Published online Mar 28, 2015. doi: 10.5412/wjsp.v5.i1.41
Peer-review started: September 29, 2014
First decision: January 8, 2015
Revised: January 28, 2015
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: March 28, 2015
There are several caval reconstruction techniques currently in use for orthotopic liver transplantation. These include caval replacement or the conventional technique, performed with or without venovenous bypass, piggyback technique with anastomosis with two or three hepatic veins with or without cavotomy and modifications of the piggyback technique including end-to-side and side-to-side cavocaval anastomosis. There are few randomized controlled trials comparing the use of these techniques and our knowledge of their comparability is based on a few multi- and many single-center retrospective and prospective reviews. Although there are advantages and disadvantages for each technique, it is advisable that the surgeon perform the technique with which they have the most the experience and at which they are the most skilled as excellent outcomes can be obtained with any of the caval reconstruction options discussed.
Core tip: There are multiple options available for caval reconstruction currently in use for orthotopic liver transplantation. Those options include caval replacement or the conventional technique, performed with or without venovenous bypass, piggyback technique with anastomosis with two or three hepatic veins with or without cavotomy and modifications of the piggyback technique including end-to-side and side-to-side cavocaval anastomosis. There is currently no consensus in regards to the best technique although there are advantages and disadvantages for each. Excellent outcomes can be obtained with any of the described techniques and the surgeon’s comfort and skill with the technique is likely the most important factor.
- Citation: Beal EW, Bennett SC, Whitson BA, Elkhammas EA, Henry ML, Black SM. Caval reconstruction techniques in orthotopic liver transplantation. World J Surg Proced 2015; 5(1): 41-57
- URL: https://www.wjgnet.com/2219-2832/full/v5/i1/41.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v5.i1.41
Conventional liver transplantation includes total hepatectomy and resection of the recipient retrohepatic inferior vena cava (IVC) with interposition and anastomosis of the donor IVC as first described by Starzl et al[1] in 1963. In the conventional orthotopic liver transplantation (OLT) the recipient retrohepatic IVC is removed above the renal veins to the hepatic vein confluence at the diaphragm and replaced with the segment of donor retrohepatic IVC in an end-to-end fashion. The conventional technique reduces cardiac preload secondary to IVC clamping which can present hemodynamic challenges during the anhepatic phase of the operation. Venovenous bypass (VVB) was first utilized to increase cardiac preload and reduce venous and portal congestion thereby improving hemodynamic stability during the transplant procedure at the cost of increasing complexity.
The piggyback technique was first described by Calne et al[2] in 1968 and further developed by Tzakis et al[3]. The piggyback technique seeks to simplify the liver transplant by generally obviating the need for VVB by allowing venous return through the IVC. This technique involves full preservation of the recipient IVC with anastomosis of the donor IVC directly to the hepatic veins of the recipient[2,3]. The middle hepatic vein (MHV) and left hepatic vein (LHV) are frequently used as a common orifice for the anastomosis. It is also possible to use the MHV, LHV and right hepatic vein (RHV) or to use the MHV and RHV. Additionally some authors make further modification by adding a 1 to 3 centimeter cavotomy, in order to enlarge the anastomosis[4,5], or make a triangular cavotomy at the level of the RHV[6].
Belghiti et al[7,8] introduced a modified version of the piggyback technique in 1992, with a side-to-side cavocaval anastomosis (STSCCA) with partial clamping of the IVC. A temporary portocaval shunt (TPCS) was used during the procedure to preserve the portal venous flow thereby reducing intestinal congestion during the anhepatic phase[7,8]. Tzakis et al[9] also introduced the use of a TPCS in patients with intraoperative hemodynamic compromise.
Although these techniques have been compared in single-center prospective and retrospective studies there are only two randomized controlled trials comparing techniques and they have differing conclusions[10,11].
The issue of whether or not to use VVB with the conventional or piggyback technique or its’ modifications or whether to use a TPCS are explored as a separate issue.
OLT begins with a bilateral subcostal incision with or without a vertical midline extension. Left and right triangular ligaments are divided. If VVB is to be used, it is introduced at this point[12].
To establish VVB, catheters may be placed by percutaneous puncture of the femoral vein and internal jugular vein or alternatively by open exposure of the axillary vein and femoral vein. A catheter is placed in the portal vein after it is divided during the native hepatectomy[12]. Vascular clamps are placed on the infra- and suprahepatic vena cava and VVB is initiated[13]. Other authors describe cannulation of the left axillary vein and left iliac vein by way of the left greater saphenous vein[12].
The portal vein, infrahepatic and suprahepatic IVC are sequentially clamped. The recipient hepatectomy is completed. A running monofilament suture is used for completion of the suprahepatic and infrahepatic venous anastomosis. The portal vein, hepatic artery and common bile duct anastomosis are performed in the standard fashion as described elsewhere[12].
The conventional technique requires cross-clamping of the IVC and the portal vein during the anhepatic phase[14], and end-to-end interposition of the donor vena cava to the recipient vena cava[15].
The piggyback technique is begun with mobilization of the native liver by division of the left and right triangular, coronary, and gastrohepatic ligaments. After dissection of the porta hepatis, the cystic duct, common hepatic duct, right hepatic artery and left hepatic arteries are ligated and divided. The portal vein is skeletonized with selective ligation and division of the anterior pancreatoduodenal vein[12].
The right lobe of the liver is reflected to the left exposing the retrohepatic IVC and the IVC ligament is divided. Several authors describe this division as the key to dissecting the retrohepatic vena cava[16]. The small hepatic veins draining the caudate lobe and right accessory vein(s) are ligated[12,14,16]. Some authors also describe the isolation and division of the RHV to prevent bleeding from the parenchymal side while mobilizing the liver[14,16], this vein is then oversewn[12]. The portal vein is clamped. The MHV and LHV are isolated and clamped[14,16]. In the piggyback technique the orifices of the LHV and MHV are joined into a common orifice[12,16]. Some authors also include the RHV or an additional cavotomy of 1 to 3 cm[4,5]. An alternative approach is suggested by Gerber et al[6] who make a triangular cavotomy at the level of the RHV. If the donor and recipient are of similar weights and size the donor suprahepatic caval opening will fit the ostium created from the recipient hepatic veins. If not, it may be necessary to enlarge the anastomotic orifice[16]. Some authors suggest that in this case it may be necessary to cross-clamp the IVC. If this is poorly tolerated, VVB may be required[16].
Robles et al[17] report differing approach to the piggyback technique depending on whether they are completing the anastomosis with two or three suprahepatic veins. When they are using the MHV and LHV they dissect and suture the RHV, clamp the portal vein, complete the piggyback on the left until the common patch between the MHV and LHV is isolated and then transversely clamp the patch without occluding the retrohepatic IVC. When they use all three veins they dissect the retrohepatic vena cava on both sides, delaying portal venous clamping as long as possible and reaching the posterior face of the three veins without separating them. They then dissect the retrohepatic vena cava above the exit of the suprahepatic veins and free them from the diaphragm. They fit a transverse clamp. An end-to-end anastomosis is then performed between the two or three suprahepatic veins and the donor vena cava[17]. There are several detailed descriptions available in the literature of how to achieve a three-vein anastomosis in the piggyback technique. Tayar et al[18] recommend creating a common ostium from the three hepatic veins to provide a wide channel that is unlikely to obstruct or lead to symptoms of blocked outflow, positioning the anastomosis on the anterior and right aspect of the vena cava and shortening the graft suprahepatic IVC to avoid redundancy and kinking[18]. The portal vein, hepatic artery and biliary anastomosis are then completed in standard fashion.
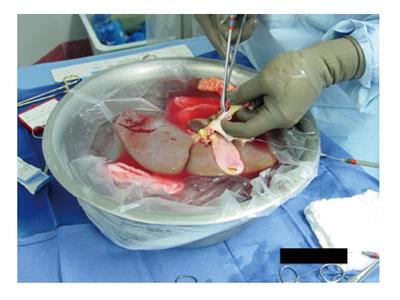
The donor hepatectomy is performed in the standard fashion. The upper cava cuff of the donor IVC is shortened flush to the hepatic veins. Both ends of the donor IVC are closed with running sutures, usually 4-0[16] or 5-0[19] polypropylene suture, or using a vascular stapler or EndoGIA stapler[20]. The IVC is flushed (Figure 1) to explore for and repair any leaks[20].
The porta hepatis is dissected and hepatic artery and common bile duct are transected. The portal vein is prepared and dissected. The hepatocaval ligament is dissected and RHV is oversewn or divided with an EndoGIA stapler. The MHV and LHV are then stapled. The recipient liver is removed[20].
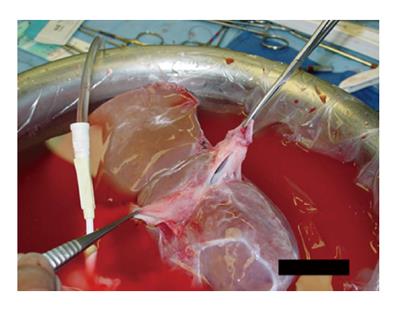
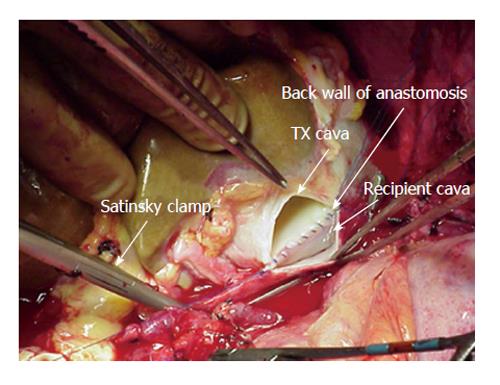
A 3-4[15] or 6[16,20] centimeter long cavotomy is made on the posterior side of the donor IVC (Figure 2) encompassing the orifices of the major hepatic veins. This will allow future transjugular biopsy or transjugular intrahepatic portosystemic stent shunting placement if necessary. The donor liver is then implanted using one large anastomosis between the recipient IVC anteriorly and the posterior wall of the donor IVC (Figure 3). The anastomosis is performed from the left side using two running 4-0 polypropylene[15,16].
Akbulut et al[21] report an additional modification of this technique in which they use a linear stapler to complete this anastomosis. The upper and lower orifices of the donor IVC are closed on the back table with running 5-0 polypropylene suture. Three stay sutures are placed in the caudal parts of recipient and donor cava with 5-millimeter venotomies. An endoscopic linear stapler is placed upward through the orifice and fired. A second stapler is placed more cranially and also fired. This creates an 8-9 cm long cavocavostomy. This anastomosis was performed in 4 min. The insertion points are then closed with running 4-0 polypropylene suture[21]. The portal vein, hepatic artery and biliary anastomosis are then completed in standard fashion.
Polak et al[22] describe the end-to-side cavocaval anastomosis. First, recipient hepatectomy is performed in a standard manner. The hepatoduodenal ligament is dissected and the common bile duct is ligated and transected. After mobilization of the liver, the RHV is oversewn, or stapled. On the back table the caudal end of the donor IVC is shortened and closed over a silastic tube with a purse string suture around it. The opening of the suprahepatic IVC is extended by longitudinal midline incision on the posterior wall. Next, the native hepatic artery and portal vein, are ligated and transected. The MHV and LHV are oversewn and the liver is removed. TPCS is only used in selective cases, by most authors, to minimize the risk of splanchnic congestion in patients without portal hypertension or in the case of a very large caudate lobe that encircled the IVC[22,23]. After tangential clamping of recipient IVC, occluding approximately a third[22] to one-half[23] of its lumen, the anterior wall of the IVC is incised longitudinally and the graft is placed orthotopically. End-to-side anastomosis is performed between donor and recipient IVC using two running sutures[22,23]. The portal vein, hepatic artery and biliary anastomosis are then completed in standard fashion.
There are two randomized controlled trials (RCTs) comparing techniques for caval reconstruction in liver transplantation. There are a multitude of prospective and retrospective multi- and single-center reviews. The results of these will now be discussed.
Isern et al[10] in 2004 in Brazil performed a RCT in which 33 patients were randomized to the piggyback technique with no TPCS and 34 patients were randomized to the standard technique with VVB. There were no differences between the two groups noted in their primary outcomes, which included post-operative mortality, chest complications, transfusion requirements and hospital stay. There was no difference reported in anesthesia time (P = 0.162), operative time (P = 0.270), cold ischemia time (P = 0.205), duration of mechanical ventilation (P = 0.429), length of hospital stay (P = 0.846) or operative mortality (P = 1.000) (Table 1). There was also no difference reported in red blood cell use (P = 0.940), fresh frozen plasma use (P = 0.890), platelet concentrate use (P = 0.209), apheresis platelet concentrate use (P = 0.486) or use of crystalloid solution (P = 0.985) (Table 1)[10].
| Variable | Conventional | Piggyback | P-value |
| n | 34 | 33 | |
| Age | 46.5 (24-73) | 48 (18-66) | 0.831 |
| Child-Pugh Score | 0.931 | ||
| A | 5 | 5 | |
| B | 21 | 19 | |
| C | 8 | 9 | |
| Anesthesia time (min) median (range) | 795 (540-1115) | 690 (510-1140) | 0.162 |
| Operative time (min) median (range) | 647 (420-925) | 600 (370-960) | 0.270 |
| Graft cold ischemia time (min) median (range) | 536 (261-900) | 497 (330-930) | 0.205 |
| Duration of mechanical ventilation (min) median (range) | 712 (200-8070) | 650 (0-26555) | 0.429 |
| Length of hospital stay (d) median (range) | 15.5 (6-72) | 17.0 (10-45) | 0.846 |
| Operative mortality (30 d) | 0 | 1.3% | 1.000 |
| Red blood cells (units) median (range) | 5.5 (0-34) | 5.0 (0-35) | 0.940 |
| Fresh frozen plasma (units) median (range) | 22.5 (0-84) | 19.0 (0-82) | 0.890 |
| Platelet concentrate (units) median (range) | 9.5 (0-40) | 0.0 (0-30) | 0.209 |
| Aferesis platelet concentrate (units) median (range) | 0.0 (0-3) | 1.0 (1-10) | 0.486 |
| Crystalloid solution | 1.5 (0-7.5) | 1.5 (0-10) | 0.985 |
Jovine et al[11] in 1997 in Italy performed a RCT in which 20 patients were randomized to the piggyback technique with no TPCS and 19 patients were randomized to the standard technique with VVB. The primary outcomes examined included primary graft nonfunction, renal failure, transfusion requirements, intensive therapy unit stay and hospital stay. They determined that there was a decrease in warm ischemia time (48.5 ± 13 min for piggy-back vs 60 ± 12 min for the conventional method) and in postoperative renal failure (zero cases in piggyback group vs four cases in conventional group) in the piggyback technique group. These results are conflicting with the RCT reported above in which there was no difference in these and other variables[11].
Additionally, a Cochrane Review published in 2011 performed a systematic review which included both of these studies[24]. They considered three trials in their review, the randomized controlled trials mentioned above: Isern et al[10] and Jovine et al[11] which both compared the standard technique with VVB to the piggyback technique without TPCS, and a randomized controlled trial by Figueras et al[25] which compared piggyback technique with and without TPCS.
For the Cochrane Review the following primary outcomes were chosen: post-operative mortality, graft failure and retransplantation. Post-operative mortality was only reported in one of the two studies, Isern et al[10], and there was no difference between the two groups (P = 0.49). Graft failure due to primary nonfunction was only reported in one study, Jovine et al[11] and there was no difference between the groups (P = 0.96). Long-term graft function and retransplantation were not reported in either trial[24].
The secondary outcomes included: adverse events, vascular morbidity, renal failure, transfusion requirements, intensive therapy unit stay, hospital stay, operating time, warm ischemia time. Specific adverse events were reported in both trials. Vascular morbidity was reported only in Jovine et al[11] and there were none. There was no difference in post-operative renal failure as defined by the need for hemodialysis (P = 0.50), transfusion of blood or platelets (P = 0.65), intensive care unit (ICU) stay (P = 0.37), hospital length of stay (P = 0.10) or operating time (P = 0.05). Warm ischemia time was only reported in Jovine et al[11] and it was lower in the piggyback group (P < 0.01). A higher proportion of chest complications were reported in the piggyback group (P = 0.01). This includes chest infections and pleural effusions[24].
These two RCTs and the associated Cochrane Review do not provide sufficient information on which to base a decision regarding which procedure to use in practice, and in fact provide conflicting information.
There are a plethora of prospective and retrospective studies available in the literature comparing the different caval reconstruction techniques.
Several authors report exclusively on their experience with the piggyback technique (Table 2). Anastomosis using the LHV and MHV is abbreviated LM. Anastomosis using the LHV, MHV and RHV is abbreviated LMR. Anastomosis using LHV and MHV and an added cavotomy is abbreviated LM+.
| Ref. | n | Anastomosis | VVB | TPCS | Complications reported | Conclusion |
| Fleitas et al[26] single center | 44 OLTs 39 patients | LM | No | No | Hepatic artery thrombosis (1), suprahepatic stricture (1), retransplant (5 - hepatic artery thrombosis, suprahepatic stricture, primary nonfunction, rejection), relaparotomy for bleeding (2), splenic steal (1) | Piggyback operation could be done in most OLTs, not restricted to certain anatomic situations. Lateral IVC clamping and unclamping results in good hemodynamic stability. Vascular complications, blood requirements, retransplantation, overall survival similar to that reported with standard technique |
| Belghiti et al[8] single center | 51 | LM | No | Yes, 100% | Four postoperative deaths (sepsis and primary nonfunction - 2, nosocomial pneumonitis at 3 and 5 mo - 2), no pulmonary embolism, NO IVC stump thrombosis | Piggyback technique was always technically feasible irrespective of graft size, VVB not required |
| Levi et al[27] single center | Era I: 945 of 1080 (87.5%) | LMR when possible | 177 (18.7%) | No | Outflow obstruction (6) | Increasingly used piggyback technique over time (P < 0.0002). Over time had shorter warm ischemia time (P = 0.0004), less frequent need for VVB (P = 0.001). Hepatic venous outflow obstruction rarely encountered |
| Era II: 851 of 920 (92.5%) | LMR when possible | 97 (11.4%) | No | Outflow obstruction (3) | ||
| Ducerf et al[5] | 88 OLTs, 81 patients | LM vs LM+ 3-cm cavotomy | No | No | No outflow obstruction (0) | Preservation of the IVC with recipient caval anastomosis with MHV and LHV is reliable. Associated cavotomy is not necessary |
| Parrilla et al[13] multi-center | 1112 | 440 LM 672 LMR | No | 6 at one center | Abdominal bleeding (2), acute outflow obstruction (9), ascites (3), intraoperative complications (28 - 2 venous tears, 26 congestion), graft failure (11) | Complications inherent to the piggyback technique including intraoperative venous congestion and acute and chronic Budd Chiari syndrome were more common when patients underwent anastomosis with two suprahepatic veins vs three (P < 0.001) |
| Cescon et al[4] | 431 | LM, LMR, LM+ 1 cm cavotomy | No | No | Complications related to anastomosis (20, 4.6%) | Increase in complications related to caval anastomosis in patients with two-vein anastomosis (LM vs LM+ P < 0.0001, LM vs LMR P = 0.065, LM+ vs LMR P = 0.4). Orifice formed with two veins is not sufficient. Advocate balloon angiography for dilation of anastomotic narrowing in most cases |
| Robles et al[17] | 171 | 87 LM 84 LMR | No | No | Hepatic venous outflow obstruction in 7 patients with LM (8%) and in 1 patient with LMR (1.2%) | Increase in hepatic venous outflow obstruction in patients with two-vein anastomosis (P < 0.05) |
In 1994 Fleitas et al[26] report a series of 39 patients who underwent 44 transplants with the piggyback technique and concluded that the piggyback operation could be performed in most patients undergoing OLT and should not be restricted based on anatomic considerations. They did a complex analysis of hemodynamic parameters including mean arterial pressure, IVC pressure, renal perfusion pressure, cardiac index and systemic vascular index and concluded that hemodynamic parameters were maintained throughout the procedures. They concluded that lateral IVC clamping and unclamping resulted in good hemodynamic stability. Vascular complications, retransplantation, blood requirements and overall survival were similar to that reported in the literature for the conventional technique[26].
Belghiti et al[8] in 1995 report the use of the piggyback technique with TPCS in 51 consecutive patients. They concluded that portocaval anastomosis was minimally time consuming with a mean time to perform of 9 min, and a range of 5 to 17 min, and that they were able to perform satisfactory portal venous anastomosis. They also conclude that the preservation of portal and caval flows as achieved with the piggyback operation with TPCS may have special importance in transplantation of partial livers[8].
Levi et al[27] performed a retrospective study comparing two different eras of their own experience. Era I was from 1994 to 2002 and era II from 2002 to 2010. They noted that they increasingly used the piggyback technique over time (P < 0.0002). Over time had shorter warm ischemia time (P = 0.0004) and less frequent need for VVB (P = 0.001). From era I to era II they noticed that their median operative time (P = 0.0000) and hospital length of stay (P = 0.0000) improved. Hepatic venous outflow obstruction was rarely encountered in their series. There were nine reported cases of hepatic venous outflow, six in era I and three in era II. The authors report that, “twice, it was recognized and corrected intraoperatively. Seven patients presented with refractory ascites. Six were successfully treated (4 balloon dilatation, 2 surgical revision), one patient died after attempted dilatation[27]”.
Several authors in this series and others report on hepatic venous outflow obstructions, which do appear to be common with the piggyback technique[5,28]. There are a multitude of potential solutions to this problem, including the ones reported above: angioplasty with or without stent placement[19,20,27,29,30], surgical revision of the anastomosis[13,20,27,30] creation of a, “neo-bed,” or suturing the peritoneum covering Gerota’s fascia of the right kidney to the diaphragm, which reduces the size of the recipients’ hepatic fossa[13,17] and retransplantation[19,20,30].
There have been many studies published on possible rescue techniques for hepatic venous outflow obstruction. These include the use of venous patches[19], lifting and suspending the liver the the diaphragm[19], conversion to termino-terminal cavo-cavostomy[31], end-to-side anastomosis[4,28,32] and side-to-side cavocavostomy[16], and side-to-side cavocavostomy with an endovascular stapler[30].
Several authors have undertaken studies comparing the piggyback operation with two-vein anastomosis vs three-vein anastomosis to analyze feasibility of these techniques and the rates of complications with somewhat conflicting results.
Ducerf et al[5] compared a group of patients who had undergone suprahepatic caval anastomosis between the graft suprahepatic IVC and the recipient left and MHVs to a group of patients that had an associated 3 centimeter vertical cavotomy with partial clamping of the recipient vena cava. Twenty patients from each group had pressure and gradient measurements done 20 mo post-operatively to assess the hepatic veins, right atria and retrohepatic vena cava. The authors concluded that preservation of the IVC with recipient caval anastomosis at the ostia of the middle and LHVs is a reliable technique. Furthermore they concluded that there is no alteration in hepatic venous outflow and that associated cavotomy is not necessary[5].
In contrast, Parilla et al[13] compare anastomosis with two hepatic veins to anastomosis with three hepatic veins and conclude that venous congestion and acute or chronic Budd Chiari syndrome are more common with anastomosis performed with two veins than when it is performed with three (P < 0.001)[13].
Cescon et al[4] compare three types of piggyback anastomosis: (1) anastomosis incorporating a cuff of the recipient LM; (2) anastomosis with the MHV and LHV plus one-centimeter cavoplasty (LM+); and (3) anastomosis with the LMR. They found an increased rate of complications with the group undergoing anastomosis with the MHV and LHV in comparison to those undergoing anastomosis with the MHV and LHV with associated cavotomy (P < 0.0001). They report their complications including four cases of thrombosis all of which required early retransplantation. For patients with anastomotic stricture, two were offered retransplantation and this was successful. The remaining cases were treated with balloon dilation during cavography (13, 65%) or anastomotic revision with end-to-side cavo-caval anastomosis between the distal stump of the donor vena cava and the recipient vena cava. Balloon dilation was successful in one session in eight patients and in two sessions in two patients. One patient in this group died from unrelated causes. In one patient balloon dilation was only partially successful and they were retransplanted. In another patient anastomotic kinking was demonstrated and cavo-caval anastomosis was attempted, but the patient required retransplant and then died soon after. Another patient had partial benefit from one session of balloon dilation and total recovery after cavo-caval anastomosis, but died of independent causes[4]. In contrast to the findings of Ducerf et al[5], they conclude that the caval anastomosis can be performed using the orifice formed by the MHV and LHV with associated cavotomy greater than one-centimeter and that the orifice formed by the two veins alone is not sufficient. Additionally, they advocate angiographic balloon dilation for anastomotic narrowing in most cases[4].
Robles et al[17] also compare the use of two-vein vs three-vein anastomosis, reporting that hepatic venous outflow complications were more common, and more serious, when two suprahepatic veins were used compared to the use of three (P < 0.05). There were six patients who presented with severe venous congestion of the graft intraoperatively which was attributed to suprahepatic vein kinking, which was treated with neo-bed creation. Five of these cases were in patients with two-vein anastomosis and one in a patient with three-vein anastomosis. Additionally, there were two patients who presented with post-operative hepatic venous outflow complications. One presented with acute Budd-Chiari syndrome due to stenosis of the anastomosis and required immediate retransplantation. A second presented with chronic Budd-Chiari syndrome with ascites and moderate graft dysfunction and was treated with diuretics. Both of these patients had two-vein anastomosis. There were no postoperative hepatic venous outflow complications in patients with three-vein anastomosis[17].
There are several retrospective and prospective studies that compare the use of the piggyback technique to the conventional technique (Table 3).
| Ref. | Comparison | Results |
| Tzakis et al[3] | 24 piggyback, selective VVB 24 standard, selective VVB | No difference in blood loss, retransplantation rate, portal vein or hepatic artery thrombosis or biliary tract complications |
| Busque et al[33] | 98 piggyback 33 standard, 15% VVB | Attempted piggyback in 131 patients. Were able to complete in 98 |
| Reddy et al[14] | 40 standard, routine VVB 36 piggyback, selective VVB | Piggyback associated with shorter anhepatic phase, shorter total operating time, less red blood cell use, trend towards shorter hospital stay, reduced hospital charges |
| Gerber et al[6] | 75 piggyback 127 standard | Piggyback here done with triangular vagotomy at level of right hepatic vein. Decreased operative time, use of blood products, caval complications in piggyback group |
| Hosein Shokouh-Amiri et al[34] | 34 piggyback 56 standard, routine VVB | Piggyback with 60% reduction in anhepatic phase, decreased operative time, higher core body temperature, decrease in fluid, plasma, platelets, RBC volume, 30% shorter ICU stay, hospital stay. Significant reduction in hospital costs |
| Barshes et al[12] | 122 piggyback 98 standard, 76% VVB | Trend towards shorter operating time and ischemia time in piggyback group. Similar amount of blood products transfused. No hepatic vein thrombosis or strictures, no IVC strictures or thrombosis, no hepatic vein obstruction, no anastomotic strictures, no hemorrhagic complications |
| Nishida et al[35] | 918 piggyback, 19.7% VVB 149 standard, 79.2% VVB | Blood transfusion, warm ischemia time, use of VVB were less in piggyback group. Liver, renal function similar |
| Sakai et al[36] | 104 standard, with VVB 148 piggyback, with VVB 174 piggyback, without VVB | Piggyback without VVB required less RBCs, FFP, cryoprecipitate, cell-saver return, less acute renal failure, better patient and graft survival. The piggyback with VVB group had shorter operative time, warm ischemia time, and less acute renal failure than the standard with VVB group |
| Vieira de Melo et al[37] | 125 standard, without VVB 70 piggyback, without VVB | Piggyback group had reduced surgical time, warm ischemia time, red blood cell use, FFP use, mortality at 30 d. No difference in cold ischemia time, length of stay, use of vasoactive drugs in ICU, period of intubation, duration of hospital stay, renal or graft function, need for reoperation, incidence of sepsis, biliary complications, vascular complications, need for retransplantation, 1-yr mortality. Cumulative survival at 1 yr significantly better in PB patients |
| Cabezuelo et al[38] | 84 standard 20 standard with VVB 80 piggyback | Standard technique in comparison to piggyback technique is an independent risk factor for post-operative renal failure. VVB does not ameliorate this effect |
Tzakis et al[3] compared 24 patients who had undergone piggyback operations to 24 matched controls who had undergone the standard operation (Table 3). They reported no difference in blood loss, retransplantation rate, portal vein or hepatic artery thrombosis or biliary tract complications (no P-values reported). They asserted that favorable anatomic conditions are required for use of the piggyback technique[3]. The least favorable circumstances tend to be with small cirrhotic livers and that the most favorable are patients with primary sclerosing cholangitis or primary biliary cirrhosis as patients with these conditions have hepatic veins which are relatively normal and accessible[3]. Tzakis et al[9] later report the use of the piggyback technique with TPCS in four hemodynamically unstable children with no established portosystemic collateral circulation[9].
Busque et al[33] report their experience with attempting the piggyback technique in 131 patients (Table 3). They were successful at performing the piggyback technique in 75% of their patients. The authors report, “reasons for conversion to the standard technique were: anatomical (22 transplants), severe portal hypertension requiring VB (8 transplants), tumor (1 transplant), and other reasons (2 transplants)[33]”. They reported that piggyback technique without caval occlusion is possible, safe and reduced the need for VVB. Additionally, the authors report that it, “avoids retrocaval dissection, facilitates retransplantation, and is associated with a short anhepatic phase, low blood product usage, and short intensive care unit stay[33]”. They report that partial outflow obstruction caused by the rotation of a small donor liver in large abdominal cavity at the hepatic vein anastomosis can be prevented by sewing the graft in counterclockwise[33].
Reddy et al[14] report their single center experience and compare the standard technique with VVB and the piggyback technique with selective VVB (Table 3). They report that the piggyback technique is safe, can be performed in the majority of patients, reduced the use of VVB (94% vs 22%), is associated with shorter anhepatic phase (P < 0.0001) and total operating time (P = 0.002), lower blood product use (P = 0.023) and a trend towards shorter hospital stay (17 d vs 11 d) and reduced hospital charges ($105439 vs $91779) when compared with the standard technique with VVB. With the piggyback technique they reduced their rate of VVB use to 20%. In their patient population 34 of 36 patients were able to undergo the piggyback operation. In 8 of these patients VVB was used. For three of these patients VVB use was elective and in the remaining 5 it was used when the patient became hemodynamically unstable during the hepatectomy[14].
Gerber et al[6] introduced a further modification of the piggyback technique in which they make a triangular venotomy at the level of the RHV of the recipient (Table 3). The LHV and MHV are oversewn. A triangular venotomy is created on the posterior wall of the donor IVC. The cavo-caval anastomosis is completed with 3 running sutures. This incision allows the liver to settle into a dependent position in the right subdiaphragmatic space. They report significantly reduced operative time (P < 0.05), use of blood products (P < 0.05) and decreased caval complications in their piggyback group (3.9% vs 1.3%)[6].
Hosein Shokouh-Amiri et al[34] performed a prospective study comparing patients undergoing standard OLT with VVB to those undergoing the piggyback technique (Table 3). They concluded that the piggyback procedure resulted in a 60% reduction of the anhepatic phase (P < 0.001), reduction in operative time (P < 0.003), higher core body temperature (P < 0.002) and associated decrease in the requirements for fluid (P≤ 0.03), plasma (P≤ 0.06), platelets (< 0.009) and red blood cells (P = 0.18), 30% shorter ICU stays (P < 0.008) and similar reduction in overall hospital stay (P≤ 0.05). A reduction in hospital charges was also seen in the piggyback group (P≤ 0.002). The authors assert that what time is added in dissection in the piggyback group is eliminated because you do not have to place bypass catheters or spend as long achieving retroperitoneal hemostasis. There is also one less anastomosis in the piggyback technique providing significant time savings[34].
Barshes et al[12] report that operative time [5:20 vs 5:47, P = non-significant (NS)] and ischemia time (5:32 vs 6:06, P = NS) were shorter in the piggyback group (Table 3). There was no significant survival difference between the two groups (P = 0.65). Two patients in the piggyback group developed graft congestion after requiring massive fluid volumes to maintain hemodynamic stability and were rescued with end-to-side donor infrahepatic IVC to recipient vena-cava cavostomy. There were no hepatic vein thrombosis or strictures, IVC strictures or thrombosis or hemorrhagic complications. Similar amounts of blood were transfused. Eight patients in each group had post-operative ascites without evidence of hepatic vein obstruction or anastomotic stricture. All of these resolved without further intervention[12].
Nishida et al[35] performed a retrospective study looking at 1067 transplants in 965 patients. Nine hundred and eighteen underwent the piggyback technique with two or three hepatic vein anastomosis vs 149 patients who underwent the conventional technique. Blood transfusions (P = 0.000202), warm ischemia time (P = 0.000000) and the use of VVB (P = 0.000000) were less with the piggyback group. Liver and renal function between the two groups in the postoperative period was similar. On univariate analysis cava reconstruction method, cold ischemia time, warm ischemia time, amount of transfusion, length of hospital stay, donor age and tumor presence were significant factors influencing graft survival (P < 0.05). On multivariate analysis cold ischemia time, donor age, amount of transfusion, and hospital stay were independent prognostic factors for graft survival (P < 0.05). Importantly caval reconstruction method as an independent marker did not show prognostic impact on graft and patient survival[35]. The authors report undertaking the conventional techniques for, “presence of tumor close to the IVC, presence of the intrahepatic cava, Budd-Chiari syndrome, or technical difficulties including the presence of the large caudate lobe or severe inflammation and adhesion between the caudate lobe and the retrohepatic IVC[35]”. Additionally, they report that, “the reasons for using VVB were as follows: hypotension due to intolerance of IVC clamping, previous TIPS procedure, previous abdominal surgery making dissection in the portal hilum difficult, anatomic reasons including fulminant liver failure without the collateral veins, or intrahepatic inferior IVC or large caudate lobes[35]”.
Sakai et al[36] compared the standard technique with VVB to the piggyback technique with and without VVB (Table 3). The choice of procedure was based on surgeon preference. The piggyback without VVB group required less intraoperative red blood cells (P = 0.006), fresh frozen plasma (P = 0.005), cryoprecipitate and cell-saver return (P = 0.007), had less acute renal failure (P = 0.001), better patient (P = 0.039) and graft survival (P = 0.003). The piggyback with VVB group had shorter operative time (P = 0.0001), warm ischemia time (P = 0.0001) and less acute renal failure (P = 0.001) than the conventional with VVB group[36].
Vieira de Melo et al[37] compared the conventional technique and piggyback technique, both without VVB or TPCS. The piggyback group had reduced surgical time, warm ischemia time, the use of red blood cells and fresh frozen plasma, and mortality at 30 d (P < 0.05). There was no difference demonstrated in cold ischemia time, length of stay or use of vasoactive drugs in ICU, period of intubation, duration of hospital stay, renal function, graft function, need for reoperation, incidence of sepsis, biliary complications, vascular complications, need for retransplantation or 1-year mortality (P-value not reported). The piggyback group had higher cumulative survival at one year (P = 0.03). There were similar rates of postoperative complications between groups[37].
Cabezuelo et al[38] compared the conventional technique, the conventional technique with VVB and the piggyback technique. They concluded that the conventional technique, in comparison with the piggyback technique, appears to be an independent risk factor for postoperative renal failure. They specifically looked at renal function in the first week after transplant and defined acute renal failure as serum creatinine > 1.5 mg/dL, an increase of 50% in the baseline creatinine or oliguria requiring renal replacement therapy. They analyzed which factors were associated with postoperative renal failure and demonstrated that intraoperative fresh frozen plasma and cryoprecipitate transfusion, intraoperative complications, postreperfusion syndrome, need for noradrenaline or dopamine, standard surgical technique verses piggyback and conventional technique with VVB vs piggyback were associated with postoperative renal failure (P < 0.01). In logistic regression analysis they demonstrated that conventional technique vs piggyback (P < 0.01), conventional technique with VVB vs piggyback (P = 0.02) and > 20 U of cryoprecipitate (P = 0.01) had independent prognostic value for the development of postoperative renal failure. They concluded that the conventional technique was an independent risk factor for postoperative renal failure and that the use of VVB did not ameliorate this effect[38].
Durand et al[39] presents their experience with s STSCCA with a specific focus on post-operative renal function (Table 4). They report that STSCCA results in low rates of postoperative renal failure in their small prospective study. STSCCA is associated with preserved renal perfusion pressure throughout the procedure and preserved cardiac index and mean arterial pressure in the anhepatic phase[39].
| Ref. | Anastomosis | Conclusion |
| Durand et al[39] | STSCCA | Low rates of postoperative renal failure. Maintained postoperative creatinine clearance. Preserved renal perfusion pressure, mean arterial pressure, cardiac index throughout procedure |
| Hesse et al[40] | STSCCA vs STSCCA with VVB vs STSCCA with TPCS | Lowest blood loss in group with VVB (no P-value reported). Highest red blood cell and fresh-frozen plasma transfusion in group without VVB or TPCS (P = 0.002). Changes in pre- and post-operative creatinine most pronounced in group with TPCS (not significant, no P-value reported) |
| Mehrabi et al[20] | STSCCA | Technique feasible in all patients, no anatomic limitations. Minimizes need for VVB or TPCS. Some patients with hepatic venous outflow obstruction managed with stenting, early revision or retransplant. Can apply technique in retransplants |
| Pisaniello et al[19] | STSCCA | Safe technique. Can be performed in most patients. Recommend post-anastomotic doppler ultrasonography |
Hesse et al[40] compared patients undergoing STSCCA with IVC preservation, STSCCA with VVB and STSCCA with TPCS (Table 4). In contrast to what is reported by other authors they reported the lowest perioperative blood loss in their VVB group and the highest rate of red blood cell and fresh frozen plasma transfusion in the group without VVB or TPCS (P = 0.002). Post-operative ICU stay and ventilation days did not differ between groups (no P-value reported). The changes in pre-operative and post-operative creatinine levels were most pronounced in patients that had construction of a temporary portacaval shunt (not significant, no P-value reported)[40].
Mehrabi et al[20] explored their single center experience with 500 OLTs performed with STSCCA (Table 4). They determined that the technique was feasible in all patients without anatomic limitations. At the beginning of their experience there were 7 cases of Budd-Chiari like syndrome due to compression of the liver on the IVC or kinking of the hepatic veins. They attributed these to long donor suprahepatic IVC or misplacement of the caval incision. These cases were managed with stenting, early revision or retransplantation. They also assert that this technique can be applied in retransplants[20].
Pisaniello et al[19] report a single center series of patients who underwent liver transplant with STSCCA, concluding that it is safe technique that can be performed in most patients even in the retransplant setting with low incidence of hepatic venous outflow obstruction (Table 4). They recommend routine postanastomotic Doppler ultrasonography[19].
Several authors report that retransplantation is easy and can performed without interfering with caval flow in patients who have undergone a STSCCA. Lerut et al[16] discuss that this is one reason that they prefer a side-to-side anastomosis to an end-to-side anastomosis, it allows removal of the failed allograft without interfering with caval flow. They also advocate for the use of a side-to-side anastomosis in delayed retransplantation because it allows the surgeon to access the most accessible and least hemorrhagic plane[16]. Mosimann et al[41] reported that patients who underwent procedures with side-to-side anastomosis could easily undergo retransplantation in a quick and safe procedure. This finding has been confirmed by other authors as well[16,33].
Zieniewicz et al[42] report their experience with the first 79 transplants in their program (Table 5). Sixty-eight of these were done with standard technique with VVB and the remaining 11 with the STSCCA. They report reduced warm ischemia time (P < 0.001) and blood loss in the STSCCA group (P < 0.001)[42].
| Ref. | Anastomosis | Conclusion |
| Zieniewicz et al[42] | STSCCA vs conventional with VVB | Reduction in warm ischemia time (P < 0.001) and blood loss in the STSCCA group (P < 0.001) |
| Remiszewski et al[43] | STSCCA vs conventional with VVB | Reduced complication rate (36% vs 30%) and reduced cost (P-value not reported) in STSCCA group |
| Khan et al[44] | STSCCA vs conventional with VVB | Reduced FFP (P = 0.03) and platelets (P = 0.04) transfused, shorter ICU stay (P = 0.005), less patients requiring ventilation after POD1 (P = 0.03) and less total days on the ventilator (P = 0.04) in STSCCA group. Comparable operating time, warm ischemia time, length of stay (P-value not reported). Outflow obstruction in 1.2% of STSCCA patients. Report hematoma formation as complication associated with VVB |
| Schmitz et al[45] | STSCCA vs conventional with VVB | Shorter warm ischemia times, reduced red blood cell (P = 0.000) and platelet transfusion (P = 0.002) in STSCCA group. Increased risk of hepatic artery stenosis (P = 0.045) and biliary leaks (P = 0.042) in the STSCCA group |
Remiszewski et al[43] compared the conventional technique with VVB to the STSCCA in a retrospective study (Table 5). They concluded that individualization is important in choosing which procedure to use for a particular patient. Their survival (P = 0.473) and lengths of stay (P = 0.63) were similar between groups. They do report a reduced complication rate (36% vs 30%, P-value not reported) and cost (P-value not reported) in the STSCCA group, although they do not provide specifics regarding cost[43].
Khan et al[44] in prospective study compared patients undergoing conventional technique with VVB to patients undergoing STSCCA (Table 5). The STSCCA group patients required less fresh frozen plasma (P = 0.03) and platelets (P = 0.04), had decreased cold ischemia time (P = 0.01), shorter ICU stays (P = 0.005), less patients requiring ventilation after post-operative day 1 (POD1) (P = 0.03) and less total days on the ventilator (P = 0.04). The two groups had comparable operating time, warm ischemia time, red cell usage, requirement for renal support and POD3 creatinine, and total hospital stay length (P = NS, value not reported). Three patients in the piggyback group developed outflow obstruction (1.2%), which the authors refer to as, “piggyback syndrome.” They also reported complications related to VVB in the conventional technique group which included 16 patients who developed hematomas at the VVB site in the axilla[44].
Schmitz et al[45] performed a retrospective study comparing patients undergoing the standard technique with VVB to patients undergoing STSCCA. The STSCCA group had shorter warm ischemia times (P = 0.000), reduced transfusion of red blood cell (P = 0.002) and fresh frozen plasma (FFP) (P = 0.004). They also demonstrated an increased risk of hepatic artery stenosis (P = 0.045) and biliary leaks in the STSCCA group (P = 0.042)[45].
Polak et al[22] demonstrated that end-to-side cavocavostomy (ETSCCA), as originally described by Cherqui et al[46], between the end of the donor suprahepatic IVC and a longitudinal incision on the anterior wall of the recipient IVC can be used successfully (Table 6). They obtained consistent patient and graft survival rates with few anastomosis related complications, performed this technique for patients who had previously had this or another type of liver transplant and had minimal intraoperative blood product requirements[22].
| Ref. | Anastomosis | Conclusion |
| Polak et al[22] | ETSCCA | Simple and safe procedure. Allows wide anastomosis and eliminates risk of venous outflow tract obstruction. Can be performed without routine TPCS. Minimal intraoperative blood products used. Can be used in first and second retransplantations |
| Wojcicki et al[23] | ETSCCA | Low risk of vascular outflow obstruction complications with ETSCCA. Partial portal and mesenteric vein thrombosis not a contraindication for OLT, can treat with eversion thrombectomy |
| Belghiti et al[47] | STSCCA, ETSCCA | Caval preservation possible in most patients. Patients tolerate transient cross-clamping of the IVC prior to reperfusion when necessary to create wide anastomosis |
Wojcicki et al[23] also report a retrospective single center study of ETSCCA (Table 6). They determined that there was a low rate of vascular complications with ETSCCA and that partial portal or mesenteric vein thrombosis is no longer a contraindication to OLT and can be managed with eversion thrombovenectomy[23].
Belghiti et al[47] present their experience with ETSCCA. They conclude that IVC preservation is feasible in almost all candidates, as it was in 90% of the patients in their series. They initial performed STSCCA, but transitioned to ETSCCA after 1993 to provide improved exposure with larger grafts and allow for post-operative transjugular biopsies when necessary. They report that they were able to perform most of their procedures with preservation of caval flow, but in cases where they were not, transient IVC cross-clamping prior to reperfusion was well tolerated. When done after reperfusion it results in a high rate of early graft failure[47].
In a single-center retrospective study Lerut et al[16] compared patients undergoing conventional technique with VVB, patients undergoing piggyback technique with VVB and patients undergoing STSCCA without VVB or TPCS (Table 7). The piggyback and STSCCA groups had reduced warm ischemia time (P < 0.001), reduced need for intraoperative blood products (P < 0.01) and lower rates of reoperation for bleeding (P < 0.01). Additionally, the STSCCA group had a significantly higher frequency of immediate extubation (P < 0.001) compared to the other two groups. The authors suggest that partial clamping of the IVC leads to reduced requirement for fluid administration which may contribute to the higher rate of immediate extubation. They assert that STSCCA preserves the advantages of piggyback OLT including reduced implantation time and need for blood products while also eliminating VVB and reducing ventilation time[16].
| Ref. | Anastomosis | Conclusion |
| Lerut et al[16] | Conventional with routine VVB, piggyback with selective VVB, STSCCA | Piggyback and STSCCA groups had reduced warm ischemia time (P < 0.001), reduced need for intraoperative blood products (P < 0.01), lower rates of reoperation for bleeding (P < 0.01). STSCCA had higher frequency of immediate extubation (P < 0.001). STSCCA preserves advantages of piggyback technique including reduced implantation time and need for blood products while also eliminating VVB and reducing ventilation time |
| Navarro et al[48] | Piggyback, STSCCA, ETSCCA | Reduced vascular complication in STSCCA compared to piggyback group with less cases of Budd Chiari syndrome and fewer releases of the cavocaval running suture (no P-value reported) |
| Hesse et al[15] | Conventional with selective VVB, piggyback with selective VVB, STSCCA | Use of packed red blood cells higher in piggyback group than standard group (P = 0.01). Use of packed red blood cells (P = 0.01), number of patients operated on for hemorrhage (0.002) and use of VVB (P = 0.02) lower in STSCCA than other two groups. Perioperative FFP, time in ICU, postoperative graft function and survival similar between the three groups (P = NS, values not reported) |
| Lai et al[49] | Conventional with VVB, piggyback, STSCCA | STSCCA group with lowest median cold (P = 0.001) and warm ischemia times (P < 0.0001), best immediate postoperative graft function (P < 0.0001), lowest transaminase peak (P = 0.007) and best bile output (P = 0.003). No complications reported |
| González et al[50] | Conventional with VVB, conventional without VVB, IVC preservation | Total operating time (P = 0.004), packed red blood cell (P = 0.009), fresh frozen plasma (P = 0.005) transfusion lower in the IVC preservation group. Postoperative kidney and renal function did not differ between groups. Incidence of complications similar between groups |
Navarro et al[48] report a retrospective multi-center study involving 17 centers in France (Table 7). They compare three groups of patients: piggyback technique, STSCCA and ETSCCA. They report an increase in the vascular complication rate in the piggyback group vs the STSCCA group with an increase in Budd Chiari syndrome and release of the cavocaval running suture (P-value not reported). They report a 1.5% rate of venous obstruction complications, which were treated with rotation of the graft, reconstruction of the caval anastomosis, placement of a Blakemore catheter, omentoplasty, placement of a second caval anastomosis, endoluminal anastomotic dilation, conversion to conventional technique with caval replacement and retransplantation. They do report some anatomical limitations to the vena cava preservation techniques including inadequate graft size. They report performing conventional OLT for a patient with fibrous stenosis of the IVC and the death of a patient with IVC agenesis[48].
Hesse et al[15] compare three groups: conventional technique with selective VVB, piggyback technique with selective VVB and STSCCA with selective VVB (Table 7). They concluded that the use of packed red blood cells was higher in the piggyback group than the conventional group, which conflicts the results of many other studies. The use of packed red blood cells post-operatively (P = 0.02), postoperative hemorrhage and the number of patients operated on for hemorrhage (P = 0.002) were lower in the STSCCA group than the other two groups. Perioperative FFP time in the ICU, postoperative graft function and survival rates were similar between the three groups (P = NS, values not reported). The STSCCA had significantly reduced use of VVB (P = 0.02). The authors concluded that preservation of the vena cava can reduce, but not avoid the use of VVB[15].
Lai et al[49] did a retrospective single-center study comparing conventional technique with VVB, piggyback and STSCCA (Table 7). They determined that STSCCA had lowest median cold ischemia time (P = 0.001), lowest warm ischemia time (P < 0.0001), best immediate postoperative graft function (P < 0.0001), lowest transaminase peak (P = 0.007) and best bile output (P = 0.003). They did not report or discuss complications[49].
González et al[50] compared the conventional technique with VVB, the conventional technique without VVB and IVC preservation (Table 7). The total operating time (P = 0.004), packed red blood cell (P = 0.009) and fresh frozen plasma (P = 0.005) requirements were all significantly lower in the IVC preservation group. Postoperative liver and renal function did not differ between the three groups. The incidence of complications was similar between the three groups[50].
One retrospective study, Mangus et al[51], published in 2008 evaluated outcomes for patients with hepatocellular carcinoma (HCC) who underwent conventional and piggyback liver transplantation (Table 8). The study does not demonstrate a difference in HCC recurrence (P = 0.47) or patient survival (1-year P = 0.49, 2-year P = 0.55) between groups, although their piggyback group did have higher median tumor size (P = 0.09) and a higher percentage of patients with bilateral tumors (24.40% vs 15.80%) (Table 8). They concluded that the two groups did not differ in survival within or outside of Milan criteria and that the presence of HCC should not preclude the use of the piggyback technique[51].
| Conventional | Piggyback | P-value | |
| n | 19, 14% | 119, 86% | |
| Age (yr) (mean, median, range) | 52, 52, 41-66 | 57, 57, 21-73 | 0.09 |
| MELD at transplant (mean, median, range) | 21, 22, 8-30 | 20, 22, 6-36 | 0.02 |
| Total cold ischemia time (h) (mean, median, range) | 8, 8, 4-13 | 7, 7, 3-17 | 0.03 |
| Total warm ischemia time (min) (mean, median, range) | 56, 59, 29-78 | 38, 29, 18-103 | < 0.001 |
| Outside milan criteria | 15.80% | 33.60% | 0.18 |
| Tumor number (mean, median, range) | 2, 1, 1-4+ | 2, 1, 1-4+ | 0.6 |
| Maximum tumor size (mean, median, range) | 2.6, 2.7, 0.4-8.0 | 3.2, 3.0, 0.4-8.2 | 0.09 |
| Tumor location bilateral | 15.80% | 24.40% | 0.41 |
| Lymphovascular invasion | 21.10% | 14.30% | 0.49 |
| Chemoembolization | 10.50% | 37.80% | 0.02 |
| 1-yr overall survival | 89.50% | 83.20% | 0.49 |
| 2-yr overall survival | 84.20% | 75.90% | 0.55 |
| Any HCC recurrence | 5.30% | 14.30% | 0.47 |
Several authors assert that the piggyback technique is cheaper, especially in comparison to the conventional technique with VVB. Specific information about costs, however, is not plentiful in the literature.
Reddy et al[14] compare the median hospital charges between patients undergoing conventional OLT and those undergoing piggyback technique with anastomosis with three suprahepatic veins. They report that the median hospital charges for patients undergoing conventional OLT was $105439 and the median hospital charges for patients undergoing the piggyback technique was $91779 (P = NS, P-value not reported)[14].
Hesse et al[15] report that the avoidance of VVB saves time and reduces cost, but do not provide specific values. Lerut et al[16] reports that the side-to-side anastomosis represents another means of reducing the cost of liver transplantation, but do not provide specific values. Zieniewicz et al[42] assert that the piggyback technique is less expensive, but do not provide specific values. Remiszewski also reports reduced cost of the piggyback technique, but does not provide specific values[43].
Hosein Shokouh-Amiri et al[34] in 2000 reported a statistically significant difference in the cost of liver transplantation. They report the mean cost for patients undergoing the piggyback technique to be $90412 ± 5753 and the cost of the conventional technique $113838 ± 4483 (P≤ 0.002). The authors suggest that the added cost of the standard technique is accounted for by the added cost of machines, catheters and personnel for VVB[34].
In 1984 Shaw et al[52] published a study in which they compared outcomes in patients who underwent liver transplantation with and without VVB. They determined that patients with VVB had better postoperative renal function (P < 0.001), required less blood during surgery (P < 0.01) and had improved 30-d survival (no P-value reported)[52]. Several of the previously mentioned studies offer a comparison of the standard technique with and without VVB in addition to comparisons with one or another type of caval preservation. It has been suggested that in patients without significant collateral circulation, excessive hemorrhage and major portal hypertension VVB or TPCS must be used to maintain hemodynamic stability[40].
Fonouni et al[53] published a review paper on the use of VVB in liver transplantation in 2008. They concluded that the piggyback technique with preservation of the IVC can be used in most cases of primary transplant and retransplant without VVB. They do recommend that VVB be used in selective cases including in patients with a intraoperative hemodynamic instability and those who fail a test of transient IVC occlusion[53]. Other authors agree with the assertion that the piggyback technique with IVC preservation obviates the need for VVB in many cases[26,40].
Proposed advantages of VVB that are reported in the literature include: maintaining cerebral, pulmonary and cardiovascular flow[51], reduced need for fluid resuscitation[34], maintaining kidney perfusion[54], maintaining hemodynamic stability during the anhepatic phase[16,34,52,55-57], providing longer anhepatic phase[24], better maintenance of core body temperature[34], reduction of intraoperative blood loss[24,56] and improving the clinical outcome[24,56].
Regarding maintaining blood flow and hemodynamic stability, Lerut et al[16] did detailed hemodynamic comparisons between patients undergoing classical OLT with VVB and those undergoing STSCCA and report that there are no significant differences in hemodynamic parameters. They suggest that this means that partial IVC clamping fulfills the function of VVB. This group determines the need for VVB based on a decrease in mean arterial pressure of more than 50% or decrease in cardiac index (> 50%) during the occlusion test[16].
Most authors agree that in patients without preoperative renal failure who tolerate a clamping trial well VVB is not required to maintain post-operative renal function and should be used selectively[54-56]. In a randomized controlled trial VVB was shown not to be associated with any clear benefit in renal function and the authors of the trial concluded that its use is not justified on this basis[54].
Complications related to VVB that have been reported in the literature include: access site problems including delayed healing of bypass access site, seroma, nerve injury[15,16,34], as well as intraoperative pulmonary or air embolism[16,52,55,58], longer operating and warm ischemia times, hypothermia[57] thrombosis of axillary and femoral veins[16] and higher cost associated with machine, catheters and personnel[34,59,60]. Many authors assert that VVB increases intraoperative blood loss. Lerut et al[16] report that there is an additional 500-1000 mL of blood loss when VVB is used.
Most authors of recent publications assert that it is most appropriate to use VVB selectively, although the criteria for its use are not always agreed upon. Hesse et al[15] in 2000 report that their decision about whether to use VVB is made based on recipient intraoperative hemodynamics, using it when the mean arterial pressure decreases by more than 30% or the cardiac index decreases by more than 50% or both during a trial clamping of the portal vein and IVC[29]. They also use VVB when excessive hemorrhage occurs due to portal hypertension and a small size recipient is preventing sufficient venous return to the heart during lateral vena cava clamping[15].
Steib et al[61] compared the conventional technique with VVB to the piggyback technique with TPCS in a small prospective study. They determined that two important intraoperative parameters, cardiac output and systemic oxygen delivery, were improved in the piggyback group with TPCS. They also determined that that graft function between the two groups was adequate and comparable[61].
It was originally asserted that the piggyback technique without VVB resulted in significant hemodynamic compromise that was not tolerated well by patients. Lázaro et al[62] completed a prospective study of the hemodynamics of a small group of patients undergoing the piggyback technique and concluded that there was a minimal hemodynamic disturbance and that this was well tolerated by patients[26].
It has been suggested that portacaval shunting helps avoid splanchnic congestion and the sequestration of third spaced fluids. An additional proposed advantage of TPCS is improved hemodynamic stability secondary to increased venous return[5].
Cherqui et al[46] proposed using TPCS systematically to achieve hemodynamic instability. Contrarily, Busque et al[33] report that they used TPCS in only 3 of 98 patients who underwent OLT with the piggyback technique. Reddy et al[14] completed 34 transplants using the piggyback technique and only performed a TPCS in one patient with severe portal hypertension, to decrease the risk of bleeding.
Belghiti et al[8] in 1995 report the use of the piggyback technique with 51 consecutive patients. The primary purpose of this paper was to report the use of a TPCS in 51 consecutive patients undergoing the piggyback technique. The mean time to perform the temporary portocaval anastomosis was 9 min with a range of 5 min to 17 min. They concluded that portocaval anastomosis was minimally time consuming and that they were able to perform satisfactory portal venous anastomosis. They also conclude that the preservation of portal and caval flows as achieved with the piggyback operation with ETSCCA and TPCS may have special importance in transplantation of partial livers[8].
Tzakis et al[9] in 1995 report the successful use of a TPCS in 4 pediatric patients experiencing hemodynamic instability in whom VVB was difficult or impossible to use.
Belghiti et al[47] in 2001 reported that they routinely use a TPCS in patients who do not have prior surgical portosystemic shunts or large spontaneous portosystemic shunts. In their series a TPCS was performed in 218 (79%) of cases. There were 57 cases in which a shunt was not performed: 45 with large spontaneous portosystemic shunt, 4 with previous portosystemic shunt, and 8 with portal vein thrombosis[47].
There is one randomized controlled trial comparing patients undergoing OLT with the piggyback technique with (n = 40) and without (n = 40) TPCS. They report that the decrease in cardiac output during the anhepatic phase is lower the TPCS group (P = 0.005). There was reduced transfusion of red blood cells in the TPCS group with 45% of patients not requiring transfusions vs 22% in the non-TPCS group (P = 0.05). The groups received similar quantities of of red blood cells (P = 0.09), fresh-frozen plasma (P = 0.76) and platelets (P = 0.88). The TPCS had greater diuresis during the anhepatic phase (P = 0.005). The authors report that patients undergoing OLT with TPCS have improved hemodynamic status, reduced intraoperative transfusion requirements and preservation of renal function[25].
There are advantages and disadvantages to each caval reconstruction technique in OLT. Although many authors conclude that there is lower operative and warm ischemia time, and decreased transfusion of blood products including red blood cells and fresh frozen plasma, with the piggyback technique and its’ modifications, especially when compared with conventional technique with VVB, there are centers where this has not been the case. The conventional technique certainly maintains a position in the surgical repertoire and is practiced by many surgeons at many centers with excellent results. Improvements in anesthesia management, ICU care, improved immunosuppression, and complication management have played at least as large a role in the excellent outcomes obtained with modern liver transplantation as the techniques described in this review. Having familiarity with the variety of options available for reconstruction of the IVC and tailoring these techniques to the individual patient when needed is advantageous. However, surgeons should utilize the techniques that they are most comfortable with and perform well as the quality of the operation is more important than the specific technique employed in achieving excellent outcomes.
P- Reviewer: Iwasaki Y, Tanoglu A S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659-676. [PubMed] [Cited in This Article: ] |
| 2. | Calne RY, Williams R. Liver transplantation in man. I. Observations on technique and organization in five cases. Br Med J. 1968;4:535-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 250] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 401] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Cescon M, Grazi GL, Varotti G, Ravaioli M, Ercolani G, Gardini A, Cavallari A. Venous outflow reconstructions with the piggyback technique in liver transplantation: a single-center experience of 431 cases. Transpl Int. 2005;18:318-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Ducerf C, Rode A, Adham M, De la Roche E, Bizollon T, Baulieux J, Pouyet M. Hepatic outflow study after piggyback liver transplantation. Surgery. 1996;120:484-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Gerber DA, Passannante A, Zacks S, Johnson MW, Shrestha R, Fried M, Fair JH. Modified piggyback technique for adult orthotopic liver transplantation. J Am Coll Surg. 2000;191:585-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Belghiti J, Panis Y, Sauvanet A, Gayet B, Fékété F. A new technique of side to side caval anastomosis during orthotopic hepatic transplantation without inferior vena caval occlusion. Surg Gynecol Obstet. 1992;175:270-272. [PubMed] [Cited in This Article: ] |
| 8. | Belghiti J, Noun R, Sauvanet A. Temporary portocaval anastomosis with preservation of caval flow during orthotopic liver transplantation. Am J Surg. 1995;169:277-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Tzakis AG, Reyes J, Nour B, Marino IR, Todo S, Starzl TE. Temporary end to side portacaval shunt in orthotopic hepatic transplantation in humans. Surg Gynecol Obstet. 1993;176:180-182. [PubMed] [Cited in This Article: ] |
| 10. | Isern MR, Massarollo PC, de Carvalho EM, Baía CE, Kavakama J, de Andrade Lima P, Mies S. Randomized trial comparing pulmonary alterations after conventional with venovenous bypass versus piggyback liver transplantation. Liver Transpl. 2004;10:425-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Jovine E, Mazziotti A, Grazi GL, Ercolani G, Masetti M, Morganti M, Pierangeli F, Begliomini B, Mazzetti PG, Rossi R. Piggy-back versus conventional technique in liver transplantation: report of a randomized trial. Transpl Int. 1997;10:109-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Barshes NR, Lee T, Kiliç M, Goss JA. Reconstruction of the hepatic venous outflow in piggyback liver transplantation. Exp Clin Transplant. 2004;2:189-195. [PubMed] [Cited in This Article: ] |
| 13. | Parrilla P, Sánchez-Bueno F, Figueras J, Jaurrieta E, Mir J, Margarit C, Lázaro J, Herrera L, Gómez-Fleitas M, Varo E. Analysis of the complications of the piggy-back technique in 1,112 liver transplants. Transplantation. 1999;67:1214-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Reddy KS, Johnston TD, Putnam LA, Isley M, Ranjan D. Piggyback technique and selective use of veno-venous bypass in adult orthotopic liver transplantation. Clin Transplant. 2000;14:370-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Hesse UJ, Berrevoet F, Troisi R, Pattyn P, Mortier E, Decruyenaere J, de Hemptinne B. Hepato-venous reconstruction in orthotopic liver transplantation with preservation of the recipients’ inferior vena cava and veno-venous bypass. Langenbecks Arch Surg. 2000;385:350-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Lerut JP, Molle G, Donataccio M, De Kock M, Ciccarelli O, Laterre PF, Van Leeuw V, Bourlier P, de Ville de Goyet J, Reding R. Cavocaval liver transplantation without venovenous bypass and without temporary portocaval shunting: the ideal technique for adult liver grafting? Transpl Int. 1997;10:171-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Robles R, Parrilla P, Acosta F, Bueno FS, Ramirez P, Lopez J, Lujan JA, Rodriguez JM, Fernandez JA, Picó F. Complications related to hepatic venous outflow in piggy-back liver transplantation: two- versus three-suprahepatic-vein anastomosis. Transplant Proc. 1999;31:2390-2391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Tayar C, Kluger MD, Laurent A, Cherqui D. Optimizing outflow in piggyback liver transplantation without caval occlusion: the three-vein technique. Liver Transpl. 2011;17:88-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Pisaniello D, Marino MG, Perrella A, Russo F, Campanella L, Marcos A, Cuomo O. Side-to-side cavocavostomy in adult piggyback liver transplantation. Transplant Proc. 2012;44:1938-1941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Mehrabi A, Mood ZA, Fonouni H, Kashfi A, Hillebrand N, Müller SA, Encke J, Büchler MW, Schmidt J. A single-center experience of 500 liver transplants using the modified piggyback technique by Belghiti. Liver Transpl. 2009;15:466-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Akbulut S, Wojcicki M, Kayaalp C, Yilmaz S. Liver transplantation with piggyback anastomosis using a linear stapler: a case report. Transplant Proc. 2013;45:1031-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Polak WG, Nemes BA, Miyamoto S, Peeters PM, de Jong KP, Porte RJ, Slooff MJ. End-to-side caval anastomosis in adult piggyback liver transplantation. Clin Transplant. 2006;20:609-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Wojcicki M, Post M, Pakosz-Golanowska M, Zeair S, Lubikowski J, Jarosz K, Czuprynska M, Milkiewicz P. Vascular complications following adult piggyback liver transplantation with end-to-side cavo-cavostomy: a single-center experience. Transplant Proc. 2009;41:3131-3134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Gurusamy KS, Pamecha V, Davidson BR. Piggy-back graft for liver transplantation. Cochrane Database Syst Rev. 2011;CD008258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Figueras J, Llado L, Ramos E, Jaurrieta E, Rafecas A, Fabregat J, Torras J, Sabate A, Dalmau A. Temporary portocaval shunt during liver transplantation with vena cava preservation. Results of a prospective randomized study. Liver Transpl. 2001;7:904-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Fleitas MG, Casanova D, Martino E, Maestre JM, Herrera L, Hernanz F, Rabanal JM, Pulgar S, Solares G. Could the piggyback operation in liver transplantation be routinely used? Arch Surg. 1994;129:842-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Levi DM, Pararas N, Tzakis AG, Nishida S, Tryphonopoulos P, Gonzalez-Pinto I, Tekin A, Selvaggi G, Livingstone AS. Liver transplantation with preservation of the inferior vena cava: lessons learned through 2,000 cases. J Am Coll Surg. 2012;214:691-698; discussion 698-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Stieber AC, Gordon RD, Bassi N. A simple solution to a technical complication in “piggyback” liver transplantation. Transplantation. 1997;64:654-655. [PubMed] [Cited in This Article: ] |
| 29. | Bilbao JI, Herrero JI, Martínez-Cuesta A, Quiroga J, Pueyo JC, Vivas I, Delgado C, Pardo F. Ascites due to anastomotic stenosis after liver transplantation using the piggyback technique: treatment with endovascular prosthesis. Cardiovasc Intervent Radiol. 2000;23:149-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Quintini C, Miller CM, Hashimoto K, Philip D, Uso TD, Aucejo F, Kelly D, Winans C, Eghtesad B, Vogt D. Side-to-side cavocavostomy with an endovascular stapler: Rescue technique for severe hepatic vein and/or inferior vena cava outflow obstruction after liver transplantation using the piggyback technique. Liver Transpl. 2009;15:49-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Zamboni F, Sampietro R. Conversion to termino-terminal cavo-cavostomy as a rescue technique for infrahepatic obstruction after piggyback liver transplantation. Transpl Int. 2008;21:1008-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Merenda R, Gerunda GE, Neri D, Barbazza F, Di Marzio E, Bruttocao A, Valmasoni M, Angeli P, Faccioli AM. Infrahepatic terminolateral cavo-cavostomy as a rescue technique in complicated “modified” piggyback liver transplantation. J Am Coll Surg. 1997;185:576-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Busque S, Esquivel CO, Concepcion W, So SK. Experience with the piggyback technique without caval occlusion in adult orthotopic liver transplantation. Transplantation. 1998;65:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Hosein Shokouh-Amiri M, Osama Gaber A, Bagous WA, Grewal HP, Hathaway DK, Vera SR, Stratta RJ, Bagous TN, Kizilisik T. Choice of surgical technique influences perioperative outcomes in liver transplantation. Ann Surg. 2000;231:814-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Nishida S, Nakamura N, Vaidya A, Levi DM, Kato T, Nery JR, Madariaga JR, Molina E, Ruiz P, Gyamfi A. Piggyback technique in adult orthotopic liver transplantation: an analysis of 1067 liver transplants at a single center. HPB (Oxford). 2006;8:182-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Sakai T, Matsusaki T, Marsh JW, Hilmi IA, Planinsic RM. Comparison of surgical methods in liver transplantation: retrohepatic caval resection with venovenous bypass (VVB) versus piggyback (PB) with VVB versus PB without VVB. Transpl Int. 2010;23:1247-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Vieira de Melo PS, Miranda LE, Batista LL, Neto OC, Amorim AG, Sabat BD, Cândido HL, Adeodato LC, Lemos RS, Carvalho GL. Orthotopic liver transplantation without venovenous bypass using the conventional and piggyback techniques. Transplant Proc. 2011;43:1327-1333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Cabezuelo JB, Ramirez P, Acosta F, Torres D, Sansano T, Pons JA, Bru M, Montoya M, Rios A, Sánchez Bueno F. Does the standard vs piggyback surgical technique affect the development of early acute renal failure after orthotopic liver transplantation? Transplant Proc. 2003;35:1913-1914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Durand F, Aschehoug J, Sauvanet A, Bernuau J, Benhamou JP, Erlinger S, Belghiti J. Preservation of renal perfusion and postoperative renal function by side-to-side cavo-caval anastomosis in liver transplant recipients. Transpl Int. 1995;8:407-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Hesse UJ, Berrevoet F, Troisi R, Mortier E, Pattyn P, de Hemptinne B. Liver transplantation by preservation of the caval flow with temporary porto-caval shunt or veno-venous bypass. Transplant Proc. 1997;29:3609-3610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Mosimann F, Gillet M. Retransplantation of the liver after side-to-side caval anastomosis. Transpl Int. 1995;8:157-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Zieniewicz K, Krawczyk M, Nyckowski P, Pawlak J, Michałowicz B, Paluszkiewicz R, Patkowski W, Grzelak I, Alsharabi A, Wróblewski T. Liver transplantation: comparison of the classical orthotopic and piggyback techniques. Transplant Proc. 2002;34:625-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Remiszewski P, Zieniewicz K, Krawczyk M. Early results of orthotopic liver transplantations using the technique of inferior vena cava anastomosis. Transplant Proc. 2006;38:237-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Khan S, Silva MA, Tan YM, John A, Gunson B, Buckels JA, David Mayer A, Bramhall SR, Mirza DF. Conventional versus piggyback technique of caval implantation; without extra-corporeal veno-venous bypass. A comparative study. Transpl Int. 2006;19:795-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Schmitz V, Schoening W, Jelkmann I, Globke B, Pascher A, Bahra M, Neuhaus P, Puhl G. Different cava reconstruction techniques in liver transplantation: piggyback versus cava resection. Hepatobiliary Pancreat Dis Int. 2014;13:242-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Cherqui D, Lauzet JY, Rotman N, Duvoux C, Dhumeaux D, Julien M, Fagniez PL. Orthotopic liver transplantation with preservation of the caval and portal flows. Technique and results in 62 cases. Transplantation. 1994;58:793-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 47. | Belghiti J, Ettorre GM, Durand F, Sommacale D, Sauvanet A, Jerius JT, Farges O. Feasibility and limits of caval-flow preservation during liver transplantation. Liver Transpl. 2001;7:983-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Navarro F, Le Moine MC, Fabre JM, Belghiti J, Cherqui D, Adam R, Pruvot FR, Letoublon C, Domergue J. Specific vascular complications of orthotopic liver transplantation with preservation of the retrohepatic vena cava: review of 1361 cases. Transplantation. 1999;68:646-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Lai Q, Nudo F, Molinaro A, Mennini G, Spoletini G, Melandro F, Guglielmo N, Parlati L, Mordenti M, Ginanni Corradini S. Does caval reconstruction technique affect early graft function after liver transplantation? A preliminary analysis. Transplant Proc. 2011;43:1103-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | González FX, García-Valdecasas JC, Grande L, Pacheco JL, Cugat E, Fuster J, Lacy AM, Taurá P, López-Boado MA, Rimola A. Vena cava vascular reconstruction during orthotopic liver transplantation: a comparative study. Liver Transpl Surg. 1998;4:133-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Mangus RS, Fridell JA, Vianna RM, Cooper AB, Jones DT, Tector AJ. Use of the piggyback hepatectomy technique in liver transplant recipients with hepatocellular carcinoma. Transplantation. 2008;85:1496-1499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Shaw BW, Martin DJ, Marquez JM, Kang YG, Bugbee AC, Iwatsuki S, Griffith BP, Hardesty RL, Bahnson HT, Starzl TE. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 346] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Fonouni H, Mehrabi A, Soleimani M, Müller SA, Büchler MW, Schmidt J. The need for venovenous bypass in liver transplantation. HPB (Oxford). 2008;10:196-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Grande L, Rimola A, Cugat E, Alvarez L, García-Valdecasas JC, Taurá P, Beltrán J, Fuster J, Lacy AM, González FJ. Effect of venovenous bypass on perioperative renal function in liver transplantation: results of a randomized, controlled trial. Hepatology. 1996;23:1418-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Chari RS, Gan TJ, Robertson KM, Bass K, Camargo CA, Greig PD, Clavien PA. Venovenous bypass in adult orthotopic liver transplantation: routine or selective use? J Am Coll Surg. 1998;186:683-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Veroli P, el Hage C, Ecoffey C. Does adult liver transplantation without venovenous bypass result in renal failure? Anesth Analg. 1992;75:489-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Acosta F, Sansano T, Rodriguez MA, Contreras RF, Reche M, Beltran R, Roques V, Robles R, Bueno FS, Ramirez P. Need for venovenous bypass in patients with familial amyloidotic polyneuropathy treated with liver transplantation. Transplant Proc. 1999;31:2394-2395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 58. | Reddy K, Mallett S, Peachey T. Venovenous bypass in orthotopic liver transplantation: time for a rethink? Liver Transpl. 2005;11:741-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Hilmi IA, Planinsic RM. Con: venovenous bypass should not be used in orthotopic liver transplantation. J Cardiothorac Vasc Anesth. 2006;20:744-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Kuo PC, Alfrey EJ, Garcia G, Haddow G, Dafoe DC. Orthotopic liver transplantation with selective use of venovenous bypass. Am J Surg. 1995;170:671-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Steib A, Saada A, Clever B, Lehmann C, Freys G, Levy S, Boudjema K. Orthotopic liver transplantation with preservation of portocaval flow compared with venovenous bypass. Liver Transpl Surg. 1997;3:518-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Lázaro JL, Charco R, Revhaug A, Murio E, Balsells J, Hidalgo E, Mora A, Cortés C, Margarit C. Hemodynamics in human liver transplantation with inferior vena cava preservation. Transplant Proc. 1997;29:2851-2852. [PubMed] [Cited in This Article: ] |