Published online Feb 4, 2013. doi: 10.5492/wjccm.v2.i1.4
Revised: December 8, 2012
Accepted: December 23, 2012
Published online: February 4, 2013
AIM: To evaluate the efficacy of intravenous glutamine on the patients with severe acute pancreatitis (SAP).
METHODS: The Cochrane Library, PubMed, EMBASE, and EBM review databases were searched up to June 2012. Randomized controlled trials (RCTs) that compared non-glutamine nutrition with intravenous glutamine supplemented nutrition in patients with SAP were included. A method recommended by the Cochrane Collaboration was used to perform a meta-analysis of those RCTs.
RESULTS: Four RCTs involving a total of 190 participants were included. Analysis of these RCTs revealed the presence of statistical homogeneity among them. Results showed that glutamine dipeptide has a positive effect in reducing the mortality rate (OR = 0.26, 95%CI: 0.09-0.73, P = 0.01), length of hospital stay (weighted mean difference = -4.85, 95%CI: 6.67--3.03, P < 0.001), and the rate of complications (OR = 0.41, 95%CI: 0.22-0.78, P = 0.006). No serious adverse effects were found.
CONCLUSION: Current best evidence demonstrates that glutamine is effective for SAP. Further high quality trials are required and parameters of nutritional condition and hospital cost should be considered in future RCTs with sufficient size and rigorous design.
Core tip: Glutamine dipeptide was given to patients with severe acute pancreatitis (SAP) in order to improve their nitrogen balance and immunonutrition. This meta-analysis aims to enhance our understanding of the clinical and economical validity of glutamine dipeptide for patients with SAP. We report the meta-analysis of four randomized controlled trials involving a total of 190 participants. Results showed that glutamine dipeptide has a positive effect in reducing the mortality rate, length of hospital stay, and the rate of complications. No serious adverse effects were found.
- Citation: Zhong X, Liang CP, Gong S. Intravenous glutamine for severe acute pancreatitis: A meta-analysis. World J Crit Care Med 2013; 2(1): 4-8
- URL: https://www.wjgnet.com/2220-3141/full/v2/i1/4.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v2.i1.4
Acute pancreatitis is a common and sometimes fatal disease that places a significant financial burden on society[1,2]. The mortality rate ranges from 10% to 15% in patients who are diagnosed with severe acute pancreatitis (SAP)[1,3]. The use of enteral nutrition (EN) for SAP was associated with a significant reduction in infectious morbidity, mortality, hospital length of stay, and a trend toward reduced organ failure morbidity[4]. However, the most common complications of enteral feeding is diarrhea, which can be detected up to 20%-30% of patients. Thus, parenteral nutrition (PN) is still a choice for the patients suffering from the SAP initially.
Glutamine (Gln) is the most abundant free amino acid in the body and plays a vital role in amino acid transport and nitrogen balance. Gln is also a primary fuel for rapidly dividing cells such as enterocytes and lymphocytes, which protect mucosa barrier and enhance immune functions[5]. The results of previous studies have shown that glutamine-enriched total PN (TPN) formulas improve the prognosis of acute pancreatitis[6,7]. It was given to patients with SAP in order to improve their nitrogen balance and immunonutrition[8-11]. Therefore, it is worth knowing whether routine supplementation of glutamine dipeptide is benefit for clinical outcomes. This meta-analysis aims to enhance our understanding of the clinical and economical validity of glutamine dipeptide for patients with SAP.
The titles and abstracts of all citations identified by the literature search were reviewed. Selection criteria were then applied to all potentially relevant studies. The meta-analysis included clinical randomized controlled trials (RCTs) of patients with SAP. The trials compared standard isonitrogen PN (or EN) and PN (or EN) intravenously supplemented with glutamine dipeptide. Editorials and expert opinions, reviews without original data, case reports and studies lacking control groups were excluded.
Trials were identified by searching the Cochrane Library (Issue 1 2012), PubMed (June 2012), EMBASE (June 2012), and CBM (Chinese Biomedical Literature Database). The query was constructed by using the combination of the following keywords: (SAP or acute pancreatitis) and (glutamine or glutamine dipeptide). Articles published in any language were considered. Abstracts of the articles selected from each of these multiple searches were reviewed and those meeting the criteria were recorded. In the case of duplicate reports, or studies obviously reporting results from the same study population, only the latest published results were used.
Data were extracted independently by two reviewers and decided by the research team. The quality of included studies was assessed independently by two authors and discrepancies were resolved by involving the third author. The quality of the studies was assessed using the scores proposed by Cochrane handbook 5 standards: randomization, allocation, concealment, blinding (participants, investigators, outcomes assessors, and data analysis), and completeness of follow-up. The following data were extracted: quantity and group dividing of patients, different doses and days of glutamine dipeptide used, and the baseline of trials. Outcome variables included: mortality rate, length of hospital stay, and rate of complications.
The statistical analysis was performed by RevMan5.0 software, which was provided by the Cochrane Collaboration. P value < 0.05 was considered statistically significant. Heterogeneity was checked by the χ2 test meta-analysis was done with fixed effects model when results of the trials had no heterogeneity. If the results had heterogeneity, random effects model was used and causes were analyzed. The result was expressed with an OR for the categorical variable and weighted mean difference (WMD) for the continuous variables, and with 95%CI.
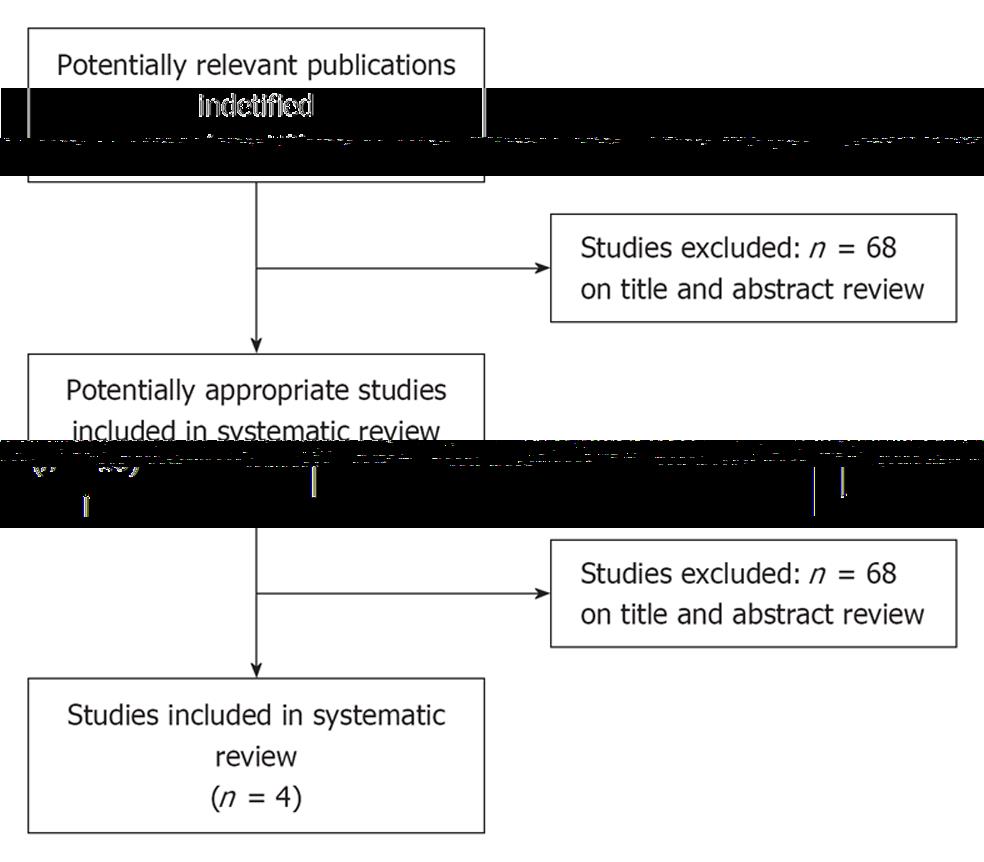
There were 78 papers relevant to the searching words. Through the steps of screening the title, reading the abstract and the entire article, only four RCTs involving 190 patients were included (Figure 1). There were three papers published in English and one in Chinese. Data regarding characteristics of the studies, including patients, baseline characteristics and quality assessment of the studies are summarized in Table 1, respectively.
| Study | Hajdú et al[8] | Han et al[9] | He et al[10] | Fuentes-Orozco et al[11] |
| No. of patients included | 45 | 60 | 41 | 44 |
| Mean age | No significant difference | No significant difference | No significant difference | No significant difference |
| Male/female | No significant difference | No significant difference | No significant difference | No significant difference |
| Body mass index on admission | No significant difference | No significant difference | No significant difference | No significant difference |
| Plasma glutamine levels on admission | No significant difference | No significant difference | No significant difference | No significant difference |
| Patients (Gln/Con) | 24/21 | 32/28 | 20/21 | 22/22 |
| Gln dipeptide | 0.5 g/kg per day, intravenously | 20 g/d, intravenously | 0.4 g/kg per day, intravenously | 0.4 g/kg per day, intravenously |
| Days of Gln (d) admininistration | 7 | 7 | 14 | 10 |
| Randomization | Yes | Yes | Yes | Yes |
| Allocation concealment | Yes | Yes | Yes | Yes |
| Double blinding | Yes | Yes | Yes | Yes |
| ITT analysis | Yes | Yes | Yes | Yes |
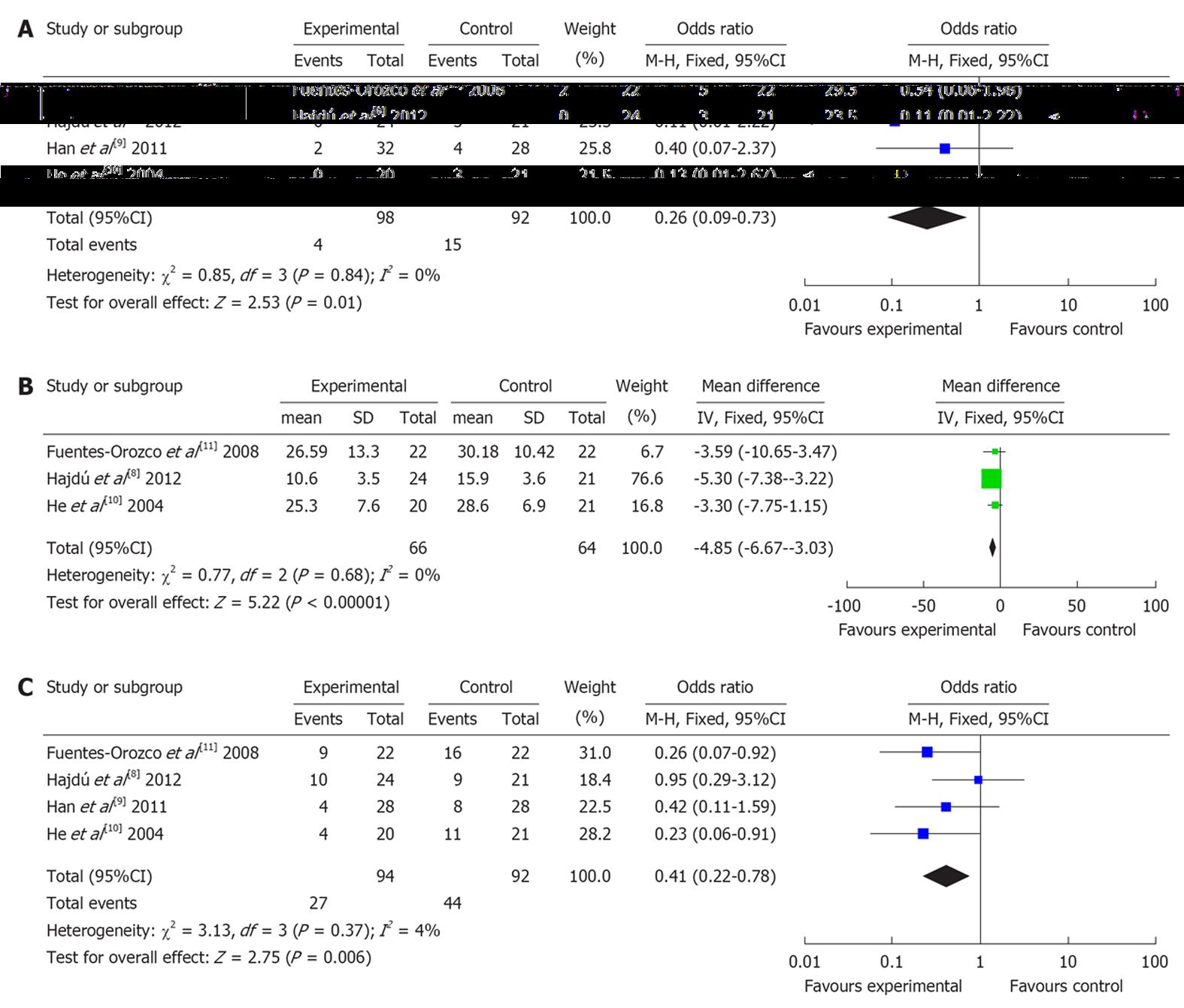
Four RCTs (involving 190 patients) reported mortality rate. There was no heterogeneity (P = 0.84). Combined analysis indicated that the use of glutamine dipeptide reduced the mortality rate (OR = 0.26, 95%CI: 0.09-0.73, P = 0.01) (Figure 2A).
Three RCTs (involving 130 patients) reported length of hospital stay. There was no heterogeneity (P = 0.77). Combined analysis indicated that the use of glutamine dipeptide reduced the length of hospital stay (WMD = -4.85, 95%CI: -6.67--3.03, P < 0.001) (Figure 2B).
Four RCTs (involving 190 patients) reported the rate of complications. There was no heterogeneity (P = 0.37). Combined analysis indicated that the use of glutamine dipeptide reduced the rate of complications (OR = 0.41, 95%CI: 0.22-0.78, P = 0.006) (Figure 2C).
The current meta-analysis demonstrated that the use of glutamine dipeptide could improve the outcome better than standard PN or EN. The use of glutamine dipeptide reduced the mortality rate, length of hospital stay, and the rate of complications. The inclusion criteria of the four RCTs were similar. There was no significant heterogeneity between any of the groups. At the same time, no serious adverse effects were found in all the included studies.
Glutamine is used as a major fuel and nucleotide substrate for rapidly dividing cells such as intestinal mucosal cells and the gut-associated immunocytes[12-14]. Glutamine can prevent atrophy of the intestinal epithelial cells through HSP 70 generation[15] and improve the intestinal immune barrier[16-18]. The deficiency of glutamine is the main cause for protein metabolism disorder, intestinal mucosal injury, enteral wall permeability destruction, bacterial translocation and immunosuppression. All these increase the secondary infection risk and hinder the recovery. A meta-analysis had revealed that glutamine could reduce the infectious morbidity and mortality in critical illness[19]. Another meta-analysis suggested that glutamine dipeptide-supplemented PN was beneficial to postoperative patients by shortening the length of hospital stay and reducing the morbidity of postoperative infectious complications[20].
In the early stage of SAP, the patients tend to be hypermetabolic due to occurrence of SIRS and subsequent multiple organ dysfunction syndromes, resulting in the greatly increased demand for nutrition[21-23]. A study had revealed that plasma glutamine levels were negatively correlated with the severity of acute pancreatitis[24]. The facts that EN is most likely superior to PN in preventing septic complications of acute pancreatitis, it may also eliminate some complications of PN (catheter sepsis, pneumothorax, and thrombosis), and costs less than TPN, make it an increasingly accepted treatment modality. According to the studies enrolled in our analysis, intravenously administered glutamine with TPN is beneficial in the prevention of infectious complications and reduce mortality rate[9-11]. At the same time, the recent RCT revealed that intravenously administered glutamine was able to achieve the same effect with early EN as well[8].
One of the disadvantages of this meta-analysis was that only four RCTs were included. All four studies had high methodological quality and generalizability, nonetheless, there may still have been bias in the final results. Besides, we didn’t analyse the parameters of nutritional condition such as concentrations of serum albumin and body weights after the use of glutamine because there is only one RCT has the data. Therefore, more multicenter cooperative studies with prospective design are needed.
In conclusion, PN supplemented glutamine dipeptide with or without EN is effective and safe to reduce the motality rate, occurence of complications, and length of hospital stay in patients with SAP. The encouraging outcomes in this analysis may demonstrate a notion in nutritional supplementation of the patients who are diagnosed with SAP. Further high quality trials are required. Parameters of nutritional condition and hospital cost should be considered in future RCTs with sufficient size and rigorous design.
Glutamine (Gln) is involved in a wide variety of metabolic and synthetic biochemical processes and acts as nitrogen and ammonium carrier to the liver and kidney. In conditions of excessive demand of Gln during episodes of severe diseases, endogenous Gln production may not be sufficient to meet the increased requirements.
The results of previous studies have shown that glutamine-enriched total parenteral nutrition formulas improve the prognosis of acute pancreatitis, however, there is no previous meta-analysis confirm the clinical validity and safety of glutamine dipeptide for patients with severe acute pancreatitis (SAP).
Authors performed a meta-analysis of four randomized controlled trials that compared non-glutamine nutrition with intravenous glutamine supplemented nutrition in patients with SAP. Authors’ results suggested that parenteral nutrition supplemented glutamine dipeptide with or without enteral nutrition is effective and safe in patients with SAP.
The encouraging outcomes may demonstrate a notion in nutritional supplementation of the patients who are diagnosed with SAP and provide a practical evidence for the use of Gln.
Gln is the most abundant free amino acid in the body and plays a vital role in amino acid transport and nitrogen balance. Deficiency of glutamine increases the secondary infection risk and hinder the recovery. Acute pancreatitis is a common disease with a very varied outcome. Patients with SAP have worse outcomes than those with mild acute pancreatitis. The two major complications of SAP are organ failure and infection, which are both considered as two major reasons for death.
This is a well written meta-analysis on the effect of intravenous glutamine in SAP. Mainly the authors systematically summarized and analyzed four randomized trial evidences regarding intravenous glutamine for SAP, and drew the conclusion that glutamine dipeptide has a positive effect in reducing the mortality rate, length of hospital stay, and the rate of complications. No serious adverse effects were found. Therefore the contribution of this meta-analysis is to provide a better knowledge of the benefits of glutamine infusion in SAP.
P- Reviewers Farré A, Jiang-Cao Kaufmann Y, Smith RC S- Editor Wen LL L- Editor A E- Editor Zheng XM
| 1. | Goldacre MJ, Roberts SE. Hospital admission for acute pancreatitis in an English population, 1963-98: database study of incidence and mortality. BMJ. 2004;328:1466-1469. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;1-20. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Cavallini G, Frulloni L, Bassi C, Gabbrielli A, Castoldi L, Costamagna G, De Rai P, Di Carlo V, Falconi M, Pezzilli R. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis. 2004;36:205-211. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Villatoro E, Mulla M, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2010;CD002941. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Hall JC, Heel K, McCauley R. Glutamine. Br J Surg. 1996;83:305-312. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Ockenga J, Borchert K, Rifai K, Manns MP, Bischoff SC. Effect of glutamine-enriched total parenteral nutrition in patients with acute pancreatitis. Clin Nutr. 2002;21:409-416. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Sahin H, Mercanligil SM, Inanç N, Ok E. Effects of glutamine-enriched total parenteral nutrition on acute pancreatitis. Eur J Clin Nutr. 2007;61:1429-1434. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Hajdú N, Belágyi T, Issekutz A, Bartek P, Gartner B, Oláh A. [Intravenous glutamine and early nasojejunal nutrition in severe acute pancreatitis -- a prospective randomized clinical study]. Magy Seb. 2012;65:44-51. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Han M, Liu T, Liu G, Wang PZ. [Clinical observation of immune nutritional agent on the treatment efect in patients with severe pancreatitis]. Tianjin Yike Daxue Xuebao. 2011;17:227-229. [Cited in This Article: ] |
| 10. | He XL, Ma QJ, Lu JG, Chu YK, Du XL. Effect of total parenteral nutrition (TPN) with and without glutamine dipeptide supplementation on outcome in severe acute pancreatitis (SAP). Clin Nutr Suppl. 2004;1:43-47. [DOI] [Cited in This Article: ] |
| 11. | Fuentes-Orozco C, Cervantes-Guevara G, Muciño-Hernández I, López-Ortega A, Ambriz-González G, Gutiérrez-de-la-Rosa JL, Gómez-Herrera E, Hermosillo-Sandoval JM, González-Ojeda A. L-alanyl-L-glutamine-supplemented parenteral nutrition decreases infectious morbidity rate in patients with severe acute pancreatitis. JPEN J Parenter Enteral Nutr. 2008;32:403-411. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Graf S, Egert S, Heer M. Effects of whey protein supplements on metabolism: evidence from human intervention studies. Curr Opin Clin Nutr Metab Care. 2011;14:569-580. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Wernerman J. Glutamine supplementation. Ann Intensive Care. 2011;1:25. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Blass SC, Goost H, Tolba RH, Stoffel-Wagner B, Kabir K, Burger C, Stehle P, Ellinger S. Time to wound closure in trauma patients with disorders in wound healing is shortened by supplements containing antioxidant micronutrients and glutamine: a PRCT. Clin Nutr. 2012;31:469-475. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Wischmeyer PE, Musch MW, Madonna MB, Thisted R, Chang EB. Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am J Physiol. 1997;272:G879-G884. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Ziegler TR, Bazargan N, Leader LM, Martindale RG. Glutamine and the gastrointestinal tract. Curr Opin Clin Nutr Metab Care. 2000;3:355-362. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Neu J, DeMarco V, Li N. Glutamine: clinical applications and mechanisms of action. Curr Opin Clin Nutr Metab Care. 2002;5:69-75. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Ikeda S, Kudsk KA, Le T, Zarzaur BL, Johnson CD. Glutamine improves impaired cellular exudation and polymorphonuclear neutrophil phagocytosis induced by total parenteral nutrition after glycogen-induced murine peritonitis. Shock. 2003;19:50-54. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Wang Y, Jiang ZM, Nolan MT, Jiang H, Han HR, Yu K, Li HL, Jie B, Liang XK. The impact of glutamine dipeptide-supplemented parenteral nutrition on outcomes of surgical patients: a meta-analysis of randomized clinical trials. JPEN J Parenter Enteral Nutr. 2010;34:521-529. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med. 2008;34:1980-1990. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130-135. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Tenner S, Sica G, Hughes M, Noordhoek E, Feng S, Zinner M, Banks PA. Relationship of necrosis to organ failure in severe acute pancreatitis. Gastroenterology. 1997;113:899-903. [PubMed] [DOI] [Cited in This Article: ] |