Published online Feb 20, 2013. doi: 10.5321/wjs.v2.i1.18
Revised: December 26, 2012
Accepted: January 11, 2013
Published online: February 20, 2013
AIM: To study the effectiveness of ozone in the elimination of cariogenic bacteria, followed with fluoride supplements.
METHODS: Sixty extracted teeth free of caries were used, and five groups were constituted. In Group I, the teeth were immersed in artificial saliva. In Group II, the teeth were inoculated with Streptococcus mutans (S. mutans) and immersed in artificial saliva. In Group III the teeth were inoculated with Lactobaccilus fermentum (L. fermentum) and immersed in artificial saliva. In Group IV the teeth were inoculated with S. mutans and L. fermentum and immersed in artificial saliva and the teeth in Group V were inoculated with S. mutans and L. fermentum, and were subjected to the application of ozone and to the action of a fluoride mineralizing gel. DIAGNOdent was used to evaluate the caries of the teeth 3 wk after inoculation of bacteria and after that the teeth of Group V were subjected to the application of ozone during 60 s, by HealOzone. After the application of ozone, products of the remineralization kit supplied by the manufacturer were applied daily, during 30 d. At the end samples were collected for analysis and evaluation of bacterial activity by polymerase chain reaction.
RESULTS: Regarding the value of caries, obtained via DIAGNOdent, in the initial measurement the groups are homogeneous (P = 0.730). There was an increase in DIAGNOdent values, presenting statistical significant difference regarding the initial measurement in all groups (P ˂ 0.001), except in group I - only artificial saliva - which shows that the artificial carie model was effective. Comparing the initial and final measurements for each of the 60 teeth, it can be observed that in 9 teeth (15.0%) there was a decrease in values between the two measurements, one (1.7%) retained the same values in the two measurements and in the remaining 50 cases (83.3%) there was increase in values between the initial and final measurements. It should also be noted that in the teeth inoculated with S. mutans + L. fermentum, there was an increase of the values in 100% of cases, and in all groups except the group with artificial saliva, there is a more frequent increase in the values. In group V, subject to the application of ozone, bacterial DNA was not detected, in group IV, bacterial DNA was detected.
CONCLUSION: Ozone was effective in the elimination of the study bacteria.
-
Citation: Marques J, Paula A, Gonçalves T, Ferreira M, Carrilho E. Ozone action on
Streptococcus mutans andLactobacillus fermentum : A pilot study. World J Stomatol 2013; 2(1): 18-23 - URL: https://www.wjgnet.com/2218-6263/full/v2/i1/18.htm
- DOI: https://dx.doi.org/10.5321/wjs.v2.i1.18
Currently dental caries can be defined as a multifactorial and infectious disease, characterized by a localized hard tissue demineralization of tooth, resulting from acidic products, through bacterial fermentation of carbohydrates[1,2].
Clarke[3] (1924) identified Streptococcus mutans (S. mutans) in carious lesions and 36 years later Fitzgerald and Keyes found that these bacteria were capable of inducing caries in hamsters. A number of studies in humans have shown that caries are a confirmation of bacterial infection, primarily mediated by S. mutans, which is transmited through the saliva within the family unit. Lactobacillus fermentum (L. fermentum) is also associated with the caries process, but is not responsible for it, since it does not have the capacity for the adherence like S. mutans[3].
Until today no single factor was found as a caries inducer, instead several factors are proposed such as: microorganisms, diet, teeth and saliva, resulting in the metabolic activity of bacteria that will ferment diet carbohydrates to produce lactic acid[1,2,4]. Metabolic activity leads to changes of pH on the interface of the tooth surface and bacterial deposits, producing an imbalance between the enamel and the fluid of the plaque, causing the loss of tooth mineral when pH decreases, or the mineral gain when the pH increases[1]. The cumulative result of these processes can be demineralization or remineralization, with mineral loss, leading to dissolution of the dental tissues and the formation of caries[1].
In strategies for management of caries, emphasis has been given to preventive treatments and remineralizing procedures in the early detected stages[1,5]. In the presence of caries we have various treatment options. The decision in cavitated teeth becomes simpler, since the treatment choice is conventional drilling and filling[5]. In the lesions that are not cavitated, the decisions become more complex, two types of approach can be selected: invasive, assuming that the lesion is active and will progress; or a conservative approach, in order to stop the progression of caries. The latter approach is where emphasis has been placed, leading to more research in this area involving fluoride products and the remineralization process[6]. These approaches can be divided into selective invasive interventions and non-invasive interventions, in which ozone is included[7]. The non-invasive interventions focus on caries prevention and the preservation of demineralized enamel and dentin, but without cavitation. Under these conditions “restitutio ad integrum” is thought to be possible, by removing bacteria and products of their metabolism and allowing the remineralizing process using fluoride[7].
The antimicrobial effects of ozone have been known for many years[5]. The direct application of this gas on coronary or root surfaces seems to have a sterilization effect[8]. It is mentioned that it is able to slow, stop, or reverse the carious process[7]. It is believed that the ozone is also useful in reducing the microbial flora in cavitated lesions, before proceeding to filling[7]. Although ozone is not a radical, it is the third most potent oxidant[9]. Due to its oxidative power, it induces the destruction of the cell walls and membranes, and cytoplasm of bacteria, fungi and certain viruses[10,11]. The use of ozone in dentistry has been advocated for the sterilization of cavities, root canal, periodontal pocket and herpetic lesions[9]. However, its major indication is in the treatment of caries[4,5]. The ozone procedure involves the application of ozone gas, the use of remineralizing agents and an oral hygiene kit to give to the patient, in order to promote the process of remineralization.
The present work aims to assess the effectiveness of ozone in the elimination of cariogenic bacteria.
Sixty extracted premolars and molars without caries were selected and stored in a saline solution. The extracted teeth were set in acrylic blocks and numbered. Before using the DIAGNOdent® pen 2190 (KAVO GmbH, Biberach, Germany) to evaluate the caries, the teeth were cleaned, dried and selected according the value obtained with DIAGNOdent. An occlusal recess of small size (0.08 mm wide, 1 mm deep and 3 mm in length) was carried out, with a 008 cylindrical diamond drill, placed in turbine with cooling air/water.
Artificial saliva solution was used and prepared based on a modification of the basal medium mucin (BMM) medium with modified Shellis artificial saliva, whose composition is described in the Table 1. The bacteria used were S. mutans (ATCC reference: 25 175) and L. fermentum (ATCC reference: 14 932). These bacteria were grown and maintained according to the instructions in the ATCC (American Type, Culture and Collection). For inoculation of the samples, bacteria were prepared in phosphate buffered solution (PBS) suspensions. This was subsequently diluted to the final value of 105 colony forming units for each bacteria of saliva used for this study. Artificial saliva was supplemented with 2% glucose (20 g/L).
| g/L | |
| Yeast extract | 5 |
| Proteose peptone | 10 |
| Potassium chloride (KCl; 74.5 g/mol) | 1.2 |
| Sodium hydrogen carbonate (NaHCO3; 84 g/L) | 0.6 |
| Potassium thiocyanate (KSCN; 97.18 g/mol) | 0.23 |
| Sodium Di-hydrogen phosphate (Na2HPO 4.12 H2O; 358 g/mol) | 0.9 |
Five groups were formed: Group I - 10 teeth, immersed in artificial saliva; Group II - 10 teeth, inoculated with L. fermentum with a bacterial load of 105 and immersed in artificial saliva; Group III - 10 teeth, inoculated with S. mutans with a bacterial load of 105 and immersed in artificial saliva; Group IV- 10 teeth, inoculated with L. fermentum and S. mutans with a total bacterial load of 2 × 105 and immersed in artificial saliva; Group V - 20 teeth inoculated with L. fermentum and S. mutans with a total bacterial load of 2 × 105, subject to the application of ozone followed by fluoride remineralizing gel (supplied by the manufacturer of HealOzone, with sodium fluoride 0, 24% w/w) and immersed in artificial saliva.
All teeth during the experimental protocol were kept in an incubator at 37 °C with permanent stirring at 150 r/min. All procedures performed during the experimental protocol were performed in a laminar flow chamber. The teeth were immersed in artificial saliva and inoculated in accordance with the specifications for each experimental group. DIAGNOdent was used to evaluate the teeth 3 wk after inoculation, as well as the pH, using a pH measuring tape (pH 1-10 Universalindikator Merck).
The application of Ozone was made with HealOzone® in group V (KAVO GmbH, Biberach, Germany) 3 wk after bacterial inoculation. Before ozone application, the teeth were removed from the medium and washed with a sterile saline solution, and ozone was applied for 60 s. For ozone application a silicone dome was used, which was selected according to the size of each tooth, and changed between each tooth. After application of the ozone a reducing solution was applied, supplied by the manufacturer, for 60 s. Then the teeth were immersed in fresh medium again. Glucose at 0.05% was added to the new medium, also composed of artificial saliva. The bottles were flushed with sterile saline solution before the new medium was inserted. 30 d later, the teeth were brushed with a tooth brush and tooth paste provided by the manufacturer of HealOzone. At the end of brushing a fluoride spray was applied, also supplied by the manufacturer, as a remineralization protocol once a day.
Seven weeks after inoculation, a collection of Groups IV and V was made, prepared by excavation of cavities, with a dentin sterile excavator. The samples were stored in PBS and 25% glycerol. The extraction of total DNA from bacteria was performed by the method of proteinase K. Extraction was performed using the protocol of bacteria Lysis Buffer from Roche. After the extraction, the DNA was quantified.
Statistical analysis of results shows the mean values and standard deviation. Comparison between groups was performed using Kruskal-Wallis test with multiple comparisons and adjusted in each group, by the Wilcoxon test. Comparisons between the initial and final measurements in each tooth and their relation with group was performed using a χ2 test with simulation by the Monte Carlo method (P < 0.001). All data analysis was performed by SPSS, version 19, at a significance level of 5%.
Regarding the value of caries, obtained with DIAGNOdent, in the initial measurement groups are homogeneous (P = 0.730) but the second measuring statistical difference exist between groups, the differences being detected between groups: group I and group V (P < 0.001), group I and group IV (P = 0.014), group IV and group V (P = 0.036).
There was an increase in DIAGNOdent values, presenting statistical significant difference regarding the initial measurement in all groups, except in the group I, only artificial saliva, see Table 2.
| Material | Med | n | Mean | Structural equation modeling | Min | Max | P25 | P50 | P75 | Med1 vs Med2 |
| Artificial Saliva | 1 | 10 | 6.10 | 0.795 | 2 | 11 | 4.75 | 6.00 | 7.50 | 0.759 |
| 2 | 10 | 8.20 | 4.54 | 0 | 45 | 0.00 | 1.00 | 14.75 | ||
| L. fermentum | 1 | 10 | 5.40 | 0.792 | 2 | 10 | 3.00 | 5.50 | 7.25 | 0.011 |
| 2 | 10 | 19.40 | 6.31 | 2 | 63 | 7.00 | 9.50 | 27.50 | ||
| S. mutans | 1 | 10 | 5.60 | 0.859 | 2 | 9 | 2.00 | 6.50 | 8.00 | 0.011 |
| 2 | 10 | 30.00 | 10.33 | 0 | 99 | 9.25 | 15.50 | 47.00 | ||
| S. mutans + L. fermentum | 1 | 10 | 6.00 | 0.978 | 2 | 12 | 4.50 | 5.00 | 8.50 | 0.005 |
| 2 | 10 | 31.90 | 3.29 | 16 | 47 | 25.00 | 29.50 | 43.75 | ||
| S. mutans + L. fermentum ozone + remin | 1 | 20 | 5.15 | 0.782 | 1 | 12 | 2.25 | 4.00 | 8.75 | < 0.001 |
| 2 | 20 | 45.95 | 5.90 | 20 | 99 | 24.50 | 37.00 | 68.50 | ||
| Comparisons between 5 groups | 1 | P = 0.730 | ||||||||
| 2 | P < 0.001 |
Comparing the initial and final measurements for each of the 60 teeth, it can be observed that in 9 teeth (15.0%) there was a decrease in values between the two measurements, one (1.7%) retained the same values in the two measurements and in the remaining 50 cases (83.3%) there was an increase in values between the initial and final measurements. Although changes are associated with the group, a decrease of the values can be expected only in the group which used artificial saliva and an increase in teeth with S. mutans or S. mutans + L. fermentum. The maintenance of values between measurements occurred in the L. fermentum group. It should also be noted that in the teeth inoculated with S. mutans + L. fermentum there was an increase of the values in 100% of cases, and in all groups except the group with artificial saliva there is a more frequent increase in the values (Table 3).
| Group | Total | |||||||
| Artificial saliva | L. fermentum | S. mutans | S. mutans + L. fermentum | S. mutans + L. fermentum + ozone | ||||
| Difference | Decreased | n(%) | 7 | 1 | 1 | 0 | 0 | 9 |
| Residue | 5.5 | -0.5 | -0.5 | -1.5 | -3.0 | |||
| Maintenance | n(%) | 0 | 1 | 0 | 0 | 0 | 1 | |
| Residue | -0.2 | 0.8 | -0.2 | -0.2 | -0.3 | |||
| Increased | n(%) | 3 | 8 | 9 | 10 | 20 | 50 | |
| Residue | -5.3 | -0.3 | 0.7 | 1.7 | 3.3 | |||
| Total | 10 | 10 | 10 | 10 | 20 | 60 | ||
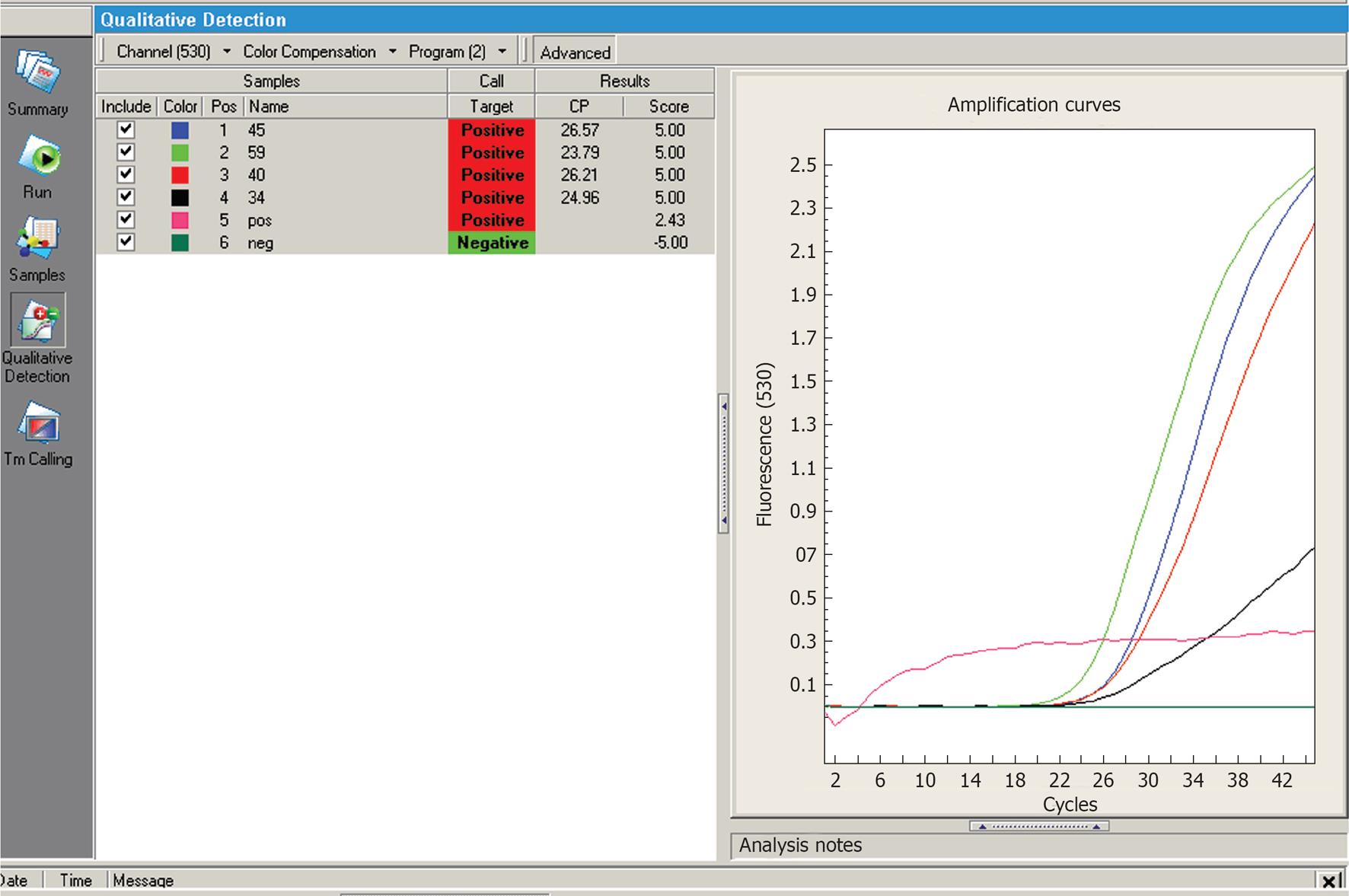
In group V, subject to the application of ozone, bacterial DNA was not detected. In group IV, bacterial DNA was detected, polymerase chain reaction amplification curves, for some teeth of group IV are presented in Figure 1.
At the beginning of the experimental work, all teeth were free from caries. The highest values accepted, quantified by DIAGNOdent® pen 2190, were 12. According to the recommendations of KaVo, this corresponds to the threshold for considering the tooth tissue normal and healthy. The diagnosis of caries lesions is made after visual and tactile examination of tooth surfaces, however it may be necessary the use auxiliary diagnostic tests[1]. A tool developed for the diagnosis and quantification of caries is DIAGNOdent, which is based on tissue fluorescence induced by laser light. In interpreting the values obtained, it is necessary to take into consideration that there may be false positives. These can occur in the following situations: teeth with plaque; fillings with fluorescent composites; proximity of the pulp; food waste; prophylactic pastes; remineralized cavities; increased natural fluorescence and patients exposed to radiation.
Three weeks after bacterial inoculation, the presence of caries was evaluated in all groups, using DIAGNOdent® pen 2190. Some authors claim that the diagnostic technologies such ECM (Electric Caries Meter) and DIAGNOdent, perform better in the early detection of caries in occlusal surfaces, compared with visual examination[12]. Goel et al[13] in 2009 conducted an in vivo study that compares the efficacy of DIAGNOdent with conventional methods (visual, tactile and bitewings radiographs). The authors concluded that DIAGNOdent showed high precision compared to conventional methods for the detection of enamel caries. However, when the cavities reach dentine, although precision is high, it is similar to other diagnostic methods. The DIAGNOdent is the diagnostic method chosen for this work since it allows the quantification of dental caries, has good accuracy, reproducibility, sensitivity and validity[12,13].
Plaque in humans is a complex biofilm comprising hundreds of microbiological species[14]. The culture plaque using the BMM medium, exhibits similar growth rates behaviour to the natural. Oral fluids are the major source of nutrients to bacterial plaque[14]. Several studies simulating these oral fluids in vitro contained as main components mucin, yeast extract and/or peptones. One of these formulations is the BMM medium, which has been widely used for in vitro studies of oral bacteria[14]. The presence of yeast extract and peptones contributes to the presence of a variable concentration of peptides, vitamins and ions in the medium[14]. Shellis in 1978 developed an artificial saliva, chemically defined, containing various ions, amino acids, vitamins, growth factors and bovine origin mucin[14]. This has as its major components potassium chloride, sodium chloride, sodium hydrogencarbonate and potassium thiocyanate[14].
The artificial saliva used in this experimental study consisted of a modification of BMM medium, with modified Shellis saliva, which since the system used was a simple one, only comprises two bacteria. To allow a greater bacterial growth prior to inoculation of bacteria on teeth, the artificial saliva was supplemented with 2% glucose, to allowing the formation of lactic acid by fermentation.
After the appearance of the tooth caries and application of ozone, the teeth were immersed in new medium, the same as used earlier, but without glucose supplementation, since the carious lesion was established and the concentration of glucose in yeast extract must correspond to 0.05% and the concentration of glucose in saliva in an adult is 0.01%[15].
In order to promote bacterial retention on site and to allow faster development of the caries process, we performed a cavity of small dimensions on the occlusal surface as the occlusal surfaces are particularly susceptible to caries[1].
The antimicrobial effect of ozone has been shown by several studies[11,16]. However there are studies that show conflicting results, such as those done by Baysan et al[17] in 2007, which demonstrated that the application of ozone in carious lesions of dentin did not significantly reduce the number of bacteria detected in infected dentin in non-cavitated caries. Castillo et al[11] in 2008 conducted an in vitro study, which used 41 strains of S. mutans including native strains obtained from the saliva of 27 children, which after being cultured, were placed in eppendorf. The ozone was applied to the eppendorf and the results show that after application of ozone for 40 s, no bacteria were viable. Polydorou et al[16] in 2006, compared the antibacterial effect of ozone with two dentin adhesive systems. The adhesive systems showed significant antibacterial activity. However, application of ozone has proved a promising in eliminating residual microorganisms in deep cavities and potentially increases the clinical success of fillings. Knight et al[18] in 2008 conducted an in vitro study to determine the effects of ozone application in dentin before the formation of a biofilm. The study showed that the ozone application prevents the formation of a biofilm of S. mutans and Lactobacillus acidophilus for a period of 4 wk.
Studies conducted to evaluate the efficacy of ozone in carious lesions in pits and fissures and roots already have a good level of scientific evidence, since they are randomized. However, there are authors who raise questions about the evaluation method, which is in most cases performed by DIAGNOdent, and as to whether the participants were aware of the treatment administered to them (only the study by Holmes is double-blind) and that many of the studies were conducted by the team members from the main precursor of this therapy, Edward Lynch[10]. Systematic reviews on the subject are unanimous about the fact that more scientific evidence is needed that this therapy can be accepted as an alternative therapeutic approach to early detected caries[5,10].
From the study it can be concluded that the caries induction protocol employed in this study was effective in the development of caries lesions. The application of ozone through HealOzone for 60 s, at a concentration of 2100 ppm was effective in eliminating caries bacteria. No bacterial DNA was detected after the application of ozone, followed by the daily application of the remineralizing products for 30 d. The treatment with ozone is advantageous in minimally invasive dentistry, it maintains the healthy tissue, and does not require anaesthesia, and with a simple and non-time-consuming process presents satisfactory results. More evidence is still needed before the cost-benefit ratio can be assessed.
To Paulo Ferreira e Nuno Brito for their help, during the experimental work.
Carious lesions occur due to acid dissolution of the enamel and/or dentin as a result of the metabolism of specific microorganisms, the main ones being Streptococcus mutans (S. mutans) and Lactobacillus fermentum (L. fermentum). Ozone is a powerful oxidant that has the ability to eliminate 99% of bacteria, fungi and viruses. Once the bacteria is eliminated, the remineralization may occurs in the treated area.
The bacterias S. mutans and L. fermentum are effective in producing tooth decay immersed in artificial saliva, and ozone was able to eliminate them. In this study the authors shows the capability of ozone in their elimination.
This is the first study to demonstrated the capability of bacteria elimination by the ozone, by polymerase chain reaction. Demonstrating that the ozone is highly effective. This study by demonstrated the bacteria elimination, shows that the ozone provides conditions for the remineralization.
Ozone therapy is advantageous in minimally invasive dentistry, because it maintains healthy tissue, does not require anesthesia and with a simple and non-time-consuming procedure gives satisfactory results.
The carie occurs due to acid dissolution of the enamel and/or dentin as a result of the metabolism of specific microorganisms, the main are S. mutans and L. fermentum. Ozone is oxidant that has the ability to eliminate bacteria, fungi and viruses.
The manuscript is good to add some information on the antibacterial effect of ozone in dental caries.
P-Reviewers Messora M, Ardila M, Pekkan G, Zhong LP, Abu El-Naaj I, Aggarwal V S- Editor Huang XZ L- Editor A E- Editor Zheng XM
| 1. | Fejerskov O, Kidd E. Cárie Dentária: A Doença e seu Tratamento Clínico. São Paulo: Livraria Santos Editora 2005; . [Cited in This Article: ] |
| 2. | Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51-59. [PubMed] [Cited in This Article: ] |
| 3. | Anderson MH, Bales DJ, Omnell KA. Modern management of dental caries: the cutting edge is not the dental bur. J Am Dent Assoc. 1993;124:36-44. [PubMed] [Cited in This Article: ] |
| 4. | Johansson E, Claesson R, van Dijken JW. Antibacterial effect of ozone on cariogenic bacterial species. J Dent. 2009;37:449-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Doméjean-Orliaguet S, Léger S, Auclair C, Gerbaud L, Tubert-Jeannin S. Caries management decision: influence of dentist and patient factors in the provision of dental services. J Dent. 2009;37:827-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Lynch E. Ozone: the revolution in dentistry. London: Quintessence Publishing Co. Ltd 2004; . [Cited in This Article: ] |
| 8. | Millar BJ, Hodson N. Assessment of the safety of two ozone delivery devices. J Dent. 2007;35:195-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Nogales CG, Ferrari PH, Kantorovich EO, Lage-Marques JL. Ozone therapy in medicine and dentistry. J Contemp Dent Pract. 2008;9:75-84. [PubMed] [Cited in This Article: ] |
| 10. | Azarpazhooh A, Limeback H. The application of ozone in dentistry: a systematic review of literature. J Dent. 2008;36:104-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Castillo A, Galindo-Moreno P, Avila G, Valderrama M, Liébana J, Baca P. In vitro reduction of mutans streptococci by means of ozone gas application. Quintessence Int. 2008;39:827-831. [PubMed] [Cited in This Article: ] |
| 12. | Pereira AC, Eggertsson H, Martinez-Mier EA, Mialhe FL, Eckert GJ, Zero DT. Validity of caries detection on occlusal surfaces and treatment decisions based on results from multiple caries-detection methods. Eur J Oral Sci. 2009;117:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Goel A, Chawla HS, Gauba K, Goyal A. Comparison of validity of DIAGNOdent with conventional methods for detection of occlusal caries in primary molars using the histological gold standard: an in vivo study. J Indian Soc Pedod Prev Dent. 2009;27:227-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Wong L, Sissons C. A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva. Arch Oral Biol. 2001;46:477-486. [PubMed] [Cited in This Article: ] |
| 15. | Di Gioia ML, Leggio A, Le Pera A, Liguori A, Napoli A, Siciliano C, Sindona G. Quantitative analysis of human salivary glucose by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;801:355-358. [PubMed] [Cited in This Article: ] |
| 16. | Polydorou O, Pelz K, Hahn P. Antibacterial effect of an ozone device and its comparison with two dentin-bonding systems. Eur J Oral Sci. 2006;114:349-353. [PubMed] [Cited in This Article: ] |
| 17. | Baysan A, Beighton D. Assessment of the ozone-mediated killing of bacteria in infected dentine associated with non-cavitated occlusal carious lesions. Caries Res. 2007;41:337-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Knight GM, McIntyre JM, Craig GG, Mulyani PS. The inability of Streptococcus mutans and Lactobacillus acidophilus to form a biofilm in vitro on dentine pretreated with ozone. Aust Dent J. 2008;53:349-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |