paraffin sections were stained with hematoxylin-eosin (HE) and examined using light microscopy. We counted the frequency of hydronephrosis on the retroperitoneal tissues. The mitotic index was defined as the mean number of mitotic figures in a 400 times magnified field from ten arbitrary microscopic fields.
VEGF was analyzed using immunohistochemical staining and the avidin-biotin-peroxidase complex technique (Vectastain ABC Kit; Vector, Burlingame, CA, United States). Briefly, 3 μm thick sections of the formalin-fixed paraffin-embedded tissue specimens were deparaffinized and dehydrated. The sections were washed with phosphate-buffered saline (PBS), treated with 0.3% hydrogen peroxide in methanol for 30 min to block endogenous peroxidase, and then incubated with primary antibody in a humidified chamber at 4 °C overnight. As the primary antibody, rabbit polyclonal antibody A-20 was used (Santa Cruz Biotechnology, Santa Cruz, CA, United States) for VEGF, diluted at 1:200. Sections were washed three times with PBS, then incubated with biotinylated horse anti-rabbit immunoglobulin G antibody for 30 min, washed again three times with PBS, and then incubated with avidin-biotinylated peroxidase complex for 30 min. After three additional washings with PBS, staining was developed by incubating the sections in 3-amino-9-ethylcarbazole (Vector) for 5 min. The sections were then counterstained with hematoxylin and mounted. The cell types showing positive staining for VEGF were defined morphologically by H and E staining, using serial sections. VEGF expression was classified as one of three categories using a method modified from the literature that was previously used on gastric tissue[9], depending on the percentage of tumor cells stained: category 1 being less than 30% of cells stained; category 2 being from 30% to 49% stained; and category 3 being 50% or more cells stained.
This study was approved by Kurume University Institutional Animal Care and Use Committee of Ethics.
Student’s t-test and the χ2 test were used to analyze the data for any significant difference and any difference was considered statistically significant when the P value was less than 0.05. The cumulative survival rate was calculated using the Kaplan-Meier method. The significance of any difference between the survival curves was determined using the log-rank test and any difference was considered significant at the 5% level.
The number of MKN-45 or MKN-45P cells was counted at 24, 48 and 72 h. There was no difference in the number of cancer cells between the two cell lines.
The concentrations of cytokines in conditioned media from MKN-45 and from MKN-45P are shown in Table 1. The concentrations of IL-6, IL-8, VEGF and MMP-2 protein in the culture supernatants from MKN-45P were each significantly higher than each of those from MKN-45 (P = 0.045, P = 0.011, P = 0.013 and P = 0.021, respectively) (Table 1).
| IL-1β(pg/mL) | IL-6 (pg/mL) | IL-8 (pg/mL) | VEGF (pg/mL) | MMP-2 (ng/mL) | TIMP-1 (ng/mL) | |
| MKN-45 | 0.9 ± 0.7 | 1.2 ± 0.7 | 381.9 ± 147.1 | 1335.0 ± 624.3 | 0.3 ± 0.1 | 2.7 ± 1.8 |
| MKN-45P | 0.4 ± 0.2 | 2.9 ± 0.6 | 891.4 ± 210.2 | 3806.0 ± 229.8 | 0.7 ± 0.5 | 6.0 ± 4.0 |
| P value | 0.109 | 0.045 | 0.011 | 0.013 | 0.021 | 0.126 |
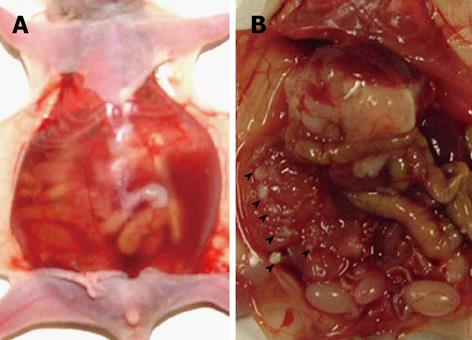
Peritoneal dissemination with bloody ascites was recognized in all five mice using the MKN-45P cell line (Figure 1A). Numerous nodules were seen on the mesentery (Figure 1B). We confirmed histologically that the nodule in the peritoneum was composed of cancer cells. All five mice in the non-therapy group were cachexic; however, there was no significant difference in body weight (P = 0.591) and no difference in the number of peritoneal nodules (P = 0.783) between the therapy and non-therapy group. The volume of ascites in the therapy group was significantly less than that in the non-therapy group (P = 0.042). No side-effects of bevacizumab were evident, such as bleeding, bowel perforation or thrombosis (Table 2).
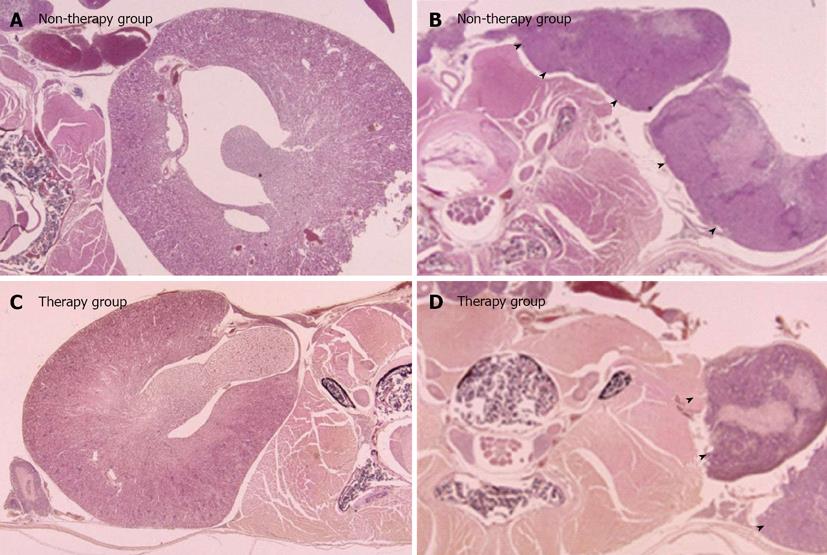
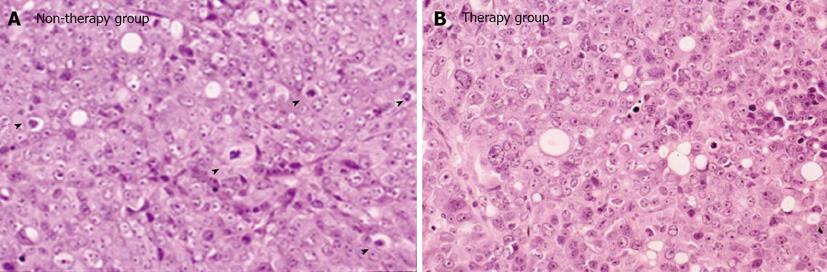
In the therapy group, two right kidneys (40%) and one left kidney (20%) showed hydronephrosis. In the non-therapy group, four right kidneys (80%) and two left kidneys (40%) showed hydronephrosis. The frequency of hydronephrosis in the therapy group was lower than that in the non-therapy group (Table 2). In the therapy group, the grade of hydronephrosis was mild and only a small amount of tissue was recognized in the retroperitoneum. In contrast, in the non-therapy group, the grade of hydronephrosis was severe and a large amount of tumor tissue was recognized in the retroperitoneum (Figure 2). High magnification examination of the tumor tissue revealed a lower number of mitoses in the therapy group than in the control group (Figure 3).
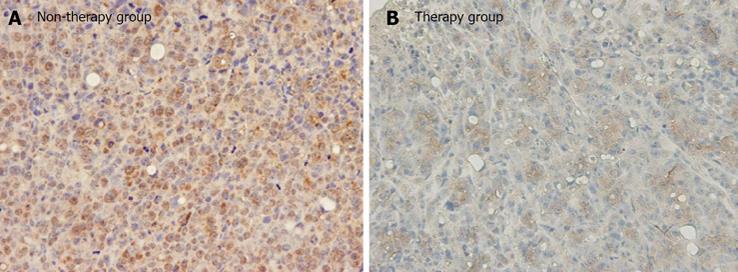
Immunoreactivity for VEGF was mainly identified as supranuclear staining or diffuse staining in the cytoplasm of the cancer cells (Figure 4). Based on the percentage of positive tumor staining, all of the five mice in the therapy group were in category 2, whereas all of the five mice in the non-therapy group were in category 3 (Figure 4).
The mitotic index was 9.6 ± 2.1 in the therapy group and this was significantly lower than that of 21.0 ± 5.7 in the non-therapy group (P < 0.01) (Table 2).
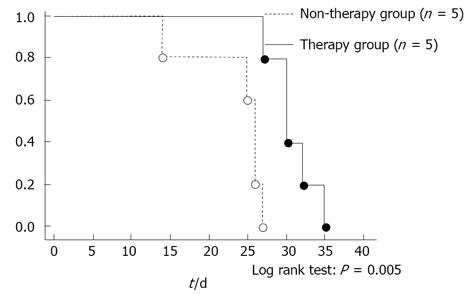
We investigated the findings for any correlation between survival and bevacizumab treatment. The median survival of the treated mice was 30.8 d and that of the untreated mice was 26.6 d The survival of the therapy group was significantly longer than that of the non-therapy group (P = 0.005) (Figure 5).
Research in the field of tumor angiogenesis has provided a foundation for radical development in the management and treatment of human cancers. VEGF is the most sensitive angiogenic factor and is expressed in cancer cells. Several clinical trials have confirmed that targeting the vascular VEGF/VEGF receptor pathway can show some clinical benefit. VEGF was initially described as a vascular permeability factor by Senger et al[12] in 1983 and was later cloned and found to be homologous to VEGF by Ferrara et al[13]. VEGF has been reported to enhance the permeability of tumor vessels[5], to induce serine protease or metalloproteases[14,15], to inhibit apoptosis in endothelial cells[16,17], and to inhibit the maturation of dendritic cells[18]. Since then, several randomized trials have shown a clinical benefit by various VEGF-targeted agents in patients with metastatic colorectal cancer, advanced non small cell lung cancer, renal cell carcinoma, hepatocellular carcinoma and metastatic breast cancer[19]. VEGF-targeted therapy has thus become an important treatment option for several human malignancies.
Peritoneal metastasis in gastric cancer takes place through a multistep process involving the detachment of cancer cells from the primary tumor, their attachment to the distant peritoneum, invasion into the subperitoneal space, proliferation and angiogenesis[1-3]. Angiogenesis is a key step in the various stages of human cancer development and dissemination. Previous reports have indicated that the presence of angiogenic factors is an essential event in the development of peritoneal metastasis[20-22].
In gastric cancer, there is a tendency for the tumor/normal ratio of VEGF mRNA to be correlated with distant metastasis[7]. Positive expression of tissue VEGF, circulating VEGF, VEGF-C and VEGF-D were each associated with poor prognosis in resected gastric cancer[8]. We have previously reported that tissue VEGF was a useful indicator of peritoneal recurrence from gastric cancer[9]. In our immunohistochemical study on clinical specimens, the VEGF score of patients with peritoneal recurrence was significantly higher than that of patients without peritoneal recurrence and the VEGF score was a significant parameter of peritoneal recurrence, suggesting that VEGF was correlated with peritoneal metastasis from gastric cancer and that VEGF was a useful indicator of peritoneal recurrence[9].
The present study reveals that the concentrations of IL-6, IL-8, VEGF and MMP-2 protein in the culture supernatant of MKN-45P are each significantly higher than each of those of MKN-45. IL-6 has been reported as a prognostic factor in gastric carcinoma and is significantly correlated with the incidence of lymph node metastasis and liver metastasis[23]. IL-8 has been reported as a prognostic factor in gastric carcinoma and is significantly correlated with the depth of invasion and vessel infiltration[24]. IL-6 and IL-8 are each related to the accomplishment of peritoneal dissemination by inducement of angiogenesis[25,26].
Degradation of the extracellular matrix is considered to be a prerequisite for peritoneal metastasis and MMPs are thought to play an important role in this process[27,28]. There are many reports that highly invasive cancer cells with a high potential for metastasis stimulate the production of MMPs[27] and that MMP-2 is significantly correlated with depth of invasion, lymph node metastasis and distant metastasis from gastric cancer[29].
These studies have provided clear evidence that VEGF is an essential element in the development of peritoneal metastasis. Accordingly, we investigated whether VEGF antibody might prevent peritoneal metastasis from gastric cancer.
Bevacizumab is a monoclonal antibody against VEGF that inhibits tumor growth by blocking angiogenesis. Cancer cells transferred with VEGF have been found to have an increased potential for the development of tumorigenesis in a xenograft model[21]. According to several reports, antiangiogenic agents can decrease tumor vessel permeability and prevent tumor growth[11,30,31]. Jain et al[31] have reported that antiangiogenic therapy normalized tumor vessels and reduced interstitial fluid pressure, which finally decreased malignant ascites. In the present study, all the mice in the non-therapy group were cachexic. However, there was no significant difference in body weight between the therapy group and the non-therapy group because the volume of ascites in the therapy group was significantly less than that in the non-therapy group. These findings suggested that bevacizumab suppressed cell proliferative activity by inhibiting angiogenesis of VEGF, thus contributing to the smaller amount of tumor tissue and the low incidence of hydronephrosis in the therapy group. Although the number of peritoneal nodules did not differ significantly between the two groups, the nodules on the mesentery in the treated group appeared to have been smaller but these were too small to be measured or weighed. The tumors on the retroperitoneum in the non-therapy group were larger than those in the therapy group and large tumors need new blood vessels for their growth. On immunohistochemical staining, the percentage of tumor cells stained for VEGF in the therapy group was lower than that in the non-therapy group. The mitotic index in the therapy group was also significantly lower than that in the non-therapy group. These results suggested that bevacizumab might suppress the vascular permeability effect and the cell proliferative activity by inhibiting angiogenesis of VEGF and thereby prolonging survival in the mice in the therapy group.
The findings from the present study indicate that the addition of bevacizumab to standard treatment might prolong the survival of gastric cancer patients, especially those with peritoneal metastasis. In conclusion, combination of bevacizumab with anticancer drugs may suppress peritoneal dissemination from gastric cancer.
The results from the present study show that VEGF was correlated with peritoneal metastasis from gastric cancer. Accordingly, using bevacizumab to inhibit VEGF may suppress peritoneal dissemination from gastric cancer. Therefore, combination of bevacizumab with anticancer drugs might suppress peritoneal dissemination from gastric cancer.
The therapy for peritoneal metastasis is the most important treatment to improve the prognosis of advanced gastric cancer. However, there is yet no effective treatment against peritoneal metastasis from gastric cancer. The relationship between vascular endothelial growth factor (VEGF) and peritoneal metastasis has been reported. Therefore, the authors investigated whether bevacizumab, a humanized monoclonal antibody against VEGF, had a suppressive effect on peritoneal dissemination from gastric cancer, experimentally, using a mouse peritoneal metastasis model.
The research hot spot is suppression of peritoneal metastasis and prolonging the survival of peritoneal metastasis by bevacizumab.
The authors proved that bevacizumab reduced the volume of ascites and decreased the proliferative activity of cancer cells on the peritoneum macroscopically and microscopically. So, this research proved the suppressive effect of bevacizumab for peritoneal metastasis from gastric cancer more clearly than other similar articles. Moreover, hydronephrosis is one of the most popular events of peritoneal metastasis from gastric cancer clinically. In this research, the authors focused on the hydronephrosis and proved the suppressive effect of bevacizumab for hydronephrosis to reduce the tumor volume on the retroperitoneum.
The results show that using bevacizumab to inhibit the VEGF may suppress peritoneal dissemination from gastric cancer. Therefore, bevacizumab could be used in preventing peritoneal recurrence and the combination of bevacizumab with anticancer drugs may suppress peritoneal dissemination from gastric cancer.
The authors think that the design of this study is good and they analyze the effect of molecular targeting therapy for VEGF against peritoneal metastasis from gastric cancer. The results are interesting and suggest that bevacizumab could be used in preventing peritoneal recurrence. The combination of bevacizumab with anticancer drugs may suppress peritoneal dissemination from gastric cancer.
P- Reviewers: Cimpean AM, Lu XM, Li Q, Tamiya M S- Editor: Gou SX L- Editor: Roemmele A E- Editor: Liu XM
| 1. | Yonemura Y, Endo Y, Yamaguchi T, Fujimura T, Obata T, Kawamura T, Nojima N, Miyazaki I, Sasaki T. Mechanisms of the formation of the peritoneal dissemination in gastric cancer. Int J Oncol. 1996;8:795-802. [PubMed] [Cited in This Article: ] |
| 2. | Yonemura Y. Peritoneal dissemination. Tokyo: Health Publishers 1996; . [Cited in This Article: ] |
| 3. | Liotta LA. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986;46:1-7. [PubMed] [Cited in This Article: ] |
| 4. | Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol. 1999;6:373-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 179] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029-1039. [PubMed] [Cited in This Article: ] |
| 6. | Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224-5229. [PubMed] [Cited in This Article: ] |
| 7. | Ryu KH, Shim KN, Jung SA, Yoo K, Joo YH, Lee JH. Significance of preoperative tissue levels of vascular-endothelial cadherin, liver-intestine cadherin and vascular endothelial growth factor in gastric cancer. Korean J Gastroenterol. 2012;60:229-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Liu L, Ma XL, Xiao ZL, Li M, Cheng SH, Wei YQ. Prognostic value of vascular endothelial growth factor expression in resected gastric cancer. Asian Pac J Cancer Prev. 2012;13:3089-3097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Aoyagi K, Kouhuji K, Yano S, Miyagi M, Imaizumi T, Takeda J, Shirouzu K. VEGF significance in peritoneal recurrence from gastric cancer. Gastric Cancer. 2005;8:155-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Miyagi M, Aoyagi K, Kato S, Shirouzu K. The TIMP-1 gene transferred through adenovirus mediation shows a suppressive effect on peritoneal metastases from gastric cancer. Int J Clin Oncol. 2007;12:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Wildiers H, Guetens G, De Boeck G, Verbeken E, Landuyt B, Landuyt W, de Bruijn EA, van Oosterom AT. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003;88:1979-1986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2734] [Cited by in F6Publishing: 2616] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 13. | Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1485] [Cited by in F6Publishing: 1453] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 14. | Pepper MS, Ferrara N, Orci L, Montesano R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun. 1991;181:902-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 439] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Unemori EN, Ferrara N, Bauer EA, Amento EP. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol. 1992;153:557-562. [PubMed] [Cited in This Article: ] |
| 16. | Shaheen RM, Davis DW, Liu W, Zebrowski BK, Wilson MR, Bucana CD, McConkey DJ, McMahon G, Ellis LM. Antiangiogenic therapy targeting the tyrosine kinase receptor for vascular endothelial growth factor receptor inhibits the growth of colon cancer liver metastasis and induces tumor and endothelial cell apoptosis. Cancer Res. 1999;59:5412-5416. [PubMed] [Cited in This Article: ] |
| 17. | Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336-30343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1473] [Cited by in F6Publishing: 1552] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 18. | Lissoni P, Malugani F, Bonfanti A, Bucovec R, Secondino S, Brivio F, Ferrari-Bravo A, Ferrante R, Vigoré L, Rovelli F. Abnormally enhanced blood concentrations of vascular endothelial growth factor (VEGF) in metastatic cancer patients and their relation to circulating dendritic cells, IL-12 and endothelin-1. J Biol Regul Homeost Agents. 2001;15:140-144. [PubMed] [Cited in This Article: ] |
| 19. | Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371-6375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 283] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Kondo Y, Arii S, Mori A, Furutani M, Chiba T, Imamura M. Enhancement of angiogenesis, tumor growth, and metastasis by transfection of vascular endothelial growth factor into LoVo human colon cancer cell line. Clin Cancer Res. 2000;6:622-630. [PubMed] [Cited in This Article: ] |
| 22. | Mori A, Arii S, Furutani M, Mizumoto M, Uchida S, Furuyama H, Kondo Y, Gorrin-Rivas MJ, Furumoto K, Kaneda Y. Soluble Flt-1 gene therapy for peritoneal metastases using HVJ-cationic liposomes. Gene Ther. 2000;7:1027-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Ashizawa T, Okada R, Suzuki Y, Takagi M, Yamazaki T, Sumi T, Aoki T, Ohnuma S, Aoki T. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005;8:124-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Kido S, Kitadai Y, Hattori N, Haruma K, Kido T, Ohta M, Tanaka S, Yoshihara M, Sumii K, Ohmoto Y. Interleukin 8 and vascular endothelial growth factor -- prognostic factors in human gastric carcinomas? Eur J Cancer. 2001;37:1482-1487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Huang SP, Wu MS, Wang HP, Yang CS, Kuo ML, Lin JT. Correlation between serum levels of interleukin-6 and vascular endothelial growth factor in gastric carcinoma. J Gastroenterol Hepatol. 2002;17:1165-1169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152:93-100. [PubMed] [Cited in This Article: ] |
| 27. | Nagase H, Woossner JF Jr. Matrix metallopreteinases. J Biol Chem. 1999;274:21492-21494. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3230] [Cited by in F6Publishing: 3096] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 28. | Mizutani K, Kofuji K, Shirouzu K. The significance of MMP-1 and MMP-2 in peritoneal disseminated metastasis of gastric cancer. Surg Today. 2000;30:614-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Mönig SP, Baldus SE, Hennecken JK, Spiecker DB, Grass G, Schneider PM, Thiele J, Dienes HP, Hölscher AH. Expression of MMP-2 is associated with progression and lymph node metastasis of gastric carcinoma. Histopathology. 2001;39:597-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1788] [Cited by in F6Publishing: 1802] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 31. | Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67:2729-2735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |