Published online Feb 8, 2016. doi: 10.5409/wjcp.v5.i1.118
Peer-review started: July 17, 2015
First decision: October 21, 2015
Revised: November 4, 2015
Accepted: November 10, 2015
Article in press: November 10, 2015
Published online: February 8, 2016
AIM: To examine the effect of carob-bean gum (CBG) thickened-formulas on reflux and tolerance indices in infants with gastro-esophageal reflux (GER).
METHODS: Fifty-six eligible infants (1-6 mo old) were randomly allocated to receive for two weeks a formula with either 0.33 g/100 mL (Formula A) or 0.45 g/100 mL (Formula B) of cold soluble CBG galactomannans respectively, or a formula with 0.45 g/100 mL of hot soluble CBG galactomannans (Formula C). No control group receiving standard formula was included in the study. Data on the following indices were obtained both at baseline and follow-up from all study participants: 24 h esophageal pH monitoring indices, anthropometrical indices (i.e., body weight and length) and tolerance indices (i.e., frequency of colics; type and frequency of defecations). From the eligible infants, forty seven were included in an intention-to-treat analysis to examine the effects of the two-week trial on esophageal 24 h pH monitoring, growth and tolerance indices. Repeated Measures ANOVA was used to examine the research hypothesis.
RESULTS: Regarding changes in 24 h pH monitoring indices, significant decreases from baseline to follow-up were observed in the “Boix Ochoa Score” (i.e., an index of esophageal acid exposure), in the total number of visible refluxes and in all symptoms related indices due to acid reflux only for infants provided with Formula A, while no significant changes were observed for infants provided with Formulas B and C. In addition, the significant decreases observed in two symptoms related pH monitoring indices (i.e., “Symptom index for reflux” and “Percentage of all reflux”) for infants provided with Formula A were also found to differentiate significantly compared to the changes observed in the other two groups (P = 0.048 and P = 0.014 respectively). Concerning changes in anthropometric indices, body weight significantly increased among infants provided with Formulas A and C, but not for infants provided with Formula B. As far as tolerance indices were concerned, the numbers of total and diarrheic defecations increased significantly only in infants provided with Formula B and these changes were significantly higher compared to the decreases observed in infants fed with Formulas A and C (P = 0.003 and P = 0.015 respectively. Lastly the number of colics significantly decreased in all infants, irrespective of the tested formula.
CONCLUSION: Formula A (i.e., 0.33 g/100 mL of cold galactomannans) was effective in reducing certain pH-monitoring indices of uncomplicated GER, increased body weight and was well-tolerated by infants.
Core tip: The present study showed that Formula A was more effective in decreasing esophageal acid exposure, the total daily number of visible and measurable refluxes, as well as acid reflux related symptoms, while such changes were not observed for the infants fed with Formulas B and C. Furthermore, a significant increase of body weight was observed for infants fed with Formulas A and C while that was not observed for infants fed with Formula B, probably due to the increased number of diarrheic and total defecations recorded in this group. These findings indicate that Formula A, containing 0.33 g/100 mL of cold soluble galactomannans, seems to be more effective in reducing certain pH-monitoring indices of uncomplicated gastro-esophageal reflux, increasing body weight and being well-tolerated by infants.
- Citation: Georgieva M, Manios Y, Rasheva N, Pancheva R, Dimitrova E, Schaafsma A. Effects of carob-bean gum thickened formulas on infants’ reflux and tolerance indices. World J Clin Pediatr 2016; 5(1): 118-127
- URL: https://www.wjgnet.com/2219-2808/full/v5/i1/118.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v5.i1.118
Gastro-esophageal reflux (GER) is defined as the involuntary passage of gastric contents (e.g., saliva, ingested foods and drinks, gastric secretions, pancreatic or biliary secretions) into the esophagus and does not refer to any specific etiology with or without regurgitation and vomiting[1]. The term regurgitation is specifically used if the reflux dribbles effortlessly out of the mouth[2]. GER is a common and global problem affecting about 50% of all babies up to the age of two months and has a peak incidence at the age of three months. Only some infants will develop pathologic gastro-esophageal reflux disease (GERD), in which clinical problems are related to excessive passage of acid gastric contents. GERD should be suspected if the regurgitating infant shows one or more other symptoms such as crying, fussing or arching of the back, refusal to feed, failure to thrive, hematemesis, occult blood in the stool, anemia or refusal to eat[2,3]. Uncomplicated GER should be suspected in infants with uncomplicated recurrent regurgitation[1]. In some cases GER may affect thrive because of caloric insufficiency and potentially lower dietary nutrients’ intake. There is often abnormal sucking and swallowing and weight gain may be poor.
Since infant regurgitation is a transient problem, treatment goals are to provide effective reassurance and symptom relief. Infants with GER may feel unhappy and parents often seek medical attention. The use of anti-reflux formulas and formulas with added thickening agents [e.g., processed rice, corn or potato starch, guar gum or carob-bean gum (CBG)] results in a decrease of visible regurgitation[4]. CBG or locust bean gum is refined from the endosperm of seeds of the carob tree (Ceratonia siliqua). Eighty-five percent of the product is in the form of galacto-mannose oligo/polysaccharide having the monosaccharides mannose and galactose in a ratio of 4:1, about 5% is protein, and the final 10% is water. This galactomannan is indigestible but fermentable by colonic bacteria[5]. Because of this fermentable characteristic, some infants may react with abdominal pain, colic and diarrhea. In fact these adverse effects are normal for fiber ingestion and not specifically associated with CBG. Nevertheless, it seems that CBG is safe for its therapeutic use in term infants to treat GER from birth onwards[6]. Commercially available anti-reflux formulas currently contain CBG galactomannans at a concentration of 0.45 g/100 mL. However, Miyazawa et al[7,8] published studies in 2006 and 2007 reporting that lower dosages of CBG (0.35 g/100 mL) are effective too, at least with regard to visible refluxes. Although for these reasons the amount of CBG could be reduced to 0.35-0.40 g/100 mL, the effect of these lower concentrations on measurable refluxes is not known.
The primary objective of the current study was to examine the efficacy of formulas containing cold vs hot soluble CBG galactomannans (at a concentration of 0.45 g per 100 mL) and the effect of feeding infants with a lower concentration of galactomannans (i.e., 0.33 g per 100 mL) on visible and measurable refluxes assessed by 24 h pH impedance monitoring. Furthermore, a secondary objective was to determine whether the decrease in the concentration of galactomannans and the change from hot to cold soluble galactomannans affects weight gain and tolerance indices (i.e., stool frequency and consistency, colic) in infants.
The current study was a randomized, partly double blind clinical trial initiated on July 2013 and completed on July 2014 at the Second Pediatric Clinic in the University Hospital “St. Marine” Varna, Bulgaria. Informed consent was obtained from the parents of all infants that were found to be eligible to be included in the study. Prior to study’s initiation and during the first screening phase, eligibility of infants to participate in the study was assessed according to the following inclusion criteria: Availability of parents/infants to participate in the study throughout the intervention period; less than ¼ of daily milk consumption coming from breast milk; no use of any anti-reflux formulae or medications that can affect gastrointestinal tract motility; no history, diagnosis or illness from cow’s milk protein allergy (i.e., positive IgE and/or positive skin prick test to cow’s milk), wheezing, aspiration caused pneumonia, apnea, anemia, bleeding, laryngitis, urinary tract infection, diarrhea, neurologic deficits and any known organic or metabolic cause of reflux. Further to the initial screening phase, a total number of 56 one to six month-old infants that were born full-term, fulfilling all above inclusion criteria and diagnosed with GER (based on a score > 7 in the GER Orenstein questionnaire[9] as filled in by parents at inclusion) were considered eligible and entered the study. Eligible infants were randomized into three study groups based on the type of formula provided to them: Formula A containing 0.33 g/100 mL cold soluble galactomannans; Formula B containing 0.45 g/100 mL cold galactomannans; and Formula C containing 0.45 g/100 mL of hot soluble galactomannans. The cold soluble form of galactomannans is heated during production to be pre-gelatinised and gets gelatinised when dissolved in lukewarm water (i.e., of approximately 45 °C). The hot soluble form of CBG galactomannans is only minimally heated during production and needs to be dissolved in hot water (i.e., of approximately 90 °C) to be gelatinised. The difference in water temperature explains why this study could not be double blind for all study groups. More specifically, parents whose infants were allocated to Formulas A and B were instructed to use lukewarm water, whereas those parents whose infants were allocated to Formula C were instructed to use hot water for the preparation of the relevant milk formulae. Further to the above although the intervention was double blind for the study groups receiving Formula A and Formula B, this was not feasible for the Formula C treatment arm.
Following the first screening and before allocation of eligible infants to the study groups all infants were fed with a standard infant formula (Frisolac Gold 1, Friesland Campina, the Netherlands) for seven consecutive days, which served as a “run-in” period before the initiation of the intervention. On day seven, baseline anthropometric and 24 h pH impedance monitoring measurements were conducted. From day eight to day 21 the infants received the intervention Formula A, B or C. Allocation of infants to each one of three treatment arms was based on a standard table developed by a statistician (StatistiCal B.V., Wassenaar, The Netherlands), randomly assigning a different numerical code to each study participant receiving one of the three test formulae. On day 22 the final anthropometric and 24 h pH impedance monitoring measurements were conducted. Formula C was the reference formula and was provided to parents in the standard Friso Comfort packaging. The other two test formulae were provided in blank sachets labeled with either “A” or “B”. The product developer kept the decoding information in a sealed envelope, which was opened after completion of the intervention and evaluation of the study results.
The measurements conducted and the data collected in the present study are summarized below.
Gastro-esophageal reflux questionnaire: The gastro-esophageal reflux questionnaire (GERQ) is an instrument developed and validated for diagnosis of GER in infants and toddlers from 1 to 14 mo old[9,10]. Based on the scoring (i.e., GERQ score) derived from the answers provided by mothers, infants with a GERQ score > 7 (i.e., score indicative of possible GER) were considered eligible to participate in the study. The appropriateness of using the Orenstein questionnaire to identify infants with GER in the current study was also confirmed by the pH monitoring indices values obtained at baseline. Specifically, all eligible children identified by Orenstein questionnaire were also found to have pH indices above the references values suggested by Kitz et al[11] at baseline.
Three-day diaries: A 3-d diary was provided to mothers both at the start and at the end of the intervention period (i.e., the diaries were filled in by mothers from day four until day six and from day 18 to day 20), in order to record “tolerance” indices (i.e., type and frequency of colic and defecations) and information regarding the amount of formula consumed by their infants during the day. Regarding colic, that was defined based on the classic definition of infantile colic and specifically an approach based on the rule of threes: i.e., fussy crying that lasts for 3 h per day and for 3 d per week[12]. Regarding defecations, the total number of infants’ defecations was recorded by mothers in the diaries, while a visual chart, i.e., the Bristol Stool chart (BSC), that classifies defecations based on 7-point stool hardness scale (1, hard; 7, watery) was used to define constipation; diarrhea and ideal-stool defecations. BSC is currently the most popular scale/tool used in many clinical trials also conducted on infants and children to assess stool consistency[13]. Regarding the amount of formula consumed by their infants during the day, mothers were asked to keep a record reporting the exact volume of milk formula prepared and the exact volume of milk formula left over after each feeding. This information was recorded during the total intervention period in relevant record sheets that were provided to mothers. Mothers received both written and verbal instructions for the correct completion of the diaries and the record sheets.
Anthropometrical measurements: Body weight of infants was measured, as an average of two separate measurements, on a calibrated scale (Digital baby weight scale Seca 374) to the nearest 10 g, without cloths and diapers. Recumbent length of infants was measured as an average of two separate measurements, using a length board (Seca 416 infantometer for measuring babies and toddlers) to the nearest 1 cm according to standard instructions.
Gastro-esophageal reflux monitoring: Gastro-esophageal reflux was quantitatively assessed via combined measurements of the intra-esophageal pH and multiple electrical impedance[14,15], using the Digitrapper pH-Z ambulatory 24 h pH and impedance recorder (Digitrapper, Sierra Scientific Instruments, Los Angeles, CA) and the relevant software (AccuView pH-Z). According to its principle of operation, this method measures the electrical impedance changes between two neighboring electrodes during the passage of a bolus inside a luminal organ (i.e., retrograde bolus movement in the esophagus in the current study). An age-appropriate catheter was used in the current study depending on the infant’s length and was placed trans-nasally above the upper boarder of the lower esophageal sphincter. The correct positioning of the catheter during the 24 h esophageal pH monitoring was assessed via X-ray at both baseline and follow-up examination. The purpose of X-ray was to ensure that the catheter was positioned above the stomach and specifically three vertebrae above the diaphragm following the guidelines from the European Society for Pediatric Gastroenterology, Hepatology and Nutrition[1].
Esophageal pH impedance monitoring was performed continuously for 19-24 h at the two time points of measurements, i.e., before the initiation and at the end of the intervention. At the end of each recording, data were analyzed using the AccuView pH-Z™ software version 5.2 (Given Imaging Ltd, Israel) and results were expressed in 10 pH impedance monitoring indices. Among the 24 h pH monitoring indices, the recording of the “symptom-related” indices required caregiver’s interference by pressing an “event button” any time the baby was crying or was showing signs of anxiety or discomfort (according to parent’s/caregiver’s perception). This was not required for all other pH indices (i.e., “non-symptom related” ones).
The sample size estimation in the present study was based on the experience gained from a previous intervention study[16] conducted also with Bulgarian infants, examining the same outcomes (i.e., the same pH monitoring indices) as the current study. Based on the observed changes in pH monitoring indices observed in this previous intervention study, a minimum sample size of 30 subjects (or 10 subjects per treatment arm) was considered adequate to provide in the present study a statistical power of 90%.
The effect of the intervention scheme on pH monitoring, growth and tolerance indices was examined using intention-to-treat (ITT) analysis. Multiple imputations were performed to estimate missing follow-up data due to drop-outs and the pooled imputed data were used in all subsequent analyses. All data were reported as mean (SD) and as mean change (95%CI) over baseline. Normality tests were used to determine normality of distribution of the examined variables. Repeated measures analysis of variance (Repeated Measures ANOVA) was used to assess the significance of the differences between groups at baseline and follow-up examination (Treatment effect), the significance of the changes observed within each group (Time effect) and the significance of the differences among groups in the changes from baseline to follow-up examination (Treatment X Time Interaction effect). The between-group factor was the study groups (i.e., Formula A vs Formula B vs Formula C); the within-group factor was the time-point of measurement (i.e., baseline, follow-up). In all analyses, adjustments were made for the average volume of milk consumed by infants per day during the intervention period. All P-values reported were two-tailed. Statistical analysis was conducted with the use of the SPSS statistical analysis software for Windows (version 21.0). The level of statistical significance was set at P≤ 0.05. The statistical methods of this study were reviewed by Dr. Kourlaba Georgia from The Stavros Niarchos Foundation-Collaborative Center for Clinical Epidemiology and Outcomes Research.
The study was approved by the Medical Ethical Committee of the “St. Marina” University Hospital of Varna (Ethical approval No. 13/03.28.2013) and was implemented in accordance to the signed protocol and the rules for good clinical practice. The study was registered in the Netherlands Trial Register: NTR4334.
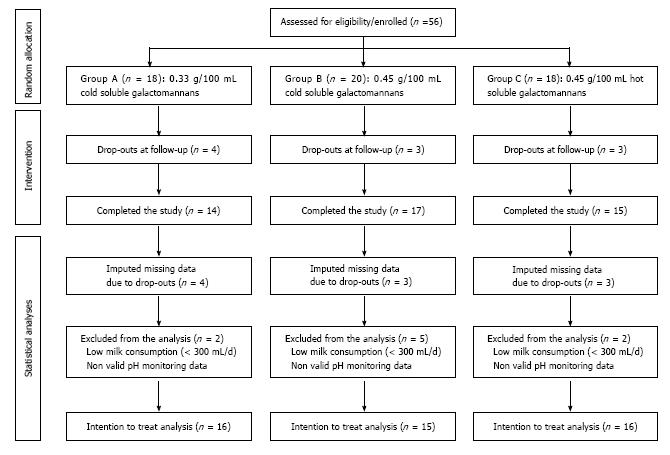
Figure 1 presents a flow diagram of infants that were included in the statistical analysis. More specifically, 56 eligible infants were identified at the initial screening phase. From these 56 infants, 10 infants either dropped out at follow-up or did not perform the 2nd 24 h esophageal pH monitoring due to parental refusal to provide consent for the follow-up pH measurement. Nevertheless, as these 10 infants were actually subjected to treatment almost throughout the intervention period, multiple imputations were conducted to estimate their missing data at follow-up examination and these infants were reinstated in the study sample for which the ITT analysis was performed. Before performing the ITT analysis, a thorough examination of the collected data revealed that for five infants at least one of the two 24 h pH impedance monitoring measurements was non-valid (i.e., mainly due to the incorrect positioning of the catheter), while in four infants the average daily milk consumption was quite low (i.e., below 300 mL per day) throughout the intervention period. These nine infants were excluded from the ITT analysis, which was finally performed for a total sample 47 infants. The results from these analyses are presented in Tables 1-3 and in Figure 2.
| Baseline | Follow up | 2-wk change | P-value(treatment x time) | ||||
| Mean | (SD) | Mean | (SD) | Mean change | (95%CI) | ||
| Reflux Index (%) | 0.484 | ||||||
| Formula A (n = 16) | 11.4 | -10.3 | 8.97 | -8.23 | -2.52 | (-9.48 to 4.45) | |
| Formula B (n = 15) | 6.47 | -5.67 | 10.1 | -13.1 | 3.44 | (-3.85 to 10.7) | |
| Formula C (n = 16) | 10.3 | -12.6 | 9.42 | -10.2 | -0.67 | (-7.77 to 6.44) | |
| P value (Treatment effect) | 0.247 | 0.901 | |||||
| Longest reflux (min) | 0.445 | ||||||
| Formula A (n = 16) | 19.3 | -15.4 | 13.9 | -8.69 | -5.16 | (-13.8 to 3.48) | |
| Formula B (n = 15) | 15.7 | -18.4 | 11.8 | -7.27 | -3.31 | (-12.3 to 5.73) | |
| Formula C (n = 16) | 14.4 | -9.47 | 17.6 | -16.6 | 2.48 | (-6.32 to 11.3) | |
| P value (Treatment effect) | 0.664 | 0.219 | |||||
| Total time below pH 4 (min) | 0.722 | ||||||
| Formula A (n = 16) | 12.2 | -12.1 | 7.85 | -7.64 | -4.5 | (-13.6 to 4.58) | |
| Formula B (n = 15) | 11.5 | -20.3 | 10.3 | -13.1 | -1.7 | (-11.2 to 7.80) | |
| Formula C (n = 16) | 9.66 | -12.9 | 9.84 | -10.3 | 0.72 | (-8.54 to 9.98) | |
| P value (Treatment effect) | 0.944 | 0.655 | |||||
| Reflux below pH 4 for more than 5 min (n/d) | 0.712 | ||||||
| Formula A (n = 16) | 6.42 | -7.19 | 4.6 | -5.21 | -1.76 | (-5.89 to 2.38) | |
| Formula B (n = 15) | 4.08 | -4.28 | 4.33 | -4.83 | 0.48 | (-3.85 to 4.80) | |
| Formula C (n = 16) | 5.53 | -8.34 | 5.96 | -5.86 | 0.15 | (-4.06 to 4.37) | |
| P value (Treatment effect) | 0.446 | 0.452 | |||||
| Boix Ochoa Score1 | 0.198 | ||||||
| Formula A (n = 16) | 107.6 | -163 | 36.6 | -29.4 | -72.03 | (-131.6 to -12.5)3 | |
| Formula B (n = 15) | 53 | -78.1 | 45 | -41.8 | -12.3 | (-74.6 to 50.0) | |
| Formula C (n = 16) | 57.2 | -80.7 | 52.1 | -49.8 | -0.08 | (-60.8 to 60.7) | |
| P value (Treatment effect) | 0.346 | 0.381 | |||||
| Total refluxes per day (n/d)2 | 0.385 | ||||||
| Formula A (n = 16) | 377.3 | -524.5 | 142.9 | -118 | -231.83 | (-437.9 to -25.8)3 | |
| Formula B (n = 15) | 169.3 | -217 | 128 | -97 | -30.9 | (-246.6 to 184.8) | |
| Formula C (n = 16) | 307.3 | -475.4 | 220.4 | -266.8 | -99.2 | (-309.5 to 111.1) | |
| P value (Treatment effect) | 0.343 | 0.234 | |||||
| Baseline | Follow up | 2-wk change | P-value(treatment x time) | ||||
| Mean | (SD) | Mean | (SD) | Mean change | (95%CI) | ||
| Symptom index for reflux (SI) | 0.0483 | ||||||
| Formula A (n = 16) | 39.7 | -26.2 | 21.6 | -14.5 | -18.23 | (-31.8 to -4.57)3 | |
| Formula B (n = 15) | 24.5 | -27.2 | 19.7 | -20.8 | -5.21 | (-19.5 to 9.03) | |
| Formula C (n = 16) | 27.7 | -20.6 | 33.7 | -24.8 | 6.49 | (-7.39 to 20.4) | |
| P value (Treatment effect) | 0.213 | 0.119 | |||||
| Symptom association probability1 | 0.096 | ||||||
| Formula A (n = 16) | 87.4 | -25.2 | 49.5 | -42.5 | -37.93 | (-64.6 to -11.3)3 | |
| Formula B (n = 15) | 54.6 | -47.3 | 58.7 | -40.2 | 3.82 | (-24.1 to 31.7) | |
| Formula C (n = 16) | 82.9 | -33.8 | 57.8 | -38.3 | -24.8 | (-52.0 to 2.37) | |
| P value (Treatment effect) | 0.031 | 0.762 | |||||
| Percentage of acid refluxes2 (%) | 0.067 | ||||||
| Formula A (n = 16) | 39.8 | -26.1 | 22.6 | -15.0 | -17.23 | (-30.8 to -3.66)3 | |
| Formula B (n = 15) | 24.6 | -27.3 | 19.4 | -20.8 | -5.53 | (-19.7 to 8.65) | |
| Formula C (n = 16) | 27.7 | -20.4 | 33.3 | -25.3 | 5.93 | (-7.89 to 19.8) | |
| P value (Treatment effect) | 0.21 | 0.152 | |||||
| Percentage of all reflux (%) | 0.0143 | ||||||
| Formula A (n = 16) | 47.9 | -22.3 | 31.0 | -18.1 | -16.93 | (-31.3 to -2.28)3 | |
| Formula B (n = 15) | 35.1 | -23.6 | 36.8 | -28.7 | 1.78 | (-13.3 to 16.9) | |
| Formula C (n = 16) | 33.7 | -22.3 | 48.2 | -28.8 | 14.4 | (-0.28 to 29.2) | |
| P value (Treatment effect) | 0.167 | 0.201 | |||||
| Baseline | Follow up | 2-wk change | P-value (treatment x time) | ||||
| Mean | (SD) | Mean | (SD) | Mean change | (95%CI) | ||
| Growth indices | |||||||
| Weight (kg) | 0.648 | ||||||
| Formula A (n = 16) | 5.8 | -1.34 | 6.37 | -1.11 | 0.573 | (0.14 to 0.99)3 | |
| Formula B (n = 15) | 5.36 | -1.44 | 5.91 | -1.37 | 0.51 | (-0.06 to 0.96) | |
| Formula C (n = 16) | 5.33 | -1.73 | 6.06 | -0.97 | 0.793 | (0.35 to 1.22)3 | |
| P value (Treatment effect) | 0.355 | 0.40 | |||||
| Length (cm) | 0.917 | ||||||
| Formula A (n = 16) | 61.2 | -5.57 | 63.2 | -5.07 | 1.933 | (0.59 to 3.27)3 | |
| Formula B (n = 15) | 59.4 | -6.23 | 61.5 | -6.02 | 2.073 | (0.67 to 3.48)3 | |
| Formula C (n = 16) | 60.2 | -3.76 | 62.5 | -3.53 | 2.333 | (0.96 to 3.70)3 | |
| P value (Treatment effect) | 0.543 | 0.631 | |||||
| Tolerance indices | |||||||
| Total number (3 d) of hard stools1 | 0.723 | ||||||
| Formula A (n = 16) | 0.5 | -2.00 | 0.48 | -1.07 | -0.01 | (-0.81 to 0.79) | |
| Formula B (n = 15) | 0.2 | -0.56 | 0.55 | -1.45 | 0.4 | (-0.44 to 1.24) | |
| Formula C (n = 16) | 0.13 | -0.50 | 0.17 | -0.47 | -0.02 | (-0.83 to 0.80) | |
| P value (Treatment effect) | 0.62 | 0.291 | |||||
| Total number (3 d) of diarrheic defecations2 | 0.0153 | ||||||
| Formula A (n = 16) | 8.38 | -11.6 | 5.31 | -5.63 | -2.88 | (-7.91 to 2.16) | |
| Formula B (n = 15) | 5.6 | -4.81 | 10.3 | -10.1 | 5.473 | (0.0 to 10.7)3 | |
| Formula C (n = 16) | 11.1 | -10.7 | 6.77 | -6.68 | -5.3 | (-10.4 to -0.16) | |
| P value (Treatment effect) | 0.163 | 0.176 | |||||
| Total number of defecations (3 d) | 0.0033 | ||||||
| Formula A (n = 16) | 10.7 | -10.4 | 7.59 | -4.23 | -2.89 | (-7.42 to 1.64) | |
| Formula B (n = 15) | 7.33 | -3.81 | 12.5 | -9.05 | 6.023 | (1.28 to 10.8)3 | |
| Formula C (n = 16) | 12.8 | -9.39 | 8.07 | -5.70 | -5.723 | (-10.3 to -1.10)3 | |
| P value (Treatment effect) | 0.153 | 0.0403 | |||||
| Number of colics per day | 0.569 | ||||||
| Formula A (n = 16) | 3.31 | -2.72 | 1.34 | -1.33 | -1.993 | (-3.02 to -0.95)3 | |
| Formula B (n = 15) | 4.42 | -2.44 | 1.59 | -1.61 | -1.873 | (-2.96 to -0.79)3 | |
| Formula C (n = 16) | 2.79 | -1.55 | 1.5 | -1.18 | -1.243 | (-2.30 to -0.19)3 | |
| P value (Treatment effect) | 0.868 | 0.735 | |||||
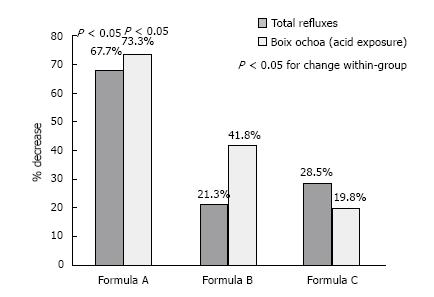
Tables 1 and 2 present the changes in the “non-symptom related” and “symptom related” 24 h pH monitoring indices, respectively. According to the data presented in Table 1, significant decreases from baseline to follow-up examination were observed for the “Boix Ochoa Score” and the “Number of refluxes per day” (by 72.0 and 231.8 respectively), only for the Formula A group (percent changes from baseline in the three study groups are also presented in Figure 2). Still, these changes did not differentiate significantly with the changes observed in the other two study groups. No other significant findings were observed for the rest of the “non-symptom related” 24 h pH monitoring indices examined in the present study. Based on the data displayed in Table 2, significant decreases were observed from baseline to follow-up examination for all “symptom related” 24 h pH monitoring indices only for infants fed with Formula A. Furthermore, in two out of the four “symptom-related” indices examined, the decreases observed for infants fed with Formula A from baseline to follow-up examination (i.e., by -18.2 in SI and by -16.9% in the percentage of all refluxes) were significantly higher compared to the relevant changes in other two groups (i.e., P < 0.05).
Table 3 summarizes the changes observed in growth and tolerance indices. As far as growth indices were concerned, body weight significantly increased for infants fed with Formula A (by 0.57 kg or 40.7 g per day) and C (by 0.79 kg or 56.4 g per day). Nevertheless these changes from baseline to follow-up examination were not found to differentiate significantly among the three study groups. Furthermore, no significant differences were observed among groups with regards to recumbent length, which increased significantly in all three study groups. Regarding tolerance indices, the number of diarrheic and total defecations, increased significantly from baseline to follow-up examination by 5.47 and 6.02, respectively, only for infants fed with Formula B. On the contrary, a significant decrease by 5.72 in the number of total defecations was observed for infants fed with Formula C. Furthermore the increases observed in the number of diarrheic and total defecations for infants fed with Formula B were significantly higher compared to the changes observed in the other two groups (P = 0.015 and 0.003, respectively). Lastly, the total number of colics decreased significantly form baseline to follow-up examination in all three study groups, but no significant differences in these changes were observed among groups.
In the current study, among the several pH monitoring indices examined, only “Boix Ochoa score” and the “Total number of refluxes per day” showed significant decreases for infants fed with Formula A, while no significant changes were observed for infants fed with Formulas B and C. Furthermore, Formula A was found to be more effective in decreasing acid reflux-related symptom indices compared to the two other formulas (Table 2). Similarly, to the present study the majority of previous clinical trials assessing the effectiveness of formulas containing different types and concentrations of various thickening agents found no significant differences on the most commonly pH indices examined, i.e., the Reflux Index, the number of reflux episodes lasting more than 5 min and the duration of the longest reflux episodes[4]. The only exception were two clinical trials, out of 14, that reported significant decreases in these three pH indices after providing formulas thickened with re-gelatinised corn-starch vs a control group receiving a standard formula, for four weeks[17,18]. However, considering the above, direct comparisons of the current study with these two studies are probably not feasible, mainly because of the shorter intervention period, the different thickening agent and the lack of a control group in the current study.
Similar favourable changes in the aforementioned three pH monitoring were also reported by Marinova and Stoimenova[16] on infants fed for two weeks with a formula containing 0.5 g/100 mL of hot-soluble CBG galactomannans. In this study infants were provided with the thickened formula after having been fed with a standard formula for two weeks. These favourable changes observed over the total intervention period of four weeks could be also partially attributed to the gastro-esophageal maturation. Although the same thickening agent was used as in the current study, these findings are not directly comparable to the current ones due to the slightly higher concentration of CBG galactomannans in the tested formula and the different equipment and analysis software used for the 24 h pH monitoring.
Reduced intake of calories and nutrients due to GER and consequently poor growth is of concern. In line with other studies[4], the present study showed increases of body weight during the 2-wk intervention period. These increases were significant for infants fed with Formulas A and C and were 40.7 and 56.4 g per day, respectively (Table 3). The findings of the present study regarding the concentration of CBG in Formula A (i.e., 0.33 g/100 mL) and the weight gain observed seem to be comparable with previous studies providing CBG in similar concentrations. More specifically, in the study of Miyazawa et al[19], when 0.35 g/100 mL CBG-galactomannans were provided a weight gain of 29.3 g per day was observed after one week of intervention. In the study of Vandenplas et al[20] when 0.33 and 0.36 g/100 mL CBG-galactomannans (i.e., calculated with 13 g of infant milk powder per 100 mL and 85% galactomannans in CBG) were provided, the weight gains observed were 37 and 24 g per day, respectively, after two weeks of intervention and 27.5 and 25 g per day, respectively, after four weeks of intervention. Taken together, it seems that the increase in body weight as seen in group A is comparable to the increases reported in other studies also using CBG thickened formulas in similar concentrations as in Formula A. However, regarding the increase in body weight observed in Formula C, this was higher compared to those reported in other studies providing similar or higher concentrations. For instance in the study of Vivatvakin and Buachum[21], when a comparable product with an even higher CBG concentration (i.e., 0.5 g/100 mL) than Formula C was provided, a weight gain of 24.5 g per day was observed after two weeks of intervention. The higher mean volume of milk formula consumed by infants fed with Formula C (i.e., 841.3 mL) compared to infants fed with Formulas A (i.e., 756.7 mL) and B (i.e., 711.9 mL) in the present study as well as to infants in the study of Vivatvakin and Buachum (i.e., 589.5 mL)[21] might provide an explanation for these differences. However, as the exact volume of breast milk consumed by infants in the present study could not be recorded or estimated, the reasoning provided above might not fully explain the observed weight gain in group C.
In studies examining the effect of other thickening agents instead of CBG on weight gain some mixed results were observed. Xinias et al[17] reported no significant differences in weight gain between the experimental and control groups after four weeks of intervention with cornstarch-thickened formulas. Furthermore, Chao and Vandenplas[22] reported no significant differences in body weight gain between the control and intervention groups during the first two weeks, but significantly higher increases at four and eight weeks of intervention compared to the control group. Similarly, in another study by Chao and Vandenplas[23], when rice-thickened formula was provided, significantly higher weight gains were observed at four and eight weeks of intervention for the intervention compared to the control group.
Regarding changes observed in tolerance indices, the present study showed a significant increase in the number of diarrheic and total defecations from baseline to follow-up for the infant fed with Formula B. In contrast, no such unfavorable adverse effects were observed for infants fed with Formulas A and C, potentially indicating that this might be an adverse effect only of Formula B providing 0.45 g/100 mL of cold soluble CBG-galactomannans. This observation could further provide an explanation for the non-significant increase of body weight recorded for infants fed with Formula B, while body weight significantly increased among infants in the other two study groups. Of course the subjective assessment and recording of these indices by parents/caregivers might have also produced bias that needs to be considered when interpreting these findings.
The results of the present study should be interpreted under the light of its strengths and limitations. Regarding strengths, the inclusion of a “run-in” period in the study protocol and the measurement of reflux by pH-monitoring increase methodological integrity and decrease possible bias in data collection and results. However, the use of fairly new pH-monitoring equipment can be considered as a limitation of the current study, since direct comparisons with previous studies and results/outcomes may not be feasible or appropriate. Furthermore, the absence of a control group can be considered as another limitation of the current study, since this might have limited the ability to have a more clear view on the effectiveness and tolerance of the three anti-reflux formulas under study. Lastly, although the number of infants examined in the present study was relatively small, the imputation of missing data as part of the ITT analysis resulted to a sufficient sample size and as such to adequate statistical power for the analyses. Nevertheless, future intervention studies with larger samples sizes should be implemented in order to shed more light on this field.
In conclusion, the present study showed that Formula A was more effective in decreasing esophageal acid exposure (as indicated by the Boix Ochoa Score), the total daily number of visible and measurable refluxes, as well as acid reflux related symptoms, while such changes were not observed for the infants fed with Formulas B and C. Furthermore, a significant increase of body weight was observed for infants fed with Formulas A and C while that was not observed for infants fed with Formula B, probably due to the increased number of diarrheic and total defecations recorded in this group. These findings indicate that Formula A seems to be more effective in reducing certain pH-monitoring indices of uncomplicated GER, increasing body weight and being well-tolerated by infants.
The authors are indebted to the research team members as well as to the parents/caregivers and infants for participating in the study.
Gastro-esophageal reflux (GER) is a common and global problem affecting about 50% of all babies up to the age of two months and has a peak incidence at the age of three months. Only some infants will develop pathologic gastro-esophageal reflux disease, in which clinical problems are related to excessive passage of acid gastric contents. Uncomplicated GER should be suspected in infants with uncomplicated recurrent regurgitation. In some cases GER may affect thrive because of caloric insufficiency and potentially lower dietary nutrients’ intake which may lead to poor weight gain. The use of anti-reflux formulas with added thickening agents, such as carob-bean gum (CBG), can decrease the frequency and intensity of GER.
Commercially available anti-reflux formulas currently contain 0.45 g/100 mL hot-soluble CBG galactomannans. With the exception of one study, there are no other randomized clinical trials available in the literature examining the effectiveness of anti-reflux formulas containing less than 0.45 g/100 mL hot-soluble CBG galactomannans on reflux and tolerance indices. In addition there are no reports examining the effectiveness of cold vs hot-soluble CBG galactomannans on reflux and tolerance indices.
The current study is the first to examine the effectiveness of formulas containing cold or hot soluble CBG galactomannans in different concentrations (i.e., 0.45 g or 0.33 g/100 mL) on reflux indices assessed by 24 h pH impedance monitoring as well as on tolerance indices (i.e., defecations and colic).
The formula containing 0.33 g/100 mL of cold-soluble CBG galactomannans was effective in reducing certain pH-monitoring indices of uncomplicated GER, increased body weight and was well-tolerated by infants.
GER is defined as the involuntary passage of gastric contents into the esophagus and does not refer to any specific etiology with or without regurgitation and vomiting. The term regurgitation is specifically used if the reflux dribbles effortlessly out of the mouth.
In their work, the authors present a very clear and well conducted, controlled randomized study analyzing the effects of three different anti-reflux formulas for infants with GER (excluding complicated cases). The study include not too many, but a sufficient number of patients, it was performed for a relatively short period of time, but probably just sufficient. It is well described, and the results are conclusive and helpful.
P- Reviewer: Alessandro I, Classen CF, Mohammed IB S- Editor: Wang JL L- Editor: A E- Editor: Lu YJ
| 1. | Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, Sondheimer J, Staiano A, Thomson M, Veereman-Wauters G. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49:498-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 473] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 2. | Vandenplas Y, Salvatore S, Hauser B. The diagnosis and management of gastro-oesophageal reflux in infants. Early Hum Dev. 2005;81:1011-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Rasquin-Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, Staiano A. Childhood functional gastrointestinal disorders. Gut. 1999;45 Suppl 2:II60-II68. [PubMed] [Cited in This Article: ] |
| 4. | Horvath A, Dziechciarz P, Szajewska H. The effect of thickened-feed interventions on gastroesophageal reflux in infants: systematic review and meta-analysis of randomized, controlled trials. Pediatrics. 2008;122:e1268-e1277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Vandenplas Y, Lifshitz JZ, Orenstein S, Lifschitz CH, Shepherd RW, Casaubón PR, Muinos WI, Fagundes-Neto U, Garcia Aranda JA, Gentles M. Nutritional management of regurgitation in infants. J Am Coll Nutr. 1998;17:308-316. [PubMed] [Cited in This Article: ] |
| 6. | Meunier L, Garthoff JA, Schaafsma A, Krul L, Schrijver J, van Goudoever JB, Speijers G, Vandenplas Y. Locust bean gum safety in neonates and young infants: an integrated review of the toxicological database and clinical evidence. Regul Toxicol Pharmacol. 2014;70:155-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Miyazawa R, Tomomasa T, Kaneko H, Morikawa A. Effect of formula thickened with locust bean gum on gastric emptying in infants. J Paediatr Child Health. 2006;42:808-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Miyazawa R, Tomomasa T, Kaneko H, Arakawa H, Morikawa A. Effect of formula thickened with reduced concentration of locust bean gum on gastroesophageal reflux. Acta Paediatr. 2007;96:910-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Orenstein SR, Cohn JF, Shalaby TM, Kartan R. Reliability and validity of an infant gastroesophageal reflux questionnaire. Clin Pediatr (Phila). 1993;32:472-484. [PubMed] [Cited in This Article: ] |
| 10. | Orenstein SR, Shalaby TM, Cohn JF. Reflux symptoms in 100 normal infants: diagnostic validity of the infant gastroesophageal reflux questionnaire. Clin Pediatr (Phila). 1996;35:607-614. [PubMed] [Cited in This Article: ] |
| 11. | Kitz R, Ahrens P, Eickmeier O, Boehles H, Rose MA. The child with chronic cough: when does double-channel pH monitoring rule out gastroesophageal reflux. Open J Pediatrics. 2011;1:21-26. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Wessel MA, Cobb JC, Jackson EB, Harris GS, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics. 1954;14:421-435. [PubMed] [Cited in This Article: ] |
| 13. | Ghanma A, Puttemans K, Deneyer M, Benninga MA, Vandenplas Y. Amsterdam infant stool scale is more useful for assessing children who have not been toilet trained than Bristol stool scale. Acta Paediatr. 2014;103:e91-e92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Wenzl TG. Investigating esophageal reflux with the intraluminal impedance technique. J Pediatr Gastroenterol Nutr. 2002;34:261-268. [PubMed] [Cited in This Article: ] |
| 15. | Wenzl TG, Moroder C, Trachterna M, Thomson M, Silny J, Heimann G, Skopnik H. Esophageal pH monitoring and impedance measurement: a comparison of two diagnostic tests for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2002;34:519-523. [PubMed] [Cited in This Article: ] |
| 16. | Marinova M, Stoimenova M. Diet therapy with Frisovom in gastroesphageal reflux in infancy. Pediatria. 1999;39:45-46. [Cited in This Article: ] |
| 17. | Xinias I, Mouane N, Le Luyer B, Spiroglou K, Demertzidou V, Hauser B, Vandenplas Y. Cornstarch thickened formula reduces oesophageal acid exposure time in infants. Dig Liver Dis. 2005;37:23-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Moukarzel AA, Abdelnour H, Akatcherian C. Effects of a prethickened formula on esophageal pH and gastric emptying of infants with GER. J Clin Gastroenterol. 2007;41:823-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Miyazawa R, Tomomasa T, Kaneko H, Morikawa A. Effect of locust bean gum in anti-regurgitant milk on the regurgitation in uncomplicated gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2004;38:479-483. [PubMed] [Cited in This Article: ] |
| 20. | Vandenplas Y, Leluyer B, Cazaubiel M, Housez B, Bocquet A. Double-blind comparative trial with 2 antiregurgitation formulae. J Pediatr Gastroenterol Nutr. 2013;57:389-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Vivatvakin B, Buachum V. Effect of carob bean on gastric emptying time in Thai infants. Asia Pac J Clin Nutr. 2003;12:193-197. [PubMed] [Cited in This Article: ] |
| 22. | Chao HC, Vandenplas Y. Comparison of the effect of a cornstarch thickened formula and strengthened regular formula on regurgitation, gastric emptying and weight gain in infantile regurgitation. Dis Esophagus. 2007;20:155-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Chao HC, Vandenplas Y. Effect of cereal-thickened formula and upright positioning on regurgitation, gastric emptying, and weight gain in infants with regurgitation. Nutrition. 2007;23:23-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |