Published online Nov 8, 2016. doi: 10.5409/wjcp.v5.i4.365
Peer-review started: June 3, 2016
First decision: July 25, 2016
Revised: September 20, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: November 8, 2016
To determine if packed red blood cell transfusions contribute to the development of parenteral nutrition associated liver disease.
A retrospective chart review of 49 premature infants on parenteral nutrition for > 30 d who received packed red blood cell (PRBC) transfusions was performed. Parenteral nutrition associated liver disease was primarily defined by direct bilirubin (db) > 2.0 mg/dL. A high transfusion cohort was defined as receiving > 75 mL packed red blood cells (the median value). Kaplan-Meier plots estimated the median volume of packed red blood cells received in order to develop parenteral nutrition associated liver disease.
Parenteral nutritional associated liver disease (PNALD) was noted in 21 (43%) infants based on db. Among the 27 high transfusion infants, PNALD was present in 17 (64%) based on elevated direct bilirubin which was significantly greater than the low transfusion recipients. About 50% of the infants, who were transfused 101-125 mL packed red blood cells, developed PNALD based on elevation of direct bilirubin. All infants who were transfused more than 200 mL of packed red blood cells developed PNALD. Similar results were seen when using elevation of aspartate transaminase or alanine transaminase to define PNALD.
In this retrospective, pilot study there was a statistically significant correlation between the volume of PRBC transfusions received by premature infants and the development of PNALD.
Core tip: The etiology of parenteral nutrition associated liver disease (PNALD), a commonly encountered morbidity in the neonatal intensive care unit (NICU) remains unknown. Potentially hepatotoxic packed red blood cell (PRBC) transfusions are routinely administered in this setting. Whether PRBC transfusions increase the prevalence of PNALD is a clinical question that has not been systematically investigated. This pilot study demonstrated that in a cohort of NICU infants who received greater volumes of PRBC, there was a significantly higher prevalence of PNALD. Further investigations to define the exact risk are warranted to minimize NICU stays, costs, and future liver damage.
- Citation: D’Souza A, Algotar A, Pan L, Schwarz SM, Treem WR, Valencia G, Rabinowitz SS. Packed red blood cell transfusions as a risk factor for parenteral nutrition associated liver disease in premature infants. World J Clin Pediatr 2016; 5(4): 365-369
- URL: https://www.wjgnet.com/2219-2808/full/v5/i4/365.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v5.i4.365
Preterm infants are among the most highly transfused patient populations, in part because of the presumption that packed red blood cells (PRBC) improve oxygen delivery[1]. Approximately 38000 premature neonates receive more than 300000 transfusions annually[2]. PRBC transfusion guidelines are based on expert opinion, rather than evidence-based data, and vary among practitioners, institutions and clinical situations[3,4]. The increased susceptibility of neonatal intensive care unit (NICU) patients to develop anemia requiring blood transfusions is attributable to multiple factors. These include prematurity itself, nutritional deficiencies, iatrogenic blood loss, and other medical conditions commonly seen in the NICU such as sepsis, hemolysis, bleeding disorders, and surgery[5].
Although PRBC transfusions are believed to be helpful in the NICU[6], they have been implicated in the development of bronchopulmonary dysplasia, acute lung injury, necrotizing enterocolitis (NEC), intraventricular hemorrhage, and retinopathy of prematurity[7,8]. Multiple transfusions may result in iron deposition leading to dysfunction in the liver, heart and other organs[9-14]. Because RBC life span in preterm infants is shorter, accelerated cell breakdown results in even greater degrees of hepatic iron deposition. Efforts to decrease transfusion requirements in low birth weight infants include utilizing erythropoietin in the first 48 hour of life[15]. This hematopoietic agent has been suggested to have a neuroprotective effect in newborns[16].
Parenteral nutrition (PN) is another recognized risk factor contributing to hepatobiliary dysfunction in newborn infants[17-20]. The clinical spectrum of parenteral nutrition-associated liver disease (PNALD) encompasses cholestasis, cholelithiasis, elevated transaminases, steatosis, fibrosis, biliary cirrhosis, portal hypertension and, potentially, hepatic failure[18]. Although PNALD’s pathogenesis may be multifactorial, omega-6 fatty acids in PN regimens now appear to be the primary etiologic agent[19]. Additional risk factors include prematurity, other nutrient excesses or deficiencies, sepsis from the central line, decreased enterohepatic circulation, intestinal stasis, and bacterial overgrowth[20].
The published literature most often defines PNALD in this setting as a direct bilirubin (db) > 2.0[20,21]. As this was a pilot study to provide preliminary data on predicting hepatobiliary disease in the premature infant, transaminase values were also examined. While alanine transaminase (ALT) has been considered a more specific marker for liver dysfunction, aspartate transaminase (AST) has recently been employed as part of a derived AST/platelet ratio index, to predict liver pathology in infants with intestinal failure[22] and biliary atresia[23].
Most low birth weight infants, especially sicker babies, are unavoidably exposed to multiple risk factors associated with hepatobiliary dysfunction, including PN and PRBC transfusions. This retrospective study was conducted to determine if there was any preliminary evidence suggesting that the volume of PRBC transfusions received was associated with the subsequent development of PNALD in this population.
This retrospective chart analysis was performed as part of a study of 49/51 premature infants maintained on PN > 30 d, at the Children’s Hospital at Downstate, State University New York, Downstate Medical Center. Two patients, one with cystic fibrosis and one with Hirschsprung disease (disorders independently associated with cholestasis), were excluded from analysis. In these 49 infants, we assessed the timing and volume of PRBC transfusions, and PNALD was primarily defined as a db level > 2.0 mg/dL[20,21].
One of the authors, SMS performed all of the biomedical statistics. Kaplan-Meier plots estimated the amount of PRBC transfused to attain PNALD onset by this db criterion. Similar analyses were performed for elevation of AST and ALT as alternative markers of PNALD. Proportional hazards regression analysis used age at PNALD onset as the dependent variable, and cumulative RBCs as the time dependent predictor of interest. Potential confounders were cumulative days on TPN (time-dependent) and birth weight. Odds ratios, hazard ratios (HR) and confidence intervals (CI) are reported. This study was approved by the Institutional Review Board of SUNY Downstate Medical Center.
In this cohort of 49 NICU infants, 21 (43%) reached the endpoint of PNALD, defined by a db > 2.0 mg/dL. To analyze any potential role of PRBC transfusions in the development of PNALD, the study population was subdivided into high and low transfusion groups. The subgroups were defined by the median volume transfused in this study cohort, specifically transfusion volumes of ≥ 75 mL (high) or < 75 mL (low). Employing this cut off value, 27/49 (55%) infants were in the high transfusion group and 22/49 (45%) were in the low transfusion cohort.
Figure 1 shows the relationship between transfusion volume and PNALD, as defined by a db > 2.0 mg/dL. Among the 27 high transfusion infants, 17 (64%) developed PNALD, while PNALD was seen in only 4/22 infants (18%) in the low transfusion group. Further, among all infants who developed PNALD, 17/21 (81%) received PRBC ≥ 75 mL while only 10/28 (36%) received < 75 mL PRBC volumes (P < 0.001). The calculated odds ratio for developing PNALD, based on being in the high verses low transfusion group, was 7.6 (95%CI: 2.01-29.1).
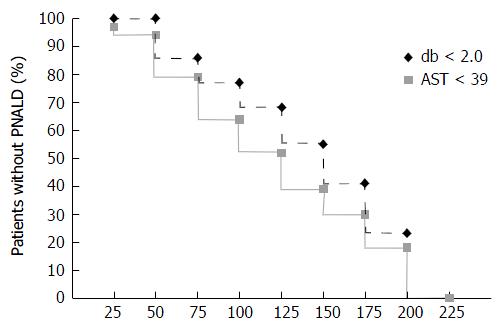
Kaplan-Meier plots provide another perspective on the relationship between PRBC transfusions and PNALD (Figure 2). With cumulative transfusion volumes of 126-150 mL, the prevalence of db > 2.0 mg/dL reached 50%. All infants who were transfused > 200 mL PRBC demonstrated PNALD. Regression analysis showed neither birth weight nor cumulative time on TPN were significant predictors of these endpoints, when used as linear covariates (data not shown). However, cumulative PRBCs were a significant predictor of db ≥ 2.0 with an estimated HR associated with each additional 15 mL/kg transfused of 1.09 (95%CI: 1.00-1.19). After controlling for birth weight and PN duration, PRBC volume transfused remained a predictor of reaching the primary end point of db ≥ 2.0. The HR related to incremental PRBC transfused volumes approached significance for each 15 mL/kg (HR = 1.11, 95%CI: 1.00-1.23, P = 0.053). However, when the NEC cases were excluded, the transfusion effect on db ≥ 2.0 became statistically significant (P = 0.026).
Five of the 49 infants in our study developed NEC. Of these five infants, three also had culture proven sepsis, and two of these three exhibited PNALD. Of the two infants with NEC but without sepsis, both demonstrated PNALD. Overall, 11/17 study subjects with culture proven sepsis demonstrated PNALD. Employing elevated transaminases, AST and ALT, to define PNALD yielded results similar to those based on db ≥ 2.0. PNALD was seen in 67% of the cohort based on AST elevations and in 41% based on ALT elevations. Of the 33 infants with AST-defined PNALD, 70% were in the high transfusion and 30% were in the low transfusion cohorts. Similarly, among the cohort of 20 infants with PNALD defined by elevated ALT, 75% were in the high transfusion and 25% were in the low transfusion groups. Similarly to db PNALD, the odds ratio for developing PNALD based on high vs low PRBC transfusion was 6.9 (95%Cl: 1.78-26.7) if defined by AST, and was 4.2 (95%CI: 1.21-14.9) if defined by ALT.
Although potentially lifesaving, blood transfusions are associated with risks in the NICU[7,8]. Several studies have attempted to better define indications for transfusion in NICU babies[24,25]. Previous investigations have sought to develop transfusion protocols, to achieve an acceptable balance between the risks and the benefits of transfusing preterm infants[1,7,25]. The PINT trial concluded that a higher hemoglobin threshold for transfusions in the NICU resulted in a greater number of transfusions without any added benefit, when compared to a restricted hemoglobin threshold[26]. In the present review of 49 premature infants on TPN, transfusion of ≥ 75 mL PRBC represented a significant risk factor for developing PNALD. If this relationship is confirmed in larger studies, the potential benefits of PRBC transfusions could be balanced with the associated risk of developing liver disease.
The pathogenesis of hepatotoxicity secondary to both PRBC transfusions and PN includes injury secondary to the generation of hepatic reactive oxidative stress (ROS)[26-30]. Since no effective mechanisms allow for excretion of parenteral iron, repeated transfusions can yield secondary Iron overload[27]. The primary sites for iron overload toxicity are those tissues where iron is stored, with liver being the main target[28]. One of the most important recognized mechanisms of liver injury in this setting is free radical mediated oxidative damage[29]. Preterm infants are especially vulnerable to this insult, as a higher proportion of their iron remains unbound to transferrin, leading to increased ROS[30]. Malonylaldehyde (MDA), a byproduct of hepatic ROS, has been employed as a marker of oxidative stress. Elevations of this compound are associated with chronic transfusion states, such as thalassemia and sickle cell disease[31]. Emerging evidence has also implicated oxidative damage as a factor in PNALD[32,33]. Animal models of PNALD using weanling rat[32] and infant rabbit[33] have correlated severity of liver injury and hepatic MDA content with time on PN.
Several important limitations are associated with this pilot study. This retrospective analysis involved a relatively small number of representative NICU patients. Additionally, the cohort was heterogeneous, as subjects were included over a wide range of gestational ages. Other potential comorbidities, previously identified as PNALD risk factors, were unable to be controlled. If this small study group were to be further subdivided, the numbers would not provide meaningful data. Finally, because this is a small cohort, the higher PRBC transfusions volumes associated with PNALD may actually be secondary to coexisting conditions which are the true primary risk factors for this condition.
In conclusion, our pilot study supports the hypothesis that repeated PRBC transfusions increase the risk of PNALD in NICU infants. This preliminary observation is being presented to stimulate further studies employing larger NICU databases. Access to this information could yield a series of well-defined PRBC transfusion recommendations and/or guidelines based on specific characteristics in this vulnerable cohort.
Parenteral nutrition is commonly associated with liver disease in the neonatal intensive care unit (NICU). There are a variety of factors that have been described as risk factors for this problem including prematurity, time on parenteral nutrition, sepsis, prolonged periods without enteral nutrition and necrotizing enterocolitis. Packed red blood cells transfusions which can generate reactive oxygen species especially in the livers of premature neonates are a potential trigger for this morbidity.
Whether the transfusion of packed red blood cells is an actual contributor to the incidence of cholestatic liver disease in the NICU infant receiving parenteral nutrition has not been systematically investigated.
This retrospective pilot study compared a cohort of NICU infants on parenteral nutrition who had received more than the median volume of packed red blood cell transfusions to a second cohort from the same nursery at the same time who received less than the median value. Higher volumes of transfusion led to a statistically significant increase in the prevalence of liver disease in this study as defined by elevated direct bilirubin, by elevated aspartate transaminase and by elevated alanine transaminase.
This preliminary observation should now be investigated in larger cohorts of NICU infants. If these results are confirmed, then guidelines addressing the safety of packed red blood cell transfusions in the NICU can be developed.
NICU: Neonatal intensive care unit; PRBC: Packed red blood cells; PNALD: Parenteral nutrition associated liver disease.
A small and succinct study, while it has some limitations, which are well acknowledged by the author. There are some interesting and statistically significant within this study.
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ingley E S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr. 2009;155:331-337.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Strauss RG. Transfusion therapy in neonates. Am J Dis Child. 1991;145:904-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Calhoun DA, Christensen RD, Edstrom CS, Juul SE, Ohls RK, Schibler KR, Sola MC, Sullivan SE. Consistent approaches to procedures and practices in neonatal hematology. Clin Perinatol. 2000;27:733-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Bednarek FJ, Weisberger S, Richardson DK, Frantz ID, Shah B, Rubin LP. Variations in blood transfusions among newborn intensive care units. SNAP II Study Group. J Pediatr. 1998;133:601-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Bain A, Blackburn S. Issues in transfusing preterm infants in the NICU. J Perinat Neonatal Nurs. 2004;18:170-182; quiz 183-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Dani C, Pratesi S, Fontanelli G, Barp J, Bertini G. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion. 2010;50:1220-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Christensen RD, Ilstrup S. Recent advances toward defining the benefits and risks of erythrocyte transfusions in neonates. Arch Dis Child Fetal Neonatal Ed. 2013;98:F365-F372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Hakeem AH, Mohamed GB, Othman MF. Retinopathy of prematurity: a study of prevalence and risk factors. Middle East Afr J Ophthalmol. 2012;19:289-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Shander A, Sazama K. Clinical consequences of iron overload from chronic red blood cell transfusions, its diagnosis, and its management by chelation therapy. Transfusion. 2010;50:1144-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Shander A, Cappellini MD, Goodnough LT. Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sang. 2009;97:185-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Ng PC, Lam CW, Lee CH, To KF, Fok TF, Chan IH, Wong E. Hepatic iron storage in very low birthweight infants after multiple blood transfusions. Arch Dis Child Fetal Neonatal Ed. 2001;84:F101-F105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Harmatz P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T, Golden D, Neumayr L, Vichinsky E. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood. 2000;96:76-79. [PubMed] [Cited in This Article: ] |
| 13. | Brown K, Subramony C, May W, Megason G, Liu H, Bishop P, Walker T, Nowicki MJ. Hepatic iron overload in children with sickle cell anemia on chronic transfusion therapy. J Pediatr Hematol Oncol. 2009;31:309-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Wahl S, Quirolo KC. Current issues in blood transfusion for sickle cell disease. Curr Opin Pediatr. 2009;21:15-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Rosebraugh MR, Widness JA, Veng-Pedersen P. Multidose optimization simulation of erythropoietin treatment in preterm infants. Pediatr Res. 2012;71:332-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Ohls RK, Christensen RD, Widness JA, Juul SE. Erythropoiesis Stimulating Agents Demonstrate Safety and Show Promise as Neuroprotective Agents in Neonates. J Pediatr. 2015;167:10-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Zambrano E, El-Hennawy M, Ehrenkranz RA, Zelterman D, Reyes-Múgica M. Total parenteral nutrition induced liver pathology: an autopsy series of 24 newborn cases. Pediatr Dev Pathol. 2004;7:425-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Kelly DA. Liver complications of pediatric parenteral nutrition--epidemiology. Nutrition. 1998;14:153-157. [PubMed] [Cited in This Article: ] |
| 19. | Hayashi N, Tashiro T, Yamamori H, Takagi K, Morishima Y, Otsubo Y, Sugiura T, Furukawa K, Nitta H, Nakajima N. Effects of intravenous omega-3 and omega-6 fat emulsion on cytokine production and delayed type hypersensitivity in burned rats receiving total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1998;22:363-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Xu ZW, Li YS. Pathogenesis and treatment of parenteral nutrition-associated liver disease. Hepatobiliary Pancreat Dis Int. 2012;11:586-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Lauriti G, Zani A, Aufieri R, Cananzi M, Chiesa PL, Eaton S, Pierro A. Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J Parenter Enteral Nutr. 2014;38:70-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 22. | Díaz JJ, Gura KM, Roda J, Perez-Atayde AR, Duggan C, Jaksic T, Lo CW. Aspartate aminotransferase to platelet ratio index correlates with hepatic cirrhosis but not with fibrosis in pediatric patients with intestinal failure. J Pediatr Gastroenterol Nutr. 2013;57:367-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Grieve A, Makin E, Davenport M. Aspartate Aminotransferase-to-Platelet ratio index (APRi) in infants with biliary atresia: prognostic value at presentation. J Pediatr Surg. 2013;48:789-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Kasat K, Hendricks-Muñoz KD, Mally PV. Neonatal red blood cell transfusions: searching for better guidelines. Blood Transfus. 2011;9:86-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 10] [Reference Citation Analysis (0)] |
| 25. | Guillén U, Cummings JJ, Bell EF, Hosono S, Frantz AR, Maier RF, Whyte RK, Boyle E, Vento M, Widness JA. International survey of transfusion practices for extremely premature infants. Semin Perinatol. 2012;36:244-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, Peliowski A, Rios A, LaCorte M, Connelly R. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 326] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 27. | Siah CW, Ombiga J, Adams LA, Trinder D, Olynyk JK. Normal iron metabolism and the pathophysiology of iron overload disorders. Clin Biochem Rev. 2006;27:5-16. [PubMed] [Cited in This Article: ] |
| 28. | Muñoz M, Villar I, García-Erce JA. An update on iron physiology. World J Gastroenterol. 2009;15:4617-4626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 211] [Cited by in F6Publishing: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Muñoz M, García-Erce JA, Remacha ÁF. Disorders of iron metabolism. Part II: iron deficiency and iron overload. J Clin Pathol. 2011;64:287-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Hirano K, Morinobu T, Kim H, Hiroi M, Ban R, Ogawa S, Ogihara H, Tamai H, Ogihara T. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;84:F188-F193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, Porter J, Evans P, Vichinsky E, Harmatz P. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 32. | Sokol RJ, Taylor SF, Devereaux MW, Khandwala R, Sondheimer NJ, Shikes RH, Mierau G. Hepatic oxidant injury and glutathione depletion during total parenteral nutrition in weanling rats. Am J Physiol. 1996;270:G691-G700. [PubMed] [Cited in This Article: ] |
| 33. | Hong L, Wang X, Wu J, Cai W. Mitochondria-initiated apoptosis triggered by oxidative injury play a role in total parenteral nutrition-associated liver dysfunction in infant rabbit model. J Pediatr Surg. 2009;44:1712-1718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |