THE CONCEPT OF FEVER: FROM FEBRILE ILLNESS TO HYPERSENSITIVITY
Fever has been considered for a long time as a primary illness in itself rather than a physiologic process occurring with disorders of various origin, marked by a raised body temperature mostly of infectious nature. In spite of this, since antiquity, some physicians believed that the high temperatures during a febrile illness could have some beneficial effects, being followed usually by a spell of sweating and subsequent resolution[1]. The idea of fever as “a mighty engine which Nature brings into the world for the conquest of her enemies” was promoted by Thomas Sydenham, even though he was unsuspecting that fever occurs as the result of immune activation caused by such “enemies” usually represented by bacteria or viruses[2].
In recent centuries, following the urbanization and the overcrowding of cities, fevers caused by epidemics such as diphtheria, flue, smallpox, measles and pertussis became a serious concern in most areas of the World, being accountable for a high death rate in early childhood. These epidemiological changes probably conditioned the notion of fever, which started to be considered a harmful reaction to infections by the body. Thus, antipyretics were initially introduced rather as an attempt to cure the fever instead of relieving sufferers from their discomfort. In actual fact, enhanced microbiological knowledge didn’t immediately result in downgrading the importance of febrile temperature vis-à-vis to the infection itself, until the beginning of the twentieth century when a new concept emerges which considers fever as a manifestation of immune activation to exogenous agents[3]. At the same time, on the other hand, it was becoming apparent that hyperthermia or fever could be beneficial in supporting the healing process following various diseases such as gonorrhea and syphilis[4].
Although the mortality rate caused by infectious febrile illnesses dramatically decreased in western countries around the mid XX century, the fear of fever, or “fever phobia” quoting a term coined by Schmitt[5] in 1980, persisted within folklore beliefs to some extent until today. Moreover, it has been argued that the unrealistic concerns about fever sprung from the patient’s and the caregiver’s misconceptions concerning what constitutes a high temperature[1,6,7]. It may be worth recalling that as far back as 1871 Wunderlich, after collecting data on one million measurements of body temperature, established 37.0 °C (range 36.2-37.5) as the normal human body temperature, suggesting that temperatures above 38.0 °C had to be considered “suspicious” of febrile nature[8].
RECURRENT FEVERS: FROM MALARIA TO AUTOINFLAMMATORY PERIODIC FEVERS
Flares of fever recurring with a repetitive clinical picture characterize recurrent fevers, suggesting that they are brought about by a single and persistent cause[9,10]. In ancient civilizations, fever caused by malaria and typhoid was probably among the febrile illnesses of major concern. Indeed, medical historicists are still debating whether Alexander the Great died of malaria or typhoid fever[11]. Especially malaria has been for many centuries the most common and alarming recurrent fever among people living around the Mediterranean Sea. Ancient Romans celebrated the Etruscan divinity Februs with a purification ritual on the month of February. The celebration was later dedicated to the divinity febris (fever), protector from recurrent seasonal fevers due to malaria. This is where the Valentine Day celebration probably later originated from, maybe in reference to “love fever”. Malaria was so rooted in the Italian wetlands that people venerated the Virgin Mary of fevers to seek for protection (Figure 1)[12].
Figure 1 The Virgin Mary of Fevers, by Nicola Mellini, XIX century, Bologna, Italy.
In 1712, a few decades after quinine had become available to cure the disease, the Italian physician Torti[13] proposed a classification of febrile illnesses based on the response to quinine, which was represented in his famous figure of the fever tree (Figure 2). The fever tree was not only about malaria, but it covered all types of fever known at that time. Regardless of the real utility of a classification based on apparent response to quinine, this figure reflected the concept of fever as a leading feature of diseases.
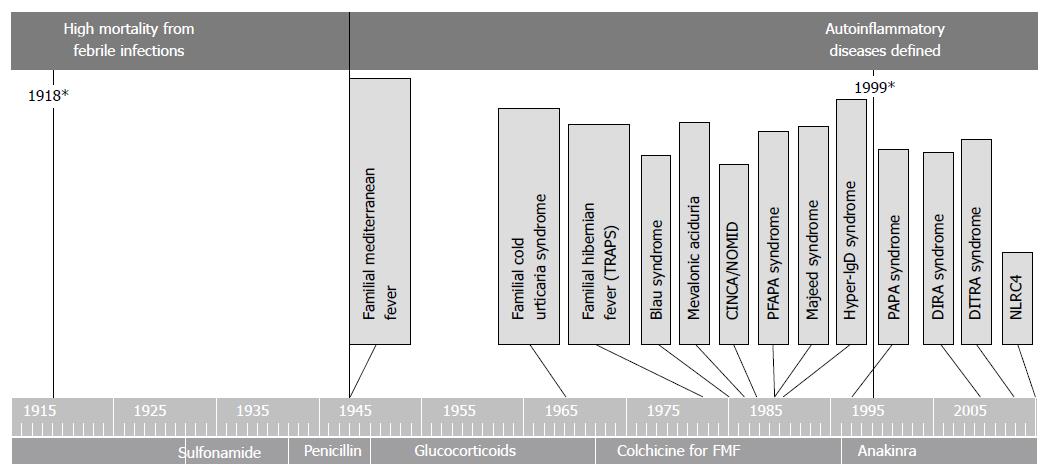
During the first decades of the XX century, eradication of malaria from most Western countries, together with improved socioeconomic conditions and the availability of vaccines and drugs for many infectious diseases, contributed to a dramatic reduction in the mortality rates caused by febrile illnesses. At the same time, the development of laboratory diagnostics and the observation of ex juvantibus’ response to antibiotics allowed the identification, in many cases, of the fever’s infectious cause. It is not surprising that only in this scenario was the recurrence of fevers beginning to be noticed in some individuals without a putative infectious cause. Indeed, only after the Second World War did physicians start describing subjects with seemingly unprovoked episodes of fever, simultaneously recurring with a set of various other complaints, usually on the skin, in the joints, muscles, abdomen, chest and lymph nodes (Figure 3).
Figure 3 Timeline discovery periodic fevers autoinflammatory disease.
PFAPA: Periodic fever adenitis pharyngitis aphthae; DITRA: Deficiency or interleukin-36-receptor antagonist deficiency; DIRA: Deficiency of interleukin-1–receptor antagonist; PAPA: Pyogenic arthritis, pyoderma gangrenosum, and acne.
It was quite clear that such recurrent fevers were the result of a pathologic immune mechanism, but the term “autoinflammatory” was only proposed at the end of the century to describe the particular pathogenesis of these disorders[14]. The pivotal role of Interleukin 1 (IL-1) in the pathogenesis of autoinflammatory disorders (ADs) was highlighted both in experimental studies as well as in therapeutic trials with IL-1 blocking agents[15]. This change in knowledge was of particular importance, as a proper identification of this category of disorders could allow better diagnostic and therapeutic choices, avoiding useless examinations and treatments such as antibiotics[16]. Moreover, the conception of fever was changing once again, from beneficial in most infections, to harmful when becoming the expression of immune dysregulation. Actually, it is not fever in itself that is harmful, but IL-1 related inflammation.
The first AD described was familial Mediterranean fever (FMF) called by the name of “benign paroxysmal peritonitis”. Clinically, FMF is characterized by recurrent and self-limited attacks of fever lasting 12-72 h with peritonitis, pleurisy, arthritis or erysipelas-like skin disease and a marked acute-phase response. Subsequent complications of untreated patients are due to renal amyloidosis. The temperature may reach 38-40 °C and can anticipate other symptoms, although some patients may only have a fever. Diagnosis of FMF is primarily clinical and based on the use of the diagnostic criteria[17-19].
After FMF, a number of other monogenic AD’s are described, presenting recurrent fever together with several other combinations of clinical features (Figure 3).
Actually, there is a great heterogeneity among AD’s concerning prevalence, severity and clinical features. On one end of the spectrum one finds the rare and often severe disorders due to mutations in a number of inflammasome-related genes, as is the case for Cryopyrinopathies, DIRA, DITRA and others, and on the opposite end there is the periodic fever adenitis pharyngitis aphthae syndrome (PFAPA). This is a relatively common disorder with likely multifactorial pathogenesis, usually characterized by mild symptoms and a benign course[20]. It is worth noting that, although it is the most common among autoinflammatory periodic fevers, PFAPA has been described relatively late, in 1989. This delay may be related to concerns raised during the previous two centuries about rheumatic fever, in addition to the higher incidence of other serious febrile illnesses of infectious origin. Indeed, in the first decades of the XX century, Rheumatic Fever, usually occurring as a sequel of Group A Streptococcus (GAS) tonsillitis, was still a prominent health problem in developed countries, being associated with the high risk of carditis. In some subjects GAS tonsillitis could recur, increasing the risk of developing chronic hearth injury. In fact, almost one third of children underwent early tonsillectomy in the years before the Second World War and we cannot know how many of them could have had PFAPA syndrome rather than GAS tonsillitis. More often than not tonsillectomy was performed as prevention in subjects who had never developed tonsillitis. Actually, during the past century, Western countries witnessed a progressive decline in the incidence of rheumatic fever, mostly irrespective of the availability of antibiotics and to the practice of tonsillectomy[21]. Nevertheless, fear of Rheumatic Fever persisted for decades and might have been in part responsible for the delayed identification of PFAPA[22].
Half way between PFAPA and rare monogenic AD, there is a group of well characterized hereditary periodic fevers that can show up with a higher frequency in certain populations, as FMF in Mediterranean people, mevalonate kinase deficiency (MKD) in Dutch and tumor necrosis factor associated periodic fever (TRAPS) in people with Northern European ancestry[23]. Patients with MKD present fever episodes lasting 3 to 7 d that typically recur every 4 to 6 wk. The fever may be accompanied by lymphadenopathy, abdominal pain with diarrhea or vomiting, headache, polyarthralgia or arthritis of large joints, skin lesions such as papular, urticarial, nodular or purpuric rash. Patients with TRAPS show attacks of extremely variable duration and intensity (from 1-2 d to 3-4 wk), characterized by fever accompanied more or less constantly by sterile peritonitis with abdominal pain, diarrhea or constipation, nausea, and vomiting. Mono- or bilateral periorbital edema is a very characteristic and almost pathognomonic sign of the disease, often associated with conjunctivitis and periorbital pain. Also very frequent are arthralgias, muscle cramps, centrifugally spreading migratory myalgias, chronic fasciitis, serpiginous rash, consisting of migratory and painful patches[24,25].
Besides a few exceptions, a common feature of periodic fever syndromes is the reversibility of symptoms with a complete sense of wellbeing outside the flares, lacking the development of sustained autoimmunity and progressive inflammatory damage. Exceptions are severe ADs which tend to present a continuous course rather than a periodic one as well as specific syndromes such as FMF and TRAPS which, if not treated, present a varying high risk of developing amyloidosis[26].
NOVEL CONCEPTS AND THE FEVER TREE REVISITED
In the last decade, some evidences have been accumulated demonstrating a poor correlation between phenotype and genotype in AD. Environmental factors can modify the clinical presentation, as in the case of FMF. This was elegantly demonstrated by the evidence represented by the people with FMF who immigrated to Germany and in spite of similar genetic backgrounds, tended to present milder symptoms compared with their cousins who remained in Turkey[27]. In addition, although it is well known that FMF is transmitted with autosomal recessive inheritance, in certain families some mutations can be associated with a dominant inheritance, this has lead to the belief that there are other possible contributions in the pathology stemming from other genetic and environmental factors.
Indeed, patients with heterozygous mutation or with multiple low penetrance variants in the MEFV gene may display attenuated phenotypes resembling more PFAPA than FMF, making it difficult to definitely confirm the diagnosis of FMF[28].
A similar behavior is also described when talking about other periodic fever syndromes, such as TRAPS. Some genetic variants for example, relatively common in the general population, may have low penetrance occurring mostly in healthy people, that can also be associated, in some individuals, with a typical manifestation of the disease[29]. In fact, the correlation between genotype and phenotype in patients with AD is often incomplete and many patients could receive the wrong diagnoses such as inflammatory bowel disease, Behçet disease or adult onset Still disease, being excluded from genetic testing for AD[16,30].
In addition, a number of recent reports showed that it could be relatively common, even following extensive molecular analysis, to find subjects with periodic fever lacking a definite genetic diagnosis[31-34]. In some cases genetic variants of uncertain significance in AD-related genes may be found, yet their relevance for diagnosis is currently unpredictable[35,36]. Recently, evidence-based provisional clinical classification criteria for autoinflammatory periodic fevers have been proposed, which may help diagnosing patients with uncertain genetic results[37]. In addition, these criteria can be used to classify patients with undifferentiated diseases based on the similarity of their clinical pictures with the known hereditary periodic fever syndromes.
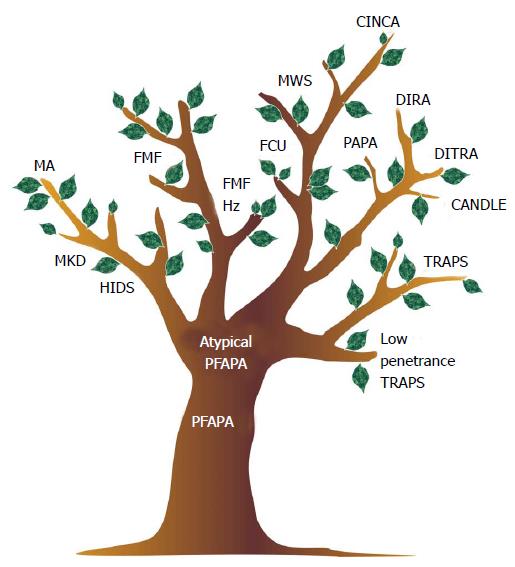
To represent this complexity, we proposed revisiting the ancient figure of the fever tree (Figure 4).
Figure 4 The fever tree revisited.
PFAPA: Periodic fever adenitis pharyngitis aphthae; MKD: Mevalonate kinase deficiency; FMF: Familial mediterranean fever; HIDS: Hyper-IgD syndrome; CINCA: Chronic infantile neurologic cutaneous and articular syndrome; MWS: Muckle wells syndrome; DIRA: Deficiency of the interleukin-1 receptor antagonist; FCU: Familial cold urticaria; DITRA: Deficiency or interleukin-36-receptor antagonist deficiency; MA: Mevalonic aciduria.
The figure of the tree can serve to represent AD as a disease continuum, from the common multifactorial PFAPA syndrome, which is figured by the trunk, to the rare monogenic diseases, represented by the tip of the branches. Going from the trunk to the major limbs and then up to the top of the smaller branches, we can find a number of intermediate phenotypes, which might be underpinned by a variety of genetic variants in the same genes, which have evolved towards more severe forms, or other genes altogether which are yet to be identified. For example, low penetrance mutations in the genes responsible for FMF, TRAPS and MKD have been associated with PFAPA-like phenotype[28,29]. Indeed, MKD encompasses a wide range of phenotypes, from the most severe end, represented by mevalonic aciduria which we can figure on the tip of the branch, to MKD and Hyper-IgD syndrome, which in milder cases can closely resemble PFAPA[38].
PFAPA itself may not be actually considered as a single disease, but rather as a heterogeneous syndrome. Atypical cases have been described, and can currently be encountered. Typically, patients with PFAPA present episodes of tonsillopharyngitis accompanied by enlarged neck lymph nodes and mouth aphthae: the periodic recurrence of fever episodes, the response to a single administration of low dose glucocorticoids, and the conclusive healing after tonsillectomy are all characteristic features of the disease. However, it is not uncommon to encounter patients with periodic fever and tonsillitis who present atypical signs, such as a poor response to glucocorticoids, a recurrence following tonsillectomy or the presence of significant cutaneous, articular or abdominal symptoms, in addition to pharyngeal involvement. In a small proportion of these atypical PFAPA cases, a definite diagnosis of hereditary periodic fever can be done (e.g., MKD or TRAPS), while in most cases the analysis of a more or less complete set of genes associated with HPF can only reveal the presence of common genetic variants of vague significance or no variants at all. Few cases in this group may still be due to yet unidentified monogenic disorders or to somatic mosaicism for mutations in autoinflammatory genes, similarly to what has been described for subjects with cryopirin associated periodic syndromes or Blau sindrome[39,40]. In most cases, however, it is reasonable to assume that this group is composed by patients with true multifactorial disorders with intermediate characteristics between PFAPA and various types of autoinflammatory diseases. In this regard, it is worth noting that some patients with atypical PFAPA may respond to colchicine, in analogy with FMF. Operatively, we could represent the disease of such patients on the fever tree positioned between the PFAPA trunk and the bottom of the FMF branch.
This picture doesn’t claim to be complete but can help to be confronted with an expanding spectrum of AD’s, including patients with atypical presentations lacking a definite genetic diagnosis. Further studies are needed to understand the role of genetic and environmental factors in these cases. Moreover, it remains to be evaluated whether a practical approach based on the clinical analogies with well defined AD’s can represent a reasonable choice in these cases.
In fact, so far, we can identify only a few groups of diseases based on the response to treatments and in particular if there is a preferential response to low dose glucocorticoids, colchicines or cytokine inhibitors (IL-1 or TNF-α).
It may be worth recording that, in regards to autoinflammatory diseases, fever in itself, is never the harm. Treatments should be aimed at relieving patients from pain and discomfort and, in some diseases in preventing long-term damage from chronic inflammation.
This review may tell just a part of the story and represent a simplification of the field. However, as physicians, we still need to be able to cure, understand, and act expediently in daily practice when dealing with the disease, without necessarily knowing the genetic makeup of each individual patient with his or her individual variability, manifestation and diseases development, keeping in mind that the personalization of medicine is not just genetically driven.
P- Reviewer: Lichtor T, Schattner M, Yves R S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK