Published online Mar 9, 2023. doi: 10.5492/wjccm.v12.i2.53
Peer-review started: December 23, 2022
First decision: January 5, 2023
Revised: January 8, 2023
Accepted: February 17, 2023
Article in press: February 17, 2023
Published online: March 9, 2023
Congestive nephropathy is kidney dysfunction caused by the impact of elevated venous pressures on renal hemodynamics. As a part of cardiorenal syndrome, the diagnosis is usually made based on history and physical examination, with findings such as jugular venous distension, a third heart sound, and vital signs as supporting findings. More recently, however, these once though objective measures have come under scrutiny for their accuracy. At the same time, bedside ultrasound has increased in popularity and is routinely being used by clinicians to take some of the guess work out of making the diagnosis of volume overload and venous congestion. In this mini-review, we will discuss some of the traditional methods used to measure venous congestion, describe the role of point-of-care ultrasound and how it can ameliorate a clinician’s evaluation, and offer a descri
Core Tip: Congestive nephropathy denotes kidney dysfunction in fluid overload states as a result of venous congestion. Conventional methods to assess congestion at the bedside lack sensitivity and diagnostic accuracy. Point-of-care ultrasound is emerging as an enhancement to physical examination for objective assessment of congestion and guide therapy. Future research should focus on its impact on practical outcomes such as freedom from congestive symptoms, quality of life, and recurrent hospitalizations.
- Citation: Turk M, Robertson T, Koratala A. Point-of-care ultrasound in diagnosis and management of congestive nephropathy. World J Crit Care Med 2023; 12(2): 53-62
- URL: https://www.wjgnet.com/2220-3141/full/v12/i2/53.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v12.i2.53
It is well known that unresolved congestion is associated with adverse outcomes in patients with heart failure, increasing the risk of re-hospitalization and death[1,2]. In 2017 alone, heart failure admissions occurred at a rate of approximately 5 per 1000 United States adults with about a quarter of those patients experiencing readmissions, which highlights the magnitude of this problem[3]. The deleterious effects of fluid overload are now being recognized outside of heart failure, with multiple studies showing a positive fluid balance being associated with increased mortality[4,5]. Though seemingly straightforward, evaluation and management of congestion require a thorough understanding of the pathophysiology and hemodynamic principles. Multiple bedside diagnostic methods and tools exist for clinicians to assess congestion including signs and symptoms, physical examination, laboratory data, and radiography, but these all have limitations. On the other hand, timely diagnosis is vital as faster rates of decongestion are associated with a reduced risk of mortality and hospitalization[6]. In addition, end-organ effects of fluid overload are being increasingly recognized, which brings us to the topic of congestive nephropathy. Congestive nephropathy is defined as renal dysfunction that occurs due to venous congestion leading to impaired organ perfusion[7]. While this term was recently coined[8], several studies have previously shown that elevated central venous pressure (CVP) is associated with worsening renal function despite preserved cardiac index[9]. This does have pathophysiologic basis as the renal perfusion pressure is the difference between mean arterial pressure and CVP; if the CVP is elevated, the perfusion pressure drops, impairing renal blood flow. In addition, activation of the renin angiotensin-aldosterone system and consequent sodium and water retention, interstitial edema, endothelial dysfunction, and increased intra-abdominal pressure all contribute to increased pressure within the encapsulated kidney (renal tamponade), ultimately leading to organ dysfunction. Further, renal dysfunction can exacerbate the existing fluid overload, resulting in a vicious cycle. In this article, we will provide a kidney-centric overview of the bedside tools available to assess congestion, focusing on advances in point-of-care ultrasonography (POCUS).
The bedside assessment of a patient’s intravascular volume is challenging. Traditionally, this assessment involves taking a thorough history and performing cardiopulmonary physical examination. A patient’s given history can often be misleading or not reflective of their hemodynamic physiology. Physical examination, including assessment of jugular venous pressure, lower extremity edema, presence of an ‘S3’, and auscultation of the lungs for evidence of pulmonary edema, has traditionally been a common way for clinicians to assess intravascular volume status at the bedside. This is wrought with subjectivity and inaccuracies, and has almost no correlation with right heart catheterization, which is the invasive gold-standard assessment[10,11]. Similarly, chest X-ray remains a common modality to diagnose pulmonary congestion resulting from heart failure or other etiologies, despite having considerable diagnostic limitations including high false negative rate[12]. The degree of venous congestion beyond that of the jugular vein, specifically the alteration of blood flow in the hepatic, portal, and renal veins leading to congestive organ injury, cannot be assessed by physical examination or an X-ray. All these traditional approaches have significant limitations and cannot reliably detect hemodynamic congestion. Diagnosis of congestive nephropathy is challenging as no gold standard exists. Traditionally, the diagnosis of congestive nephropathy has been based on clinician gestalt after a trial-and-error period without any objective way to evaluate renal hemodynamics. POCUS using vascular Doppler analysis is emerging as a promising modality to assess for venous congestion along the continuum from the heart to the kidneys.
POCUS is a limited ultrasound examination performed at the bedside and interpreted by the treating physician. It is used to answer focused clinical questions, and is integrated with the patient’s history, physical examination, and other available data to narrow the differential diagnosis and inform management. POCUS is becoming more accessible to clinicians owing to the recent advances in ultrasound technology and availability of the low-cost, highly portable equipment. Compared to conventional examination, POCUS offers substantially higher diagnostic accuracy[13]. In the context of heart failure and congestion, POCUS not only aids in the diagnosis, but also guides decongestive therapy with potential implications for patient outcomes. In this section, we will outline the various components of sonographic evaluation of a patient with suspected fluid overload/venous congestion.
Lung ultrasound (LUS) has shown superiority over chest X-ray for nearly all clinical indications[14] and can detect extravascular edema prior to the onset of clinical symptoms. From diagnosing pneumonia[15] to identifying pulmonary edema[16], LUS has proven to be more accurate, and in some settings, more accessible. In a meta-analysis of six studies and more than 1800 patients, LUS had better sensitivity (88% vs 73%) when compared to chest X-ray for the diagnosis of cardiogenic pulmonary edema[17]. LUS findings are shown to have prognostic significance in various clinical scenarios including heart failure and end-stage renal disease[18,19]. With respect to guiding therapy, in the recent LUST trial[20], LUS-guided ultrafiltration strategy was associated with a reduction in the recurrence of decompensated heart failure and other cardiovascular events in hemodialysis patients. Similarly, in heart failure patients, LUS-guided management has shown to reduce acute decompensation events and urgent care visits[21,22]. LUS is an important diagnostic, prognostic, and management tool in the assessment of clinical or subclinical fluid overload. While it does not directly diagnose congestive nephropathy, it influences the treatment by establishing fluid tolerance vs intolerance. For example, in a patient with acute kidney injury, presence of extravascular lung water on LUS would sway away the clinician from administering empiric intravenous fluids, thus avoiding iatrogenic fluid overload. Figure 1 illustrates normal and abnormal LUS findings seen in fluid overload.
Focused cardiac ultrasound (FoCUS) is a POCUS examination of the heart and inferior vena cava (IVC). Essentially, it is a limited and problem-focused evaluation performed by any clinician trained in POCUS analogous to auscultation and not restricted to cardiologists. On the contrary, consultative echocardiography involves a comprehensive evaluation documenting a predefined set of parameters and measurements. FoCUS has a much higher diagnostic accuracy than conventional physical examination[23] and quickly provides vital information related to cardiac structure and function. Pathologies requiring immediate attention such as pericardial effusion, impaired contractility, gross chamber enlargement, and valvular anomalies can be diagnosed at the bedside and promptly addressed. In addition, IVC ultrasound allows non-invasive estimation of the CVP/right atrial pressure (RAP). As mentioned, elevated CVP is the starting point of venous congestion and is associated with impaired renal function as well as mortality[24]. In spontaneously breathing patients, current guidelines recommend stratifying RAP as follows. RAP is estimated to be 3 mmHg (0-5 mmHg) if the maximal anteroposterior diameter of the IVC is < 2.1 cm with > 50% collapse during a sniff. If the IVC is > 2.1 cm and collapses < 50%, RAP is documented as 15 mmHg (10-20 mmHg). An intermediate value of 8 mmHg (5-10 mmHg) is assigned where IVC parameters do not fit this paradigm. Elevated RAP estimated by IVC ultrasound is associated with hospital readmissions and mortality[25,26]. Despite its simplicity and apparent clinical utility, isolated IVC ultrasound has several pitfalls. First, estimation of RAP by IVC ultrasound is not accurate in mechanically ventilated patients. Even in those who are spontaneously breathing, strength of ‘sniff’ considerably varies among patients, leading to false impressions. Moreover, trained athletes and active young adults can have a chronically dilated IVC without elevated RAP whereas patients with elevated intra-abdominal pressure may have a collapsed IVC despite high RAP. In addition, IVC POCUS in long axis is subject to cylinder effect, which means when the ultrasound beam bisects the three-dimensional vessel (presumably a cylinder) in the periphery rather than the center, a falsely low diameter will be recorded. This leads to incorrect interpretation during follow-up studies, particularly when different operators are performing the study. Therefore, the IVC must be examined in both long and short axis views, where feasible[27,28]. Also, in conditions such as cirrhosis, IVC size/shape may be altered by the local structural changes, making it unreliable to predict RAP. Furthermore, it must be noted that isolated IVC POCUS does not provide real-time information on end-organ congestion, which in turn depends on both RAP and venous compliance. In other words, a plethoric IVC increases the probability of congestive organ injury but cannot objectively demonstrate it.
Venous excess ultrasound score: Venous excess ultrasound score (VExUS) stands for venous excess Doppler ultrasound. It involves Doppler evaluation of the abdominal veins (hepatic, portal, and intrarenal) to assess the flow pattern and thereby detect venous congestion that effects organ perfusion. While the Doppler patterns in these individual veins have been studied long before[29-32], the concept of VExUS is fairly new and first documented by Beaubien-Souligny et al[33] in 2020. In their study including 145 cardiac surgery patients, the investigators found that severe flow abnormalities in at least two of the three above-mentioned veins together with a dilated IVC (≥ 2 cm) predicts the risk of acute kidney injury (i.e., congestive nephropathy) with a hazard ratio of 3.69, outperforming isolated CVP measurement. Therefore, adding VExUS to IVC ultrasound improves the risk prediction of organ dysfunction. Based on the degree of flow alteration in individual veins, a scoring system was proposed to quantify systemic venous congestion, which is illustrated in Figure 2. In addition to diagnosing congestion, VExUS allows objective monitoring of congestion while the patient is receiving decongestive therapy as these waveforms are dynamic[34]. For example, Argaiz et al[35] have demonstrated that improvement in portal vein pulsatility coincides with improvement in renal function in patients with heart failure receiving diuretic therapy. In addition, several case reports exist demonstrating this phenomenon in multiple veins[36-41]. While there have not been published randomized clinical trials to date, outcome data for VExUS is emerging in the literature. For example, a high VExUS score, indicating severe hemodynamic congestion, has been shown to be associated with development of acute kidney injury in various clinical settings[34,42]. Specifically in heart failure patients, altered renal vein flow has been shown to confer worse outcomes[32,43,44]. In isolation, all these waveforms have limitations, which we have discussed in detail previously and is beyond the scope of this manuscript[45,46]. Of particular note, VExUS cannot distinguish between volume and pressure overload. For instance, a patient with precapillary pulmonary hypertension can have the same Doppler stigmata of congestion as a patient with iatrogenic fluid overload. It is up to the clinician to interpret the findings in the appropriate clinical context and in conjunction with other sonographic parameters (e.g., Doppler echocardiography). Having said that, congestion from any cause (pressure or volume) still leads to congestive nephropathy. In a large cohort of patients with pulmonary hypertension, Husain-Syed et al[47] showed that intrarenal venous congestion correlates with renal dysfunction as well as mortality/ morbidity end point, which exemplifies this concept.
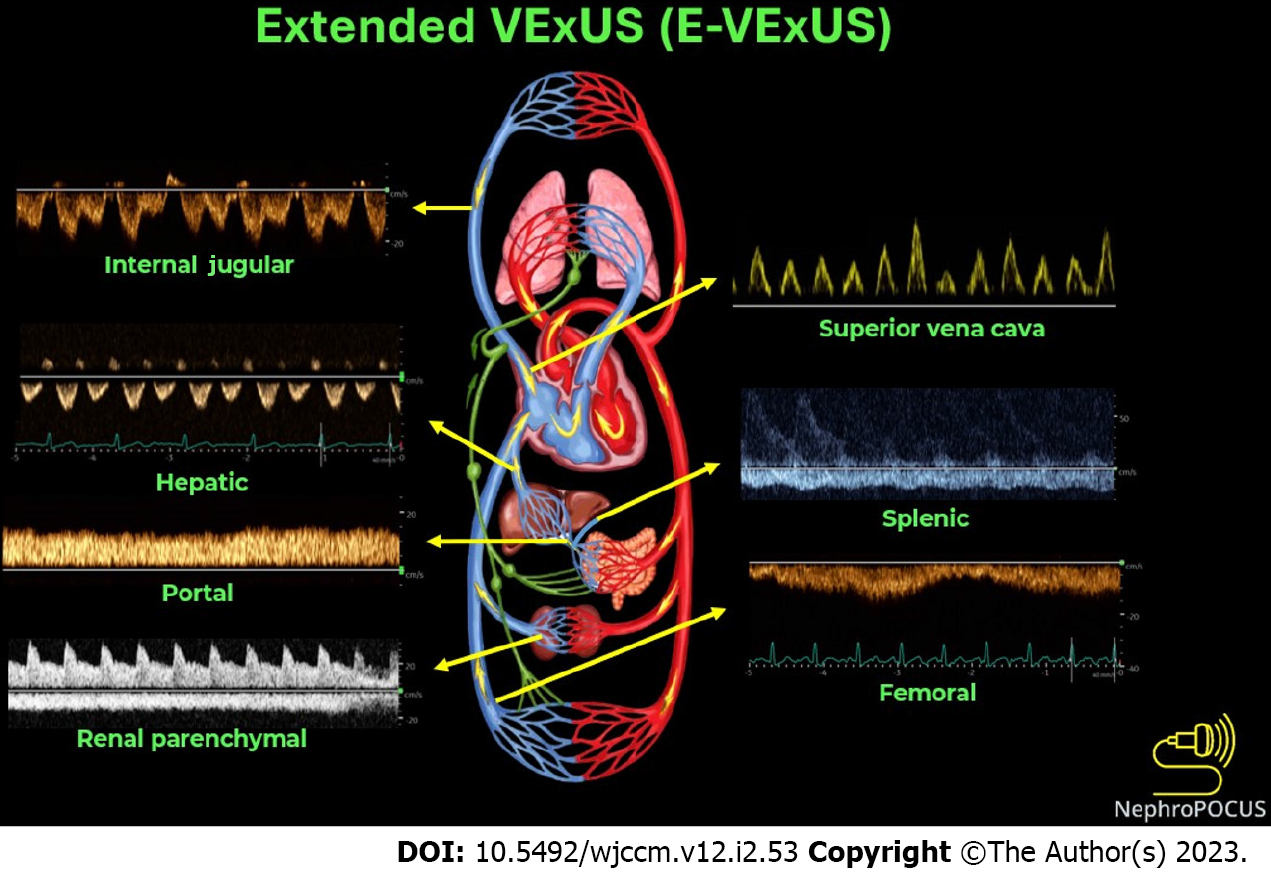
The term extended venous excess ultrasound score (E-VExUS) or extended VExUS has been proposed to include Doppler interrogation of additional veins such as the internal jugular, superior vena cava, splenic, and femoral veins in situations where the primary veins (e.g., hepatic, portal in cirrhosis, and intrarenal in advanced kidney disease) suffer from limitations[28,48]. This also includes estimation of RAP by greyscale POCUS of the internal jugular vein where IVC is not accessible or unreliable. Doppler components of E-VExUS are illustrated in Figure 3. Similar to the components of original VExUS, these veins have also been studied individually and shown to be useful to gauge the effects of elevated RAP[49-53]. Of late, femoral vein Doppler is gaining attention due to relative ease of image acquisition. In a recent study including 57 patients undergoing right heart catheterization, femoral vein flow alteration graded by stasis index showed excellent diagnostic performance to detect elevated RAP (specificity: 92.3% [80.0-99.3]; diagnostic accuracy: 90.4 [77.4-97.3]; positive likelihood ratio: 12.5 [3.01-51.97])[54]. However, caution must be exercised in ‘excluding’ elevated RAP/venous congestion based on the femoral vein alone as earlier studies showed a relatively low sensitivity[55]. This VExUS expansion is still in its early stages of adoption, so there is need for more data to establish its clinical utility in routine practice. Figure 4 is the sonographic representation of chain of venous congestion from the right heart to femoral vein. Table 1 summarizes the key sonographic findings and limitations of each application in the context of congestive nephropathy.
| Sonographic application | Possible findings in the context of congestive nephropathy | Limitations |
| Lung ultrasound | Elevated extravascular lung water (B-lines) and pleural effusion | B-lines are non-specific and can be seen in non-cardiogenic pulmonary edema, lung fibrosis, contusion, and alveolar hemorrhage |
| Focused cardiac ultrasound (basic) | LV systolic dysfunction (qualitative and M-mode); RV systolic dysfunction (qualitative and M-mode); Pericardial effusion; Gross chamber enlargement (e.g., RV dilation leading to interventricular septal flattening); Gross valvular dysfunction (e.g., tricuspid regurgitation on color Doppler); Elevated right atrial pressure (plethoric IVC) | Lack of spectral Doppler provides limited information. Qualitative assessment relies on operator experience. IVC cannot reliably estimate RAP in mechanically ventilated patients. IVC can be small in intra-abdominal hypertension despite elevated RAP. IVC can be dilated without elevated RAP in trained athletes |
| Focused cardiac ultrasound (advanced) | Reduced stroke volume assessed by LV outflow tract velocity time integral. Elevated LV filling pressures assessed by mitral inflow Doppler and mitral annular tissue Doppler. Elevated pulmonary artery pressures/right ventricular systolic pressure assessed by continuous wave Doppler through the RV outflow tract and tricuspid valve. Elevated right atrial pressure assessed by tricuspid inflow and tissue Doppler | Requires higher operator skill level and training than basic cardiac ultrasound. Suboptimal views/Doppler angle limit the accuracy of measurements obtained. Some of the parameters lack validation in critical illness |
| Hepatic vein Doppler | Reduced amplitude or reversal of the systolic wave (Normally, systolic wave is larger than the diastolic wave) | Prone to erroneous interpretation without EKG. Cannot differentiate pressure and volume overload (applies to all components of VExUS and E-VExUS). Influenced by factors other than RAP (e.g., atrial fibrillation, RV systolic excursion). Diminished pulsatility in cirrhosis; may not accurately reflect the degree of congestion |
| Portal vein Doppler | Increased pulsatility (normal waveform is near-continuous) | Pulsatile portal vein can be seen in cirrhosis and healthy, young individuals without an elevated RAP. Can appear falsely normal despite elevated RAP in patients with portal hypertension |
| Intra-renal venous Doppler | Increased pulsatility, systolic wave reversal (normal waveform is near-continuous) | Most technically challenging of the three components of VExUS. Sampling a larger vessel such as the main renal vein instead of interlobar vein leads to mistaken interpretation |
| E-VExUS | IJ vein: Reduced amplitude or reversal of the systolic wave (normally, systolic wave is larger than the diastolic wave); Splenic vein: Increased pulsatility (normal waveform is near-continuous); SVC: Reduced amplitude or reversal of the systolic wave (normally, systolic wave is larger than the diastolic wave); Femoral: Increased pulsatility and elevated velocity of the retrograde component (normal waveform is near-continuous) | Not validated as a combination score though individual components are studied. EKG is required when there is no simultaneous arterial trace to delineate cardiac cycles. IJ vein: Susceptible to probe pressure due to its relatively superficial location. Splenic vein: Similar limitations as portal vein. SVC: Technically challenging to access via transthoracic windows. Femoral: Relatively less sensitive to detect elevated RAP. Severe intra-abdominal hypertension may influence the waveform |
While POCUS has gained a lot of traction over the last several years, it is sometimes met with a degree of skepticism. Detractors are quick to point out that a significant mortality benefit with use of POCUS has not been shown. For example, the SHoC-ED trial randomized almost 300 patients with undifferentiated shock into a POCUS plus standard of care vs standard of care without ultrasonography to help diagnose the etiology of shock and help manage the condition. This showed no mortality benefit, no decrease in length of stay, decrease in intravenous fluid use, or decrease in rates of computed tomography scanning[55]. Conversely, the supporters of POCUS are quick to point out that achieving a mortality benefit in an intervention that is not therapeutic is a mountain that may prove too high to climb; in essence, unfair to expect of a diagnostic modality. In most cases, POCUS and VExUS scoring help quantify congestion in an objective manner and allow clinicians to rely much less on other unreliably recorded measures such as daily weights and intake-output documentation. Several randomized controlled trials incorporating VExUS are currently underway to determine its efficacy not only in the diagnosis but also in guiding the management such as for dosing diuretics. The use of elements of the extended VExUS examination needs to be further validated in population wide studies before becoming mainstays of the evaluation. Due to the medical community’s long-standing affinity for objective scoring systems, VExUS will without a doubt become more commonplace. However, there will continue to be significant demand from clinicians for a show of mortality reduction before the practice becomes widely adopted. In the meantime, it is important to give weight to other outcomes such as time to diagnosis, readmission rates, recovery of renal function, symptom burden from heart failure and congestion, and quality of life in the judgment of this emerging technique. On the other hand, we do acknowledge that POCUS training remains an unmet need currently. Applications such as Doppler echocardiography, VExUS, and E-VExUS require solid technical skills that can only be garnered by longitudinal training. Especially in nephrology, there are a very few fellowship programs that offer training in comprehensive hemodynamic assessment at this time[56,57]. This is ironic given that most of the consults in a typical nephrology practice revolve around managing fluid disorders. While the situation is slightly better in critical care medicine, guideline-mandated training requirements remain vague. As such, professional organizations must step up and establish robust POCUS certification and competency assessment standards. Otherwise, performance of advanced sonographic applications by inadequately trained physicians may potentially result in patient harm.
It is well known that hemodynamic congestion has adverse effects on multi-organ function and is associated with adverse clinical outcomes. Ultrasonographic techniques have long been used to quantify venous congestion and have been validated extensively in the medical literature. The combination of Doppler findings from several organ systems into an objective evaluation is a process that has been undergoing significant study in recent years. While VExUS has its limitations, it has promise as a dependable tool in the management of congestive nephropathy and is superior to any other bedside noninvasive assessment. As with other diagnostic tools, it is critical that clinicians analyze their findings as just one part of the larger clinical puzzle in conjunction with other objective data points. In the correct clinical context, using VExUS findings to apply individualized changes to care plans may ultimately help deliver more accurate care to patients with suspected congestive nephropathy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chan AHY, Canada; Hatamnejad MR, Iran S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Lucas C, Johnson W, Hamilton MA, Fonarow GC, Woo MA, Flavell CM, Creaser JA, Stevenson LW. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. 2000;140:840-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 292] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 2. | Rubio-Gracia J, Demissei BG, Ter Maaten JM, Cleland JG, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison BA, Givertz MM, Bloomfield DM, Dittrich H, Damman K, Pérez-Calvo JI, Voors AA. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol. 2018;258:185-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 3. | Agarwal MA, Fonarow GC, Ziaeian B. National Trends in Heart Failure Hospitalizations and Readmissions From 2010 to 2017. JAMA Cardiol. 2021;6:952-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 4. | Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van Regenmortel N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46:361-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 5. | Cardoso FS, Pereira R, Laranjo A, Gamelas V, Bagulho L, Germano N, Karvellas CJ. Positive fluid balance was associated with mortality in patients with acute-on-chronic liver failure: A cohort study. J Crit Care. 2021;63:238-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | McCallum W, Tighiouart H, Testani JM, Griffin M, Konstam MA, Udelson JE, Sarnak MJ. Rates of In-Hospital Decongestion and Association with Mortality and Cardiovascular Outcomes Among Patients Admitted for Acute Heart Failure. Am J Med. 2022;135:e337-e352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | D' Marco L. Congestive Nephropathy. Int J Environ Res Public Health. 2022;19. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 8. | Husain-Syed F, Gröne HJ, Assmus B, Bauer P, Gall H, Seeger W, Ghofrani A, Ronco C, Birk HW. Congestive nephropathy: a neglected entity? ESC Heart Fail. 2021;8:183-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Tabucanon T, Tang WHW. Right Heart Failure and Cardiorenal Syndrome. Cardiol Clin. 2020;38:185-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Koratala A, Ronco C, Kazory A. Diagnosis of Fluid Overload: From Conventional to Contemporary Concepts. Cardiorenal Med. 2022;12:141-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944-1956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 352] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 12. | Long B, Koyfman A, Gottlieb M. Diagnosis of Acute Heart Failure in the Emergency Department: An Evidence-Based Review. West J Emerg Med. 2019;20:875-884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Koratala A, Kazory A. An Introduction to Point-of-Care Ultrasound: Laennec to Lichtenstein. Adv Chronic Kidney Dis. 2021;28:193-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Chiu L, Jairam MP, Chow R, Chiu N, Shen M, Alhassan A, Lo CH, Chen A, Kennel PJ, Poterucha TJ, Topkara VK. Meta-Analysis of Point-of-Care Lung Ultrasonography Versus Chest Radiography in Adults With Symptoms of Acute Decompensated Heart Failure. Am J Cardiol. 2022;174:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Amatya Y, Rupp J, Russell FM, Saunders J, Bales B, House DR. Diagnostic use of lung ultrasound compared to chest radiograph for suspected pneumonia in a resource-limited setting. Int J Emerg Med. 2018;11:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Koratala A, Ronco C, Kazory A. The Promising Role of Lung Ultrasound in Assessment of Volume Status for Patients Receiving Maintenance Renal Replacement Therapy. Blood Purif. 2020;49:643-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Maw AM, Hassanin A, Ho PM, McInnes MDF, Moss A, Juarez-Colunga E, Soni NJ, Miglioranza MH, Platz E, DeSanto K, Sertich AP, Salame G, Daugherty SL. Diagnostic Accuracy of Point-of-Care Lung Ultrasonography and Chest Radiography in Adults With Symptoms Suggestive of Acute Decompensated Heart Failure: A Systematic Review and Meta-analysis. JAMA Netw Open. 2019;2:e190703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 18. | Reisinger N, Koratala A. Quantitative Lung Ultrasonography for the Nephrologist: Applications in Dialysis and Heart Failure. Kidney360. 2022;3:176-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, Hempel D, Wilson C, Frasure SE, Jhund PS, Cheng S, Solomon SD. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J. 2016;37:1244-1251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 20. | Zoccali C, Torino C, Mallamaci F, Sarafidis P, Papagianni A, Ekart R, Hojs R, Klinger M, Letachowicz K, Fliser D, Seiler-Mußler S, Lizzi F, Wiecek A, Miskiewicz A, Siamopoulos K, Balafa O, Slotki I, Shavit L, Stavroulopoulos A, Covic A, Siriopol D, Massy ZA, Seidowsky A, Battaglia Y, Martinez-Castelao A, Polo-Torcal C, Coudert-Krier MJ, Rossignol P, Fiaccadori E, Regolisti G, Hannedouche T, Bachelet T, Jager KJ, Dekker FW, Tripepi R, Tripepi G, Gargani L, Sicari R, Picano E, London GM. A randomized multicenter trial on a lung ultrasound-guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int. 2021;100:1325-1333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 21. | Rivas-Lasarte M, Álvarez-García J, Fernández-Martínez J, Maestro A, López-López L, Solé-González E, Pirla MJ, Mesado N, Mirabet S, Fluvià P, Brossa V, Sionis A, Roig E, Cinca J. Lung ultrasound-guided treatment in ambulatory patients with heart failure: a randomized controlled clinical trial (LUS-HF study). Eur J Heart Fail. 2019;21:1605-1613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 22. | Marini C, Fragasso G, Italia L, Sisakian H, Tufaro V, Ingallina G, Stella S, Ancona F, Loiacono F, Innelli P, Costantino MF, Sahakyan L, Gabrielyan S, Avetisyan M, Margonato A, Agricola E. Lung ultrasound-guided therapy reduces acute decompensation events in chronic heart failure. Heart. 2020;106:1934-1939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Marbach JA, Almufleh A, Di Santo P, Jung R, Simard T, McInnes M, Salameh JP, McGrath TA, Millington SJ, Diemer G, West FM, Domecq MC, Hibbert B. Comparative Accuracy of Focused Cardiac Ultrasonography and Clinical Examination for Left Ventricular Dysfunction and Valvular Heart Disease: A Systematic Review and Meta-analysis. Ann Intern Med. 2019;171:264-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 624] [Cited by in F6Publishing: 631] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 25. | Khandwalla RM, Birkeland KT, Zimmer R, Henry TD, Nazarian R, Sudan M, Mirocha J, Cha J, Kedan I. Usefulness of Serial Measurements of Inferior Vena Cava Diameter by Vscan(TM) to Identify Patients With Heart Failure at High Risk of Hospitalization. Am J Cardiol. 2017;119:1631-1636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Cubo-Romano P, Torres-Macho J, Soni NJ, Reyes LF, Rodríguez-Almodóvar A, Fernández-Alonso JM, González-Davia R, Casas-Rojo JM, Restrepo MI, de Casasola GG. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J Hosp Med. 2016;11:778-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Koratala A, Kazory A. Point of Care Ultrasonography for Objective Assessment of Heart Failure: Integration of Cardiac, Vascular, and Extravascular Determinants of Volume Status. Cardiorenal Med. 2021;11:5-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 28. | Koratala A, Reisinger N. Point of Care Ultrasound in Cirrhosis-Associated Acute Kidney Injury: Beyond Inferior Vena Cava. Kidney360. 2022;3:1965-1968. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 29. | Nagueh SF, Kopelen HA, Zoghbi WA. Relation of mean right atrial pressure to echocardiographic and Doppler parameters of right atrial and right ventricular function. Circulation. 1996;93:1160-1169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 174] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Arisawa J, Morimoto S, Ikezoe J, Naitoh H, Yamagami H, Kozuka T, Sano T, Shimazaki Y, Matsuda H. Pulsed Doppler echocardiographic assessment of portal venous flow patterns in patients after the Fontan operation. Br Heart J. 1993;69:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Bateman GA, Giles W, England SL. Renal venous Doppler sonography in preeclampsia. J Ultrasound Med. 2004;23:1607-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, Kawakami Y, Aonuma K. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart Fail. 2016;4:674-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 33. | Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R, Denault AY. Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. 2020;12:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 34. | Rola P, Miralles-Aguiar F, Argaiz E, Beaubien-Souligny W, Haycock K, Karimov T, Dinh VA, Spiegel R. Clinical applications of the venous excess ultrasound (VExUS) score: conceptual review and case series. Ultrasound J. 2021;13:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 35. | Argaiz ER, Rola P, Gamba G. Dynamic Changes in Portal Vein Flow during Decongestion in Patients with Heart Failure and Cardio-Renal Syndrome: A POCUS Case Series. Cardiorenal Med. 2021;11:59-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Taleb Abdellah A, Koratala A. Nephrologist-Performed Point-of-Care Ultrasound in Acute Kidney Injury: Beyond Hydronephrosis. Kidney Int Rep. 2022;7:1428-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 37. | Argaiz ER, Koratala A, Reisinger N. Comprehensive Assessment of Fluid Status by Point-of-Care Ultrasonography. Kidney360. 2021;2:1326-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Samant S, Koratala A. Point-of-care Doppler ultrasound in the management of hyponatremia: Another string to nephrologists' Bow. Clin Case Rep. 2021;9:e04687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Koratala A, Sturgill D. Point-of-care venous Doppler ultrasound in the management of heart failure and hyponatremia. Clin Nephrol. 2021;96:63-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Singh S, Koratala A. Utility of Doppler ultrasound derived hepatic and portal venous waveforms in the management of heart failure exacerbation. Clin Case Rep. 2020;8:1489-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Koratala A, Ronco C, Kazory A. Multi-Organ Point-Of-Care Ultrasound in Acute Kidney Injury. Blood Purif. 2022;51:967-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Spiegel R, Teeter W, Sullivan S, Tupchong K, Mohammed N, Sutherland M, Leibner E, Rola P, Galvagno SM Jr, Murthi SB. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit Care. 2020;24:615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 43. | Ohara H, Yoshihisa A, Horikoshi Y, Ishibashi S, Matsuda M, Yamadera Y, Sugawara Y, Ichijo Y, Hotsuki Y, Watanabe K, Sato Y, Misaka T, Kaneshiro T, Oikawa M, Kobayashi A, Takeishi Y. Renal Venous Stasis Index Reflects Renal Congestion and Predicts Adverse Outcomes in Patients With Heart Failure. Front Cardiovasc Med. 2022;9:772466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 44. | Yamamoto M, Seo Y, Iida N, Ishizu T, Yamada Y, Nakatsukasa T, Nakagawa D, Kawamatsu N, Sato K, Machino-Ohtsuka T, Aonuma K, Ohte N, Ieda M. Prognostic Impact of Changes in Intrarenal Venous Flow Pattern in Patients With Heart Failure. J Card Fail. 2021;27:20-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Galindo P, Gasca C, Argaiz ER, Koratala A. Point of care venous Doppler ultrasound: Exploring the missing piece of bedside hemodynamic assessment. World J Crit Care Med. 2021;10:310-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (4)] |
| 46. | Koratala A, Reisinger N. Venous Excess Doppler Ultrasound for the Nephrologist: Pearls and Pitfalls. Kidney Med. 2022;4:100482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 47. | Husain-Syed F, Birk HW, Ronco C, Schörmann T, Tello K, Richter MJ, Wilhelm J, Sommer N, Steyerberg E, Bauer P, Walmrath HD, Seeger W, McCullough PA, Gall H, Ghofrani HA. Doppler-Derived Renal Venous Stasis Index in the Prognosis of Right Heart Failure. J Am Heart Assoc. 2019;8:e013584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 48. | Koratala A. Hemodynamic POCUS in cirrhosis: think beyond the IVC. NephroPOCUS.com. Last accessed: 12/21/2022. Available from: https://nephropocus.com/2022/11/28/hemodynamic-pocus-in-cirrhosis-think-beyond-the-ivc/. [Cited in This Article: ] |
| 49. | Sivaciyan V, Ranganathan N. Transcutaneous doppler jugular venous flow velocity recording. Circulation. 1978;57:930-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 64] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Appleton CP, Hatle LK, Popp RL. Superior vena cava and hepatic vein Doppler echocardiography in healthy adults. J Am Coll Cardiol. 1987;10:1032-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 134] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Ghio S, Recusani F, Sebastiani R, Klersy C, Raineri C, Campana C, Lanzarini L, Gavazzi A, Tavazzi L. Doppler velocimetry in superior vena cava provides useful information on the right circulatory function in patients with congestive heart failure. Echocardiography. 2001;18:469-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Alimoğlu E, Erden A, Gürsel K, Olçer T. Correlation of right atrial pressure and blood flow velocities in the common femoral vein obtained by duplex Doppler sonography. J Clin Ultrasound. 2001;29:87-91. [PubMed] [DOI] [Cited in This Article: ] |
| 53. | Bolognesi M, Quaglio C, Bombonato G, Gaiani S, Pesce P, Bizzotto P, Favaretto E, Gatta A, Sacerdoti D. Splenic Doppler impedance indices estimate splenic congestion in patients with right-sided or congestive heart failure. Ultrasound Med Biol. 2012;38:21-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Abu-Yousef MM, Kakish ME, Mufid M. Pulsatile venous Doppler flow in lower limbs: highly indicative of elevated right atrium pressure. AJR Am J Roentgenol. 1996;167:977-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Atkinson PR, Milne J, Diegelmann L, Lamprecht H, Stander M, Lussier D, Pham C, Henneberry R, Fraser JM, Howlett MK, Mekwan J, Ramrattan B, Middleton J, van Hoving DJ, Peach M, Taylor L, Dahn T, Hurley S, MacSween K, Richardson LR, Stoica G, Hunter S, Olszynski PA, Lewis DA. Does Point-of-Care Ultrasonography Improve Clinical Outcomes in Emergency Department Patients With Undifferentiated Hypotension? Ann Emerg Med. 2018;72:478-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 56. | Koratala A, Olaoye OA, Bhasin-Chhabra B, Kazory A. A Blueprint for an Integrated Point-of-Care Ultrasound Curriculum for Nephrology Trainees. Kidney360. 2021;2:1669-1676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | Reisinger NC, Koratala A. Incorporating Training in POCUS in Nephrology Fellowship Curriculum. Clin J Am Soc Nephrol. 2022;17:1442-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |