Published online Jun 9, 2023. doi: 10.5409/wjcp.v12.i3.57
Peer-review started: September 27, 2022
First decision: October 17, 2022
Revised: November 7, 2022
Accepted: March 17, 2023
Article in press: March 17, 2023
Published online: June 9, 2023
Coronavirus disease 2019 (COVID-19) typically presents with fever and respiratory symptoms in children. Most children develop an asymptomatic and mild illness, with a minority requiring specialist medical care. Gastrointestinal manifestations and liver injury can also occur in children following infection. The mechanisms of liver injury may include infection following direct viral hepatic tissue invasion, immune response, or medication effects. Affected children might develop mild liver dysfunction which has a benign course in most children with no pre-existing liver disease. However, the presence of non-alcoholic fatty liver disease or other pre-existing chronic liver disorders is associated with a higher risk of developing severe COVID-19 illness with poor outcomes. On the other hand, the presence of liver manifestations is associated with the severity of COVID-19 disease and is considered an independent prognostic factor. Respi
Core Tip: Liver injury presenting with elevated levels of alanine aminotransferase and aspartate aminotransferase is common in children infected with the coronavirus disease 2019 (COVID-19) virus. The mechanism of liver injury is not fully understood and is likely secondary to the viral invasion of the liver, hepato-toxic medications, and the patient’s own immune-mediated response. Liver injury in children is generally mild and resolves spontaneously but is usually seen in children with more severe illnesses. In addition, children with underlying non-alcoholic fatty liver disease or another chronic liver disease may have a higher risk of severe COVID-19 illness. Management of liver injury after COVID infection is supportive. Proactive vaccination may reduce the transmission of infection and the severity of the disease.
- Citation: Bitar R, Elghoudi AA, Rawat D, Azaz A, Miqdady M, Narchi H. COVID-19-induced liver injury in infants, children, and adolescents. World J Clin Pediatr 2023; 12(3): 57-67
- URL: https://www.wjgnet.com/2219-2808/full/v12/i3/57.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v12.i3.57
Coronavirus disease 2019 (COVID-19) typically presents with fever, weakness, myalgia, malaise, and pulmonary symptoms[1]. In addition, a significant percentage of those infected with the virus develop gut manifestations such as loss of appetite, colics, and lose stools[2]. Similarly, hepatic involvement has been reported in many studies[3,4]. Previous waves of the Middle East respiratory syndrome (MERS) have shown an association with elevated serum transaminases and bilirubin and concentrations with decreased serum albumin levels[5,6]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shares up to 80% genomic sequence similarity with MERS and, consequently, hepatic injury is not an unexpected manifestation of COVID-19[7].
This review aims to describe liver injury in children with COVID-19, detailing its epidemiology, underlying mechanisms, clinical manifestations, management, and prognosis. We will also describe liver involvement in high-risk groups, including children with pre-existing liver disease.
Acute liver injury in adults and children with SARS-CoV-2 infection is defined as an elevation in the serum concentration of aminotransferases (transaminases). The spectrum of liver injury ranges from asymptomatic elevated serum transaminase levels to severe liver injury, with reports of acute-on-chronic liver failure in patients with underlying liver disease. Usually, 14%-53% of adult patients develop mild to moderate elevation of liver enzymes[8,9].
Abnormal transaminase levels have also been linked to the severity of COVID-19[9-13]. Higher morbidity and mortality have been reported in those COVID-positive and altered liver function, with liver injury being an independent prognostic factor of COVID-19[14,15]. A review by Bende et al[15] of post-acute COVID-19 syndrome of 97 subjects demonstrated increased liver stiffness, viscosity, and steatosis in around one-third of the patients, worse in subjects with pulmonary injury compared to those without. COVID-19 in children and adolescents may be asymptomatic or cause only mild symptoms. These include fever, cough, upper respiratory tract symptoms, diarrhoea, nausea, and vomiting. In a multinational, multicentre cohort study, 22% of patients had gastrointestinal symptoms, of whom 7% had no respiratory symptoms[16]. In early pediatric reports, the rate of transaminase elevation was 14%-50%[12,17,18]. However, these data may not be representative because transaminase levels and liver function tests were only reported in a small proportion of patients. In a study involving 280 children ≤ 17 years of age with COVID-19, the elevation of serum transaminases was mild, with a prevalence of 29%, and predominantly children < 3 years of age[19]. Those with chronic liver diseases have developed more aggressive diseases with an increased risk of hepatic failure[20].
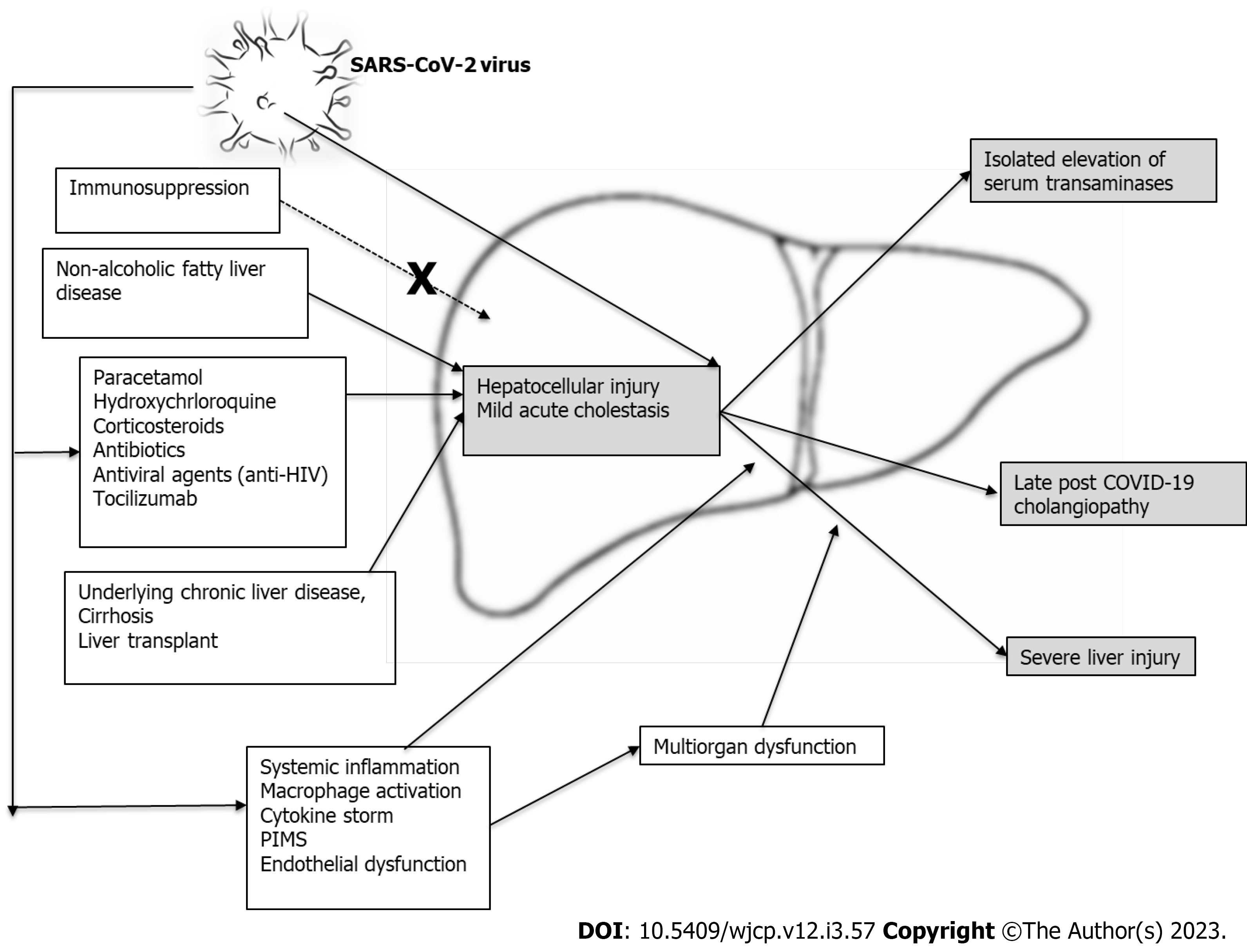
The causes of elevated liver transaminases in COVID-19 still need to be fully understood[21]. Some possible risk factors, illustrated in Figure 1, include:
There is evidence that the virus infects the organ cells by exploiting the angiotensin-converting enzyme (ACE) 2 receptor, followed by intracellular replication. ACE2 receptors are found in the respiratory tissues, gut, liver, kidney, and heart. However, compared to the viral load in the lungs of acutely infected children, the viral load in the hepatic cells was found to be low. Evidence of hepatic cell injury with a high level of serum transaminases has been found in > 70% of patients with severe COVID-19 disease[22,23]. Moreover, the virus has also been isolated from patients’ stools, indicating that the infection spreads to the gastrointestinal tract and not exclusively the pulmonary cells[22,23]. This direct viral invasion of liver tissue and replication inside the hepatocytes can explain the resulting hepatic injury.
Another mechanism for liver injury associated with SARS-CoV-2 is the polypharmacy commonly used during COVID-19 illness. Antiviral drugs, antibiotics, and steroids used to treat moderate and severe COVID-19 may also cause liver toxicity in their own right[24,25]. Antipyretics, such as paracetamol, do not play a significant role in COVID-19 liver injury. Other drugs include chloroquine, isoniazid, antivirals, particularly anti-human immunodeficiency virus medications (lopinavir/ritonavir), and biological agents such as tocilizumab, which also be considered potential co-factors in the pathophysiology of liver injury in children with COVID-19[9,22,23]. In a study of 147 patients with COVID-19-induced liver injury treated as outpatients, the prevalence of hepatic injury was the same as in those with normal or impaired liver function. However, it was observed to be higher in those patients who received hospital care and with a higher rate of utilization of ritonavir[26].
The resulting immune response is immature in infants and children with COVID-19 infection, and while it is mild in most patients, it can be severe. Hepatic injury caused by the SARS-CoV-2 virus in children is associated with systemic and local inflammatory responses of varying severity. During the acute stage of the infection, the immune system attempts to limit the reproduction of the virus through both immediate and delayed immune responses by producing specific antibodies against it. When exaggerated, this immune-mediated response can damage the hepatic tissue in children with more aggressive and prolonged disease[1]. The inflammatory markers such as cytokines, T helper 17 cells, cytotoxic DC8 T cells, interleukins 1, 2, and 6, serum ferritin, tumor necrosis factor, interferon, and other mediators[9], as well as C-reactive protein, are commonly elevated. In some patients, this may result in a “cytokine storm”, leading to rapid clinical deterioration with multiple organ failure and endothelial dysfunction, further aggravating liver damage[2,26-32]. Endothelial dysfunction is associated with the stimulation of neutrophil extracellular traps through immunological mechanisms[19], causing the development of microthrombi in the lungs[33]. The liver is similarly affected, and hepatic damage may be accelerated by a hypercoagulative disease state, which can involve other organs and tissues. Autopsy reports have confirmed the presence of hepatic sinusoidal congestion and micro thrombosis[20,27].
Acute liver injury is also a prominent feature of multi-system inflammatory diseases in children, occurring as a late complication of SARS-CoV-2 infection[34]. The exact mechanism still needs to be fully understood. Some researchers suggest an abnormal immune response to the SARS-CoV-2, similar to Kawasaki disease[35], or immunoglobulin G antibodies enhancement of monocytes and cytotoxic CD8+ T cells[8], with downregulation of neutrophil and lymphocyte functions[6,7]. The pediatric multi-system inflammatory syndrome temporally associated with COVID-19, also known as a multi-system inflammatory syndrome in children (MIS-C), is a persistent acute febrile illness progressing to multi-organ dysfunction, conferring over a 2-fold increased risk of elevated serum transaminases. Although affected children often have an underlying medical condition, such as obesity, immunocompromised state (including malignancy), or chronic liver disease (CLD)[36], it may also occur in previously healthy children and adolescents[37]. A few weeks after contracting SARS-CoV-2, affected children present with either a Kawasaki-like picture, shock, or macrophage activation syndrome, usually warranting admission to the pediatric intensive care unit (PICU). The clinical manifestations includes fever and multi-organ involvement, including gastrointestinal, cardiovascular, mucocutaneous, and neurological symptoms, with laboratory evidence of severe inflammatory activity and coagulopathy[38,39]. Although liver involvement is reported, it occurs as part of intense multi-organ involvement[24,25].
Liver injury in children infected with COVID-19 is suspected in the presence of elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) serum levels in 13% to 50% of patients[12,18,24,25,40]. AST levels > 50 UI/L are observed in 20%-50% of cases, and ALT levels > 45 UI/L in up to 35%[12,40]. Abnormal serum bilirubin levels may also occur but to a lesser extent than with transaminase. Raised alkaline phosphatase and gamma-glutamyl transferase levels are rarely observed. Liver injury is generally mild and resolves over time, with severe liver dysfunction being uncommon. Abnormal transaminase levels have also been linked to the severity of COVID-19, with liver injury occurring in 58.8% of patients with moderate and 66.7% of those with severe disease[10,11,41]. Other studies demonstrated elevated liver enzymes in 40%-60% of those with severe illness as compared to asymptomatic or patients with mild disease (18%-25%)[9,12,13]. In a meta-analysis and systematic review, significantly higher levels of aminotransferases and significantly lower albumin levels were more common in severe cases of COVID-19[42].
Severe acute liver injury, defined as serum ALT values five times above the upper limit of normal[9,36,43], is commonly associated with severe COVID-19 infection, shock, respiratory compromise, higher serum inflammatory markers, and longer overall length of stay in hospital[20]. However, acute liver failure (ALF) is rare and is more common in patients with severe illness and multi-organ dysfunction[20,22]. In one published case report, an early teenager died with acute fulminant hepatic failure secondary to COVID-19 infection. However, a similar child’s life was saved with liver transplant (LT)[44,45].
Post-COVID-19 cholangiopathy: This proliferative cholangiopathy is associated with lymphocytic portal and parenchymal infiltration, after excluding other possible causes such as adenovirus infection. It occurs at least several weeks after full recovery from asymptomatic or mild acute infection[40]. Two clinical patterns of presentations have been described: ALF in infants and acute hepatitis and cholestasis in older children. The group with hepatitis and cholestasis showed an excellent response to systemic steroids, while patients with ALF needed LT.
Secondary sclerosing cholangitis: Secondary sclerosing cholangitis (SSC) has been described in adults following COVID-19 as prolonged cholestasis with severe cholangiopathy. Unlike children, it occurs mainly in patients who have been through a complex and critical course of COVID-19 requiring admission to an intensive care unit (ICU), with a mean time of 118 d (range 138-319) between COVID-19 disease and the diagnosis of cholangiopathy. Compared to adults, ultrasound findings include strictures of intrahepatic bile ducts along with intraluminal sludging and casts formation[44,46]. Biliary duct dilatation, periportal and gallbladder wall oedema with thickening are more common in children.
Adult patients with post-COVID-19 cholangiopathy and SSC show progressive disease unresponsive to treatment with ursodiol therapy, with those most severely affected requiring LT. Corticosteroids, immunomodulators, or immunosuppressant therapies have not been studied in those patients.
Obesity and non-alcoholic fatty liver disease: In a meta-analysis of 285004 children infected with the novel virus, 9353 (3.3%) suffered from one or more comorbidities including obesity. Among 507 obese children, 64 had either severe COVID-19 or required ICU admission, with a calculated risk of severity of 2.87 (95%CI, 1.16-7.07)[46]. Obesity is thus the most common comorbidity reported in children with severe SARS-CoV-2 infection[20,47-50]. Although the proportion of patients with non-alcoholic fatty liver disease (NAFLD) in published obese cohorts remains unknown, children with NAFLD, especially those with obesity, should be considered a risk group for severe COVID-19[20].
The link between immune deficiency and severe gastrointestinal and liver involvement has not been proven[47,51,52]. Furthermore, some immunosuppressive medications even mitigate against severe COVID-19. These include calcineurin inhibitors, which potentially inhibit coronavirus replication[53]. European surveillance disproved any link between the use of calcineurin inhibitors and severe SARS-CoV-2 infections[54]. Moreover, drugs with antimetabolic activities such as mycophenolic acid have been reported, in laboratory studies, to interfere with the virus activity[55]. In addition, the regular use of immunosuppressants is not associated with a severe form of the disease[56,57]. In a study involving 180 children with an LT, 30 required non-ICU hospital admission (median 5 d) and three required ICU admission. However, none of them required inotropes or invasive ventilatory support[20]. Children on post-transplant immunosuppressive regimens have been shown to experience mild disease, similar to the general paediatric population[57-59].
While liver involvement is commonly associated with COVID-19 infection in children, most children demonstrate mildly abnormal liver function, which usually normalises without any specific treatment[9].
Like most other viral illnesses with inflammatory liver involvement, the management of hepatic involvement with COVID-19 infection is supportive. It includes stabilisation of vital signs, fluid and electrolytes correction, and ensuring adequate liver oxygenation. Avoiding hypo-perfusion and hypoxia (especially in patients with respiratory distress) is essential. Liver recovery will likely be enhanced with the ongoing improvement of the systemic inflammatory status. Some patients might require poly-pharmacotherapy depending on the severity of their lung injury and the other organs involved. Avoidance of hepatotoxic medications is crucial. All specific virus-targeted therapies are still employed exclusively in clinical research trial settings[13].
Nutrition is essential, especially in children with a prolonged critical illness. They are at higher risk of developing malnutrition, associated with increased morbidity and mortality. Hence, early oral or nasogastric tube feeding is recommended. It is preferred to parenteral nutrition, except in patients with severe gastrointestinal dysfunction. During the acute phase, the energy requirements do not need to exceed resting energy expenditure. Based on the tolerance and the patient’s general condition, the European Society of Pediatric and Neonatal Intensive Care recommends a gradual increase toward the target caloric need. Enteral nutritional support must be maintained as long as required until adequate oral intake is reliably attained to support physical and nutritional rehabilitation[60,61].
Infected patients with underlying primary liver diseases and other metabolic liver diseases should continue to receive treatment for their underlying condition[9]. Elevated liver enzymes are not a reason to discontinue antiviral treatment as long as liver function is monitored[10]. Post-LT patients are advised to continue their immunosuppressants and modulator medications.
The COVID-19 pandemic has profoundly impacted transplantation worldwide, both donor’s and recipients’ viral transmission and healthcare resources[58]. There is no indication for delaying or interrupting oncological treatments, withdrawing immune suppression, or postponing any required treatments to those patients with liver-transplant[57].
Mortality is higher in COVID-19 children with deranged hepatic function and the proportion of liver injury is directly related to the poor prognosis[14]. A literature review of 12 studies with a total of 6976 patients, whose laboratory tests were obtained at admission, showed high levels of transaminases and low albumin levels that were significantly more common in severe cases of COVID-19[42]. In a meta-analysis of 12 studies with a total of 5135 COVID-19 subjects with collected data on raised AST and outcomes, increased AST values were associated with three times higher risk of poor effects (pooled odds ratio: OR, 2.98; 95%CI, 2.35-3.77; P < 0.00001)[62]. Similarly, ten studies documented reported elevated ALT and outcomes, including 5091 patients, showed a marked increase in poor outcomes (pooled OR: 1.73; 95%CI: 1.32-2.27; P < 0.0001). Furthermore, a meta-analysis of four studies with a total sample size of 485 patients demonstrated that those with acute liver injury had higher odds of poor outcomes with a pooled OR of 1.68 (95%CI, 1.04-2.70; P = 0.03)[62].
Studies of COVID-19 outcomes among children with CLD are limited. Data from adult patients have reported mixed results and occasionally conflicting conclusions, making it difficult to determine a prognosis[63-65]. An earlier meta-analysis of 17 studies with a sample size of 8800 COVID-19 patients revealed that chronic liver disease did not significantly affect the outcomes (pooled OR, 0.96; 95%CI, 0.71-1.29; P = 0.78)[62]. A similar conclusion was also reached in a review where there were no major differences in COVID-19 severity and mortality between patients with liver disease and those without[66]. However, given the limitations of these studies, their results must be cautiously interpreted.
A more recent and comprehensive meta-analysis of 40 studies with 908032 participants concluded that CLD markedly affected clinical outcomes among COVID-19 patients[67]. For disease severity, the pooled OR was 2.44 with 95%CI of 1.89-3.16; for mortality, it was 2.35 (95%CI, 1.84-3.00). Subgroup analysis indicated that NAFLD, metabolic-associated fatty liver disease, and cirrhosis had the highest odd ratios of 5.6, 3.2, and 3.09 for COVID-19 severity. In other studies, cirrhosis was implicated as a significant risk factor for hospitalization, intensive care admission, and mortality. Mortality among cirrhotic patients was 32% compared to 8% among non-cirrhotic patients[68]. Other reports have also confirmed a higher risk of COVID-19 severity and mortality in CLD patients, up to four and two times, respectively, compared with those without CLD[62,69]. In children, a systematic review and meta-analysis inferred that those with COVID-19 have preserved effector and immunosuppressive components, and encountered a milder disease compared to adults[33].
Most children with MIS-C achieve full clinical recovery with a death rate of < 1%. However, in those where the clinical course was severe and required intensive care interventions for ALF, the possibility of death was 11 times higher than in those without these complications[70].
Preventative measures should be implemented for vulnerable patients at risk of exposure to SARS-CoV-2, as they may develop severe illness.
General measures for distancing, maintaining good hygiene and avoiding contamination with the virus are important for healthy children and the general public as well as for children with an underlying liver condition.
Children suffering from CLD and/or LT are recommended to be administered the COVID-19 vaccination, which is generally safe in this group[71]. However, CLD among adults with non-cirrhotic compensated cirrhotic or decompensated cirrhotic was associated with lower rates of development of positive SARS-CoV-2 neutralizing antibodies compared with healthy individuals (77 vs 90 percent)[72]. Other studies suggested that patients with liver disease who received COVID-19 vaccination have a negligible risk of infection and COVID-19-associated mortality[73]. Likewise, children awaiting a LT also would need to be prioritized for receiving COVID-19 vaccine. The type of vaccine would depend on the active strain of the virus as well as the chance to choose from different vaccines as per the local infectious disease department recommendations and regardless of the time available prior to the transplant to allow the child to receive the 2 doses as in certain protocols[74]. Routine serology tests to check for the COVID-19 virus antibodies are not indicated[71].
The spectrum of liver injury in children with COVID-19 ranges from being asymptomatic with elevated serum transaminase levels to severe liver injury, with reports of acute-on-chronic liver failure in patients with underlying liver disease. The mechanism of liver injury is multifactorial. Most children demonstrate a mild self-resolving liver injury; the level of severity of the damage is linked to how severe the infection is with COVID-19 disease and is considered an independent prognostic factor. Treatment is supportive and LT is required only in very few patients. Children with underlying NAFLD and other pre-existing chronic liver diseases carry a higher risk of developing severe COVID-19 illness and poor outcomes.
Thanks to Tasneem Abul Qasim, a senior librarian from the education department of SEHA, for her help and assistance with conducting the literature search.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Arab Emirates
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tudoran C, Romania; Wijaya JH, Indonesia S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13871] [Cited by in F6Publishing: 12445] [Article Influence: 3111.3] [Reference Citation Analysis (1)] |
| 2. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1160] [Cited by in F6Publishing: 1137] [Article Influence: 284.3] [Reference Citation Analysis (0)] |
| 3. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 315] [Article Influence: 78.8] [Reference Citation Analysis (1)] |
| 4. | Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 203] [Article Influence: 67.7] [Reference Citation Analysis (2)] |
| 5. | Yang Z, Xu M, Yi JQ, Jia WD. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int. 2005;4:60-63. [PubMed] [Cited in This Article: ] |
| 6. | Song JE, Kang MK, Lee YR, Lee CH, Park JG, Kweon YO, Tak WY, Park SY, Jang SY, Hwang JS, Jang BK, Jang WY, Suh JI, Chung WJ, Kim BS; Daegu-Gyeongbuk Liver Study Group (DGLSG). Multicenter Analysis of Clinical Features and Prognosis of COVID-19 Patients with Hepatic Impairment. Gut Liver. 2021;15:606-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J Gen Intern Med. 2020;35:1545-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 809] [Cited by in F6Publishing: 676] [Article Influence: 169.0] [Reference Citation Analysis (0)] |
| 8. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6024] [Cited by in F6Publishing: 6200] [Article Influence: 1550.0] [Reference Citation Analysis (0)] |
| 9. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1184] [Cited by in F6Publishing: 1225] [Article Influence: 306.3] [Reference Citation Analysis (4)] |
| 10. | Huang W, Li C, Wang Z, Wang H, Zhou N, Jiang J, Ni L, Zhang XA, Wang DW. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Sci China Life Sci. 2020;63:1678-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 11. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19202] [Cited by in F6Publishing: 18107] [Article Influence: 4526.8] [Reference Citation Analysis (5)] |
| 12. | Parri N, Lenge M, Buonsenso D; Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group. Children with Covid-19 in Pediatric Emergency Departments in Italy. N Engl J Med. 2020;383:187-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 450] [Cited by in F6Publishing: 454] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 13. | Calitri C, Fumi I, Ignaccolo MG, Banino E, Benetti S, Lupica MM, Fantone F, Pace M, Garofalo F. Gastrointestinal involvement in paediatric COVID-19 - from pathogenesis to clinical management: A comprehensive review. World J Gastroenterol. 2021;27:3303-3316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Chen LY, Chu HK, Bai T, Tu SJ, Wei Y, Li ZL, Hu LL, Zhu R, Zhang L, Han CQ, Xiao L, He Q, Song J, Liu WH, Zhu QJ, Chen H, Yang L, Hou XH. Liver damage at admission is an independent prognostic factor for COVID-19. J Dig Dis. 2020;21:512-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Bende F, Tudoran C, Sporea I, Fofiu R, Bâldea V, Cotrău R, Popescu A, Sirli R, Ungureanu BS, Tudoran M. A Multidisciplinary Approach to Evaluate the Presence of Hepatic and Cardiac Abnormalities in Patients with Post-Acute COVID-19 Syndrome-A Pilot Study. J Clin Med. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V, Krivec U, Lo Vecchio A, Shingadia D, Soriano-Arandes A, Melendo S, Lanari M, Pierantoni L, Wagner N, L'Huillier AG, Heininger U, Ritz N, Bandi S, Krajcar N, Roglić S, Santos M, Christiaens C, Creuven M, Buonsenso D, Welch SB, Bogyi M, Brinkmann F, Tebruegge M; ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 692] [Cited by in F6Publishing: 785] [Article Influence: 196.3] [Reference Citation Analysis (0)] |
| 17. | Mansourian M, Ghandi Y, Habibi D, Mehrabi S. COVID-19 infection in children: A systematic review and meta-analysis of clinical features and laboratory findings. Arch Pediatr. 2021;28:242-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 18. | Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y, Wu C, Liu H, Liu D, Shao J, Peng X, Yang Y, Liu Z, Xiang Y, Zhang F, Silva RM, Pinkerton KE, Shen K, Xiao H, Xu S, Wong GWK; Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382:1663-1665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1629] [Cited by in F6Publishing: 1617] [Article Influence: 404.3] [Reference Citation Analysis (0)] |
| 19. | Zhou YH, Zheng KI, Targher G, Byrne CD, Zheng MH. Abnormal liver enzymes in children and infants with COVID-19: A narrative review of case-series studies. Pediatr Obes. 2020;15:e12723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Nicastro E, Ebel NH, Kehar M, Czubkowski P, Ng VL, Michaels MG, Lobritto SJ, Martinez M, Indolfi G. The Impact of Severe Acute Respiratory Syndrome Coronavirus Type 2 on Children With Liver Diseases: A Joint European Society for Pediatric Gastroenterology, Hepatology and Nutrition and Society of Pediatric Liver Transplantation Position Paper. J Pediatr Gastroenterol Nutr. 2022;74:159-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Anirvan P, Bharali P, Gogoi M, Thuluvath PJ, Singh SP, Satapathy SK. Liver injury in COVID-19: The hepatic aspect of the respiratory syndrome - what we know so far. World J Hepatol. 2020;12:1182-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 22. | Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 546] [Cited by in F6Publishing: 538] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 23. | Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, Lang C, Xiao Q, Xiao K, Yi Z, Qiang M, Xiang J, Zhang B, Chen Y. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). Cold Spring Harbor Laboratory 2020. [DOI] [Cited in This Article: ] |
| 24. | Ahmed J, Rizwan T, Malik F, Akhter R, Malik M, Ahmad J, Khan AW, Chaudhary MA, Usman MS. COVID-19 and Liver Injury: A Systematic Review and Meta-Analysis. Cureus. 2020;12:e9424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Ali N, Hossain K. Liver injury in severe COVID-19 infection: current insights and challenges. Expert Rev Gastroenterol Hepatol. 2020;14:879-884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 189] [Article Influence: 47.3] [Reference Citation Analysis (2)] |
| 27. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 293] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 28. | Li Y, Xiao SY. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92:1491-1494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 29. | Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front Immunol. 2020;11:1708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 768] [Cited by in F6Publishing: 651] [Article Influence: 162.8] [Reference Citation Analysis (0)] |
| 30. | Wang J, Lu Z, Jin M, Wang Y, Tian K, Xiao J, Cai Y, Zhang X, Chen T, Yao Z, Yang C, Deng R, Zhong Q, Deng X, Chen X, Yang XP, Wei G, Wang Z, Tian J, Chen XP. Clinical characteristics and risk factors of COVID-19 patients with chronic hepatitis B: a multi-center retrospective cohort study. Front Med. 2022;16:111-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Wang M, Yan W, Qi W, Wu D, Zhu L, Li W, Wang X, Ma K, Ni M, Xu D, Wang H, Chen G, Yu H, Ding H, Xing M, Han M, Luo X, Chen T, Guo W, Xi D, Ning Q. Clinical characteristics and risk factors of liver injury in COVID-19: a retrospective cohort study from Wuhan, China. Hepatol Int. 2020;14:723-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Debes JD, Anugwom CM, Aby ES. Systematic analysis of acute liver injury during SARS-CoV-2 infection. Dig Liver Dis. 2020;52:953-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 33. | Di Giorgio A, Hartleif S, Warner S, Kelly D. COVID-19 in Children With Liver Disease. Front Pediatr. 2021;9:616381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Carter MJ, Fish M, Jennings A, Doores KJ, Wellman P, Seow J, Acors S, Graham C, Timms E, Kenny J, Neil S, Malim MH, Tibby SM, Shankar-Hari M. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26:1701-1707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 260] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 35. | Lee PY, Day-Lewis M, Henderson LA, Friedman KG, Lo J, Roberts JE, Lo MS, Platt CD, Chou J, Hoyt KJ, Baker AL, Banzon TM, Chang MH, Cohen E, de Ferranti SD, Dionne A, Habiballah S, Halyabar O, Hausmann JS, Hazen MM, Janssen E, Meidan E, Nelson RW, Nguyen AA, Sundel RP, Dedeoglu F, Nigrovic PA, Newburger JW, Son MBF. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942-5950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 253] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 36. | Perez A, Cantor A, Rudolph B, Miller J, Kogan-Liberman D, Gao Q, Da Silva B, Margolis KG, Ovchinsky N, Martinez M. Liver involvement in children with SARS-COV-2 infection: Two distinct clinical phenotypes caused by the same virus. Liver Int. 2021;41:2068-2075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Cavalcanti A, Islabão A, Magalhães C, Veloso S, Lopes M, do Prado R, Aquilante B, Terrazas AM, Rezende MF, Clemente G, Terreri MT. Paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS): a Brazilian cohort. Adv Rheumatol. 2022;62:6. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 38. | Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, Kucera F, Brierley J, McDougall M, Carter M, Tremoulet A, Shimizu C, Herberg J, Burns JC, Lyall H, Levin M; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020;324:259-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1345] [Cited by in F6Publishing: 1108] [Article Influence: 277.0] [Reference Citation Analysis (0)] |
| 39. | Jenke A, Steinmetz M. Paediatric inflammatory multisystem syndrome - temporally associated with SARS-CoV-2 (PIMS-TS) - a German single centre real-life evaluation of the Swiss and UK consensus statements. Cardiol Young. 2022;1-5. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 40. | Chiappini E, Licari A, Motisi MA, Manti S, Marseglia GL, Galli L, Lionetti P. Gastrointestinal involvement in children with SARS-COV-2 infection: An overview for the pediatrician. Pediatr Allergy Immunol. 2020;31 Suppl 26:92-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Fan H, Cai J, Tian A, Li Y, Yuan H, Jiang Z, Yu Y, Ruan L, Hu P, Yue M, Chen N, Li J, Zhu C. Comparison of Liver Biomarkers in 288 COVID-19 Patients: A Mono-Centric Study in the Early Phase of Pandemic. Front Med (Lausanne). 2020;7:584888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Abdulla S, Hussain A, Azim D, Abduallah EH, Elawamy H, Nasim S, Kumar S, Naveed H. COVID-19-Induced Hepatic Injury: A Systematic Review and Meta-Analysis. Cureus. 2020;12:e10923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 234] [Article Influence: 58.5] [Reference Citation Analysis (2)] |
| 44. | Haji Esmaeil Memar E, Mamishi S, Sharifzadeh Ekbatani M, Alimadadi H, Yaghmaei B, Chegini V, Janani S, Mahmoudi S. Fulminant hepatic failure: A rare and devastating manifestation of Coronavirus disease 2019 in an 11-year-old boy. Arch Pediatr. 2020;27:502-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 45. | Yohanathan L, Campioli CC, Mousa OY, Watt K, Friedman DZP, Shah V, Ramkissoon R, Hines AS, Kamath PS, Razonable RR, Badley AD, DeMartino ES, Joyner MJ, Graham R, Vergidis P, Simonetto DA, Sanchez W, Taner T, Heimbach JK, Beam E, Leise MD. Liver transplantation for acute liver failure in a SARS-CoV-2 PCR-positive patient. Am J Transplant. 2021;21:2890-2894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Tsankov BK, Allaire JM, Irvine MA, Lopez AA, Sauvé LJ, Vallance BA, Jacobson K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int J Infect Dis. 2021;103:246-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 47. | Kehar M, Ebel NH, Ng VL, Baquero JER, Leung DH, Slowik V, Ovchinsky N, Shah AA, Arnon R, Miloh T, Gupta N, Mohammad S, Kogan-Liberman D, Squires JE, Sanchez MC, Hildreth A, Book L, Chu C, Alrabadi L, Azzam R, Chepuri B, Elisofon S, Falik R, Gallagher L, Kader H, Mogul D, Mujawar Q, Namjoshi SS, Valentino PL, Vitola B, Waheed N, Zheng MH, Lobritto S, Martinez M. Severe Acute Respiratory Syndrome Coronavirus-2 Infection in Children With Liver Transplant and Native Liver Disease: An International Observational Registry Study. J Pediatr Gastroenterol Nutr. 2021;72:807-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 48. | Gao F, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Chen YP, Targher G, Byrne CD, George J, Zheng MH. Obesity Is a Risk Factor for Greater COVID-19 Severity. Diabetes Care. 2020;43:e72-e74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 246] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 49. | Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, Banker SL, Giordano M, Manice CS, Diamond R, Sewell TB, Schweickert AJ, Babineau JR, Carter RC, Fenster DB, Orange JS, McCann TA, Kernie SG, Saiman L; Columbia Pediatric COVID-19 Management Group. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children's Hospital in New York City, New York. JAMA Pediatr. 2020;174:e202430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 331] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 50. | Korakas E, Ikonomidis I, Kousathana F, Balampanis K, Kountouri A, Raptis A, Palaiodimou L, Kokkinos A, Lambadiari V. Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab. 2020;319:E105-E109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 51. | Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, Safa K, Kotton CN, Blumberg EA, Besharatian BD, Tanna SD, Ison MG, Malinis M, Azar MM, Rakita RM, Morilla JA, Majeed A, Sait AS, Spaggiari M, Hemmige V, Mehta SA, Neumann H, Badami A, Goldman JD, Lala A, Hemmersbach-Miller M, McCort ME, Bajrovic V, Ortiz-Bautista C, Friedman-Moraco R, Sehgal S, Lease ED, Fisher CE, Limaye AP; UW COVID-19 SOT Study Team. Coronavirus Disease 2019 in Solid Organ Transplant: A Multicenter Cohort Study. Clin Infect Dis. 2021;73:e4090-e4099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 262] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 52. | Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 53. | Carbajo-Lozoya J, Müller MA, Kallies S, Thiel V, Drosten C, von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165:112-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 54. | Belli LS, Fondevila C, Cortesi PA, Conti S, Karam V, Adam R, Coilly A, Ericzon BG, Loinaz C, Cuervas-Mons V, Zambelli M, Llado L, Diaz-Fontenla F, Invernizzi F, Patrono D, Faitot F, Bhooori S, Pirenne J, Perricone G, Magini G, Castells L, Detry O, Cruchaga PM, Colmenero J, Berrevoet F, Rodriguez G, Ysebaert D, Radenne S, Metselaar H, Morelli C, De Carlis LG, Polak WG, Duvoux C; ELITA-ELTR COVID-19 Registry. Protective Role of Tacrolimus, Deleterious Role of Age and Comorbidities in Liver Transplant Recipients With Covid-19: Results From the ELITA/ELTR Multi-center European Study. Gastroenterology. 2021;160:1151-1163.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 55. | Schoot TS, Kerckhoffs APM, Hilbrands LB, van Marum RJ. Immunosuppressive Drugs and COVID-19: A Review. Front Pharmacol. 2020;11:1333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 56. | Nicastro E, Di Giorgio A, Zambelli M, Ginammi M, Bravi M, Stroppa P, Casotti V, Palladino R, Colledan M, D'Antiga L. Impact of the Severe Acute Respiratory Syndrome Coronavirus 2 Outbreak on Pediatric Liver Transplant Recipients in Lombardy, Northern Italy. Liver Transpl. 2020;26:1359-1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 57. | D'Antiga L. Coronaviruses and Immunosuppressed Patients: The Facts During the Third Epidemic. Liver Transpl. 2020;26:832-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 497] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 58. | Doná D, Torres Canizales J, Benetti E, Cananzi M, De Corti F, Calore E, Hierro L, Ramos Boluda E, Melgosa Hijosa M, Garcia Guereta L, Pérez Martínez A, Barrios M, Costa Reis P, Teixeira A, Lopes MF, Kaliciński P, Branchereau S, Boyer O, Debray D, Sciveres M, Wennberg L, Fischler B, Barany P, Baker A, Baumann U, Schwerk N, Nicastro E, Candusso M, Toporski J, Sokal E, Stephenne X, Lindemans C, Miglinas M, Rascon J, Jara P; ERN TransplantChild. Pediatric transplantation in Europe during the COVID-19 pandemic: Early impact on activity and healthcare. Clin Transplant. 2020;34:e14063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 59. | Nikoupour H, Kazemi K, Arasteh P, Ghazimoghadam S, Eghlimi H, Dara N, Gholami S, Nikeghbalian S. Pediatric liver transplantation and COVID-19: a case report. BMC Surg. 2020;20:224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Oba J, Carvalho WB, Silva CA, Delgado AF. Gastrointestinal manifestations and nutritional therapy during COVID-19 pandemic: a practical guide for pediatricians. Einstein (Sao Paulo). 2020;18:eRW5774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Tume LN, Valla FV, Joosten K, Jotterand Chaparro C, Latten L, Marino LV, Macleod I, Moullet C, Pathan N, Rooze S, van Rosmalen J, Verbruggen SCAT. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensive Care Med. 2020;46:411-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 62. | Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, Honganur NS, Akbar A, Deol A, Francis B, Patel S, Mehta D, Jaiswal R, Singh J, Patel U, Malik P. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol. 2021;21:100273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 63. | Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 157] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 64. | Frager SZ, Szymanski J, Schwartz JM, Massoumi HS, Kinkhabwala M, Wolkoff AW. Hepatic Predictors of Mortality in Severe Acute Respiratory Syndrome Coronavirus 2: Role of Initial Aspartate Aminotransferase/Alanine Aminotransferase and Preexisting Cirrhosis. Hepatol Commun. 2021;5:424-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | . Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 66. | Lippi G, de Oliveira MHS, Henry BM. Chronic liver disease is not associated with severity or mortality in Coronavirus disease 2019 (COVID-19): a pooled analysis. Eur J Gastroenterol Hepatol. 2021;33:114-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 67. | Nagarajan R, Krishnamoorthy Y, Rajaa S, Hariharan VS. COVID-19 Severity and Mortality Among Chronic Liver Disease Patients: A Systematic Review and Meta-Analysis. Prev Chronic Dis. 2022;19:E53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 30] [Reference Citation Analysis (0)] |
| 68. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 332] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 69. | Wu ZH, Yang DL. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res. 2020;25:54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 70. | Center for Disease Control and Prevention, Center for Preparedness and Response: Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19), Clinician Outreach and Communication (COCA) Webinar. [cited 19 May 2020]. Available from: https://emergency.cdc.gov/coca/calls/2020/callinfo_051920.asp?deliveryName=USCDC_1052-DM28623. [Cited in This Article: ] |
| 71. | Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, Hameed B, Kaul DR, Kulik LM, Kwok RM, McGuire BM, Mulligan DC, Price JC, Reau NS, Reddy KR, Reynolds A, Rosen HR, Russo MW, Schilsky ML, Verna EC, Ward JW, Fontana RJ; AASLD COVID‐19 Vaccine Working Group. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74:1049-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 125] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 72. | Ai J, Wang J, Liu D, Xiang H, Guo Y, Lv J, Zhang Q, Li J, Zhang X, Li Q, Liang J, Guo X, Feng Y, Liu L, Qin W, Wang X, Rao W, Tian Q, Zhang Y, Xie F, Jiang S, Yan Y, Qiu Y, Wu H, Hou Z, Zhang N, Zhang A, Ji J, Yang J, Huang J, Zhao Z, Gu Y, Bian L, Zhang Z, Zou S, Ji H, Ge G, Du X, Hou A, Zhu Y, Cong Q, Xu J, Zu H, Wang Y, Yan Z, Yan X, BianBa Y, Ci Q, Zhang L, Yang S, Gao X, Zhong L, He S, Liu C, Huang Y, Liu Y, Xu D, Zhu Q, Xu X, Lv M, Zhang W, Qi X. Safety and Immunogenicity of SARS-CoV-2 Vaccines in Patients With Chronic Liver Diseases (CHESS-NMCID 2101): A Multicenter Study. Clin Gastroenterol Hepatol. 2022;20:1516-1524.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 73. | Bondarev VN, Lakotkina EA, Gorvat NA. [Some data on the incidence of allergic reactions and the course of vaccinal process in healthy children and in those with reactivity changes]. Pediatriia. 1971;50:15-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 74. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10556] [Cited by in F6Publishing: 9404] [Article Influence: 2351.0] [Reference Citation Analysis (1)] |