Peer-review started: September 1, 2016
First decision: November 10, 2016
Revised: December 20, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: March 28, 2017
Processing time: 209 Days and 0.2 Hours
The pathophysiology of chronic obstructive pulmonary disease (COPD) and Alpha one antitrypsin deficiency is increasingly recognised as complex such that lung function alone is insufficient for early detection, clinical categorisation and dictating management. Quantitative imaging techniques can detect disease earlier and more accurately, and provide an objective tool to help phenotype patients into predominant airways disease or emphysema. Computed tomography provides detailed information relating to structural and anatomical changes seen in COPD, and magnetic resonance imaging/nuclear imaging gives functional and regional information with regards to ventilation and perfusion. It is likely imaging will become part of routine clinical practice, and an understanding of the implications of the data is essential. This review discusses technical and clinical aspects of quantitative imaging in obstructive airways disease.
Core tip: Phenotyping emphysematous patients radiologically allow physicians to diagnose and deliver tailored and targeted therapies that are not possible with spirometry. When patients are divided into chronic bronchitis or emphysema on computed tomography (CT), they have significantly different clinical features and spirometry, demonstrating its ability to characterise phenotypic differences. CT offers accurate mapping and measurement of emphysema whereas magnetic resonance imaging (MRI) can provide functional information relating to ventilation and perfusion. This unique feature of MRI can help prognosticate patients in whom surgery is being considered. CT and MRI have both been sufficiently validated clinically and pathologically.
- Citation: Crossley D, Turner A, Subramanian D. Phenotyping emphysema and airways disease: Clinical value of quantitative radiological techniques. World J Respirol 2017; 7(1): 1-16
- URL: https://www.wjgnet.com/2218-6255/full/v7/i1/1.htm
- DOI: https://dx.doi.org/10.5320/wjr.v7.i1.1
The pathophysiology of chronic obstructive pulmonary disease (COPD) and Alpha one antitrypsin deficiency (AATD) is increasingly recognised as complex and lung function alone is insufficient for early detection, categorising and dictating management. Up to one third of the lung can be destroyed before respiratory impairment is detected by spirometry[1], meaning those with early disease may remain undiagnosed. Patients with emphysema and airways disease have significant clinical and physiological differences[2,3] and therefore phenotyping radiologically should allow for more individualised treatment with outcomes that are more meaningful to the patient.
The typical clinical phenotype of the patient with emphysema is that of significant breathlessness, hyperinflation and low body mass index. By contrast, the phenotype associated with predominant airways disease, i.e., chronic cough and infective exacerbations, has a different clinical spectrum within the umbrella term of COPD and requires separate recognition. Severity of symptoms and exacerbation rates are factors that directly impact patient’s quality of life, and therefore diagnosing and tailoring treatment early on will have the best outcome for symptom resolution and slowing disease progression.
Quantitative imaging techniques can phenotype patients into predominant airways disease or emphysema, providing an objective tool to detect disease earlier and more accurately. This is of increasing significance as targeted treatments beyond inhaled therapy (such as endobronchial valves and alpha one augmentation therapy) become available, which require careful patient selection. Computed tomography (CT) provides detailed information relating to structural and anatomical changes seen in COPD, whereas MRI/nuclear imaging gives functional and regional information with regards to ventilation and perfusion. Optical coherence tomography (OCT) gives microscopic detail of the airway wall where differences in the contribution of active inflammation and airway remodelling could be a useful biomarker and drug target.
This review article discusses these three imaging modalities, how they can be used to phenotype patients radiologically into emphysema and airways disease, and therefore individualise management. The clinical and pathological validation of each is demonstrated as well as the methods of quantification. Their individual merits and how they compare against one another is discussed, and trials that have used imaging as an outcome measure for treatments in COPD already are highlighted. It is the strengths of these techniques make it likely imaging will become part of clinical practice, and an understanding of the implications of the data is therefore essential for healthcare workers.
Spirometry measures such as the forced expiratory volume in 1 second (FEV1) alone are insensitive to early emphysematous change, and only moderately correlate to quality of life measures[4]. Therefore using symptoms and exacerbations alongside FEV1 to categorise COPD seems logical, which led to adoption of these methods in the most recent GOLD guidelines[5]. However this is not the only conceivable way in which severity could be described; CT scanning has potential to delineate additional phenotypes complementing GOLD severity stage.
Studies have shown measures of airways disease on CT such as increased wall thickening are distinct from those of low density and parenchymal destruction seen in emphysema and therefore can be used to subdivide COPD patients into phenotypes[3,6]. When patients have been classified by CT into emphysema or airways predominant phenotypes, there are significant differences between the groups for lung function, symptoms and exacerbation rates. Table 1 lists relevant trials that have divided patients radiologically and the clinically different variables between the groups. Han et al[7] demonstrated differences in the rate of exacerbations between the emphysema and airway predominant phenotypes, and that the risks were independent between the two groups. This adds evidence to the increasing recognition that the two disease states are separate and the driving pathology behind them may be different.
| Ref. | HRCT defined phenotypes | Variables studied | Significant variable difference |
| Kitaguchi et al[8], 2006 | A: Little or none of either emphysema or BWT E: Emphysema but no BWT M: Emphysema and BWT | Gas exchange Gas transfer Lung function Response to beta-agonist Response to treatment with ICS Sputum cell differentiation | A: ↑ BMI ↑DLCO ↓ hyperinflation ↑ reversibility ↑response to ICS ↑ % of sputum eosinophils E: No response to ICS M: ↑ response to ICS ↑ % of sputum eosinophils |
| Fujimoto et al[9], 2006 | A: Little or none of either emphysema or BWT E: Emphysema but no BWT M: Emphysema and BWT | Exacerbation rates Gas exchange Gas transfer Hospital admissions Lung function Response to beta-agonist Symptoms | M: ↑ volume of sputum, exacerbation rate and admission to hospital |
| Pistolesi et al[10], 2008 | From derivation set, created new validation set Group A and B | CT parameters Gas exchange Gas transfer Lung function | A: ↓ FEV1, ↑ TLC ↓ DLCO. ↑ pixel index (threshold -950HU) B: ↑ BMI purulent sputum worse bronchial wall thickening |
| Han et al[7], 2011 | Emphysema predominant or Airway predominant | BWT Exacerbation rates lung function % emphysema | Emphysema Predominant (> 35% -950HU): ↓ FEV1 and 6MWD ↑ SGRQ and MRC grade For every 5% ↑ in emphysema, 1.18 fold ↑ exacerbation frequency Airways predominant: For 1 mm ↑ in segmental BWT 1.84 fold ↑ in exacerbation frequency |
| Subramanian et al[3], 2016 | Emphysema dominant, airways disease dominant, mixed pathology and mild disease | Blood parameters CT parameters Gas exchange Gas transfer Lung volumes Spirometry | Compared with airway disease dominant group, emphysema dominant group had ↑ lung volumes, ↓ gas transfer ↓ pO2 + pCO2↓BMI ↑Hb No difference between age, and smoking history between the groups |
| Da Silva et al[2], 2016 | Emphysema or airways disease | Clinical + functional evaluation HRCT | Emphysema group: ↑ airflow obstruction ↓ BMI ↓ 6MWD |
Table 2 summarises the current treatment recommendations from BTS and GOLD once patients have been phenotyped. There is of course overlap between the groups, with those patients with an emphysematous predominant phenotype experiencing more frequent exacerbations, and patients should continue to be evaluated individually. This overlap is highlighted in the table.
| CT phenotype | CT defining features | Clinical features | Findings | Treatments | Ref. |
| Emphysema | ↓ Perc15 Emphysema Centrilobular Panlobular Paraseptal Bullous | Health status | ↓ BMI[2] ↑ SGRQ + MRC[7] | Rehabilitation Nutritional support Palliative care | GOLD 2016[5] |
| Exercise tolerance | ↓ 6MWD[2] ↓ pO2↓ pCO2[3] | Rehabilitation Maintenance of physical activity Oxygen | GOLD 2016[5] | ||
| Lung function | ↑ TLC ↓ KCO ↓ FEV1/FVC | LAMA/LABA LVRS/BVLS Transplant Bullectomy[11] LVRS[11] | GOLD 2016[5] NICE 2010[11] | ||
| Symptoms | ↑ Hb[3] No significant response to ICS[8] | Theophylline Rehabilitation typically MRC > 3 | GOLD 2016[5] NICE 2010[11] | ||
| Airways disease | Exacerbation frequency/ severity | ↑ Exacerbations hospital admissions[7] | LABA/phosphodiesterase-4 inhibitor LAMA/phosphodiesterase-4 inhibitor Mucolytics Add in ICS Prophylactic antibiotics | GOLD 2016[5] NICE 2010[11] Brown et al[12], 2007 Fabbri et al[13], 2009 Calverley et al[14], 2009 Herath et al[15], 2013 | |
| Lower wall area/body Surface area ratio (WA/BSA) Lower luminal area/BSA Higher %WA | Symptoms | Significant response to ICS+ Significantly higher % of sputum eosinophils[8] Peribronchial thickening[10] Air trapping | Physiotherapy and active breathing techniques Mucolytics Roflumilast Bronchodilators | NICE 2010[11] |
Emphysema as a result of smoking/inhalation of noxious gases most frequently results in the centrilobular distribution of emphysema which begins in the upper zones. However, their relative high V/Q ratio means they contribute significantly less to the overall PFT result and therefore in usual COPD isolated to purely the upper zones, the PFTs may seem relatively normal earlier on. Nakano et al[16] showed accordingly that the correlation between FEV1 and %LAA was weakest in the upper zones, but as the emphysema often begins in the upper zones, there is a higher association for DLCO here and centrally rather than peripherally. Similar findings were demonstrated by Parr et al[17] in AATD patients that basal distribution is associated with greater impairment of FEV1 (P = 0.002), but less impairment of gas exchange (P = 0.016), and Aa gradient (P = 0.007). Given the lung function variation between different lung regions the authors warn of using a single physiological parameter as a measure of severity as it may introduce bias.
Castaldi et al[18] found that panlobular rather than centrilobular distribution was associated with stronger associations with lung function and QoL than CT lung density, demonstrating that the distribution of disease has an independent effect on severity. AATD typically occurs in a panlobular distribution with basal predominance, and Dawkins et al[19] showed that for these patients, basal distribution carried a higher mortality risk. Finally, in patients randomised to the medical arm of the National Emphysema Treatment Trial, the authors demonstrated that a greater proportion of emphysema in the lower lung zone vs upper lung zone was predictive of mortality (P = 0.005)[20].
Lung volume reduction surgery: Using CT measurements both visually and quantitatively allow for more careful selection of COPD patients when considering lung volume reduction surgery (LVRS). Selecting patients appropriately to either medical or surgical treatments can reduce the associated mortality. The National Emphysema Treatment Trial randomised 1218 severe emphysema patients to either LVRS or medical management[21]. They visually scored the CT scans of patients as being either predominantly upper lobe or lower lobe, and assessed exercise capacity. They found that in a carefully selected population of those with upper lobe emphysema and a low exercise capacity, those in the surgical treatment arm had a significantly lower mortality (RR for death 0.47, P = 0.005). However, in those with predominantly lower lobe emphysema but a high exercise capacity, those randomised to the surgical arm did worse (RR for death 2.06, P = 0.02). Therefore, LVRS confers a survival advantage in carefully selected patients, but there is associated higher mortality with no significant increase in functional status in those with non-upper zone predominant disease. Gierada et al[22] have also demonstrated that those upper lobe predominant emphysema, in a heterogeneous have a two-fold or more average increase in FEV1 following LVRS.
Predicting post-operative FEV1: CT density masking to quantify the severity of emphysema is linked to favourable post-operative outcomes. Sverzellati et al[23] applied a density mask to 9 COPD patients awaiting lobectomy for lung cancer, along with spirometry. With specific equations, they predicted the post-operative FEV1 using both values and found quantitative CT was superior to lung function (r = 0.9). Gierada et al[24] used various LAA measurements and determined that a 75% LAA or greater for -900HU threshold, or 25% at -950HU were associated with improved outcomes post-operatively including a > 50% improvement in FEV1 and 2 fold increased six minute walk distance.
Finally the ratio of upper to lower lobe emphysema is of particular importance in assessing predicted post-operative FEV1 following bilateral LVRS. Consistent with the fact that upper lobe predominance is associated with better outcomes, Flaherty et al[25] found that the CT emphysema ratio (CTR) was the best single predictor of a successful 12% increase in FEV1 (absolute value 200 mL). Importantly, the highest CTR scores (> 2.5) were associated with a greater than 90% specificity at each time point up to 36 mo, although the sensitivity was low. The positive predictive value of this threshold was at least 75% up to 36 mo after surgery. The negative predictive value remained moderate at all thresholds throughout 36 mo of follow-up.
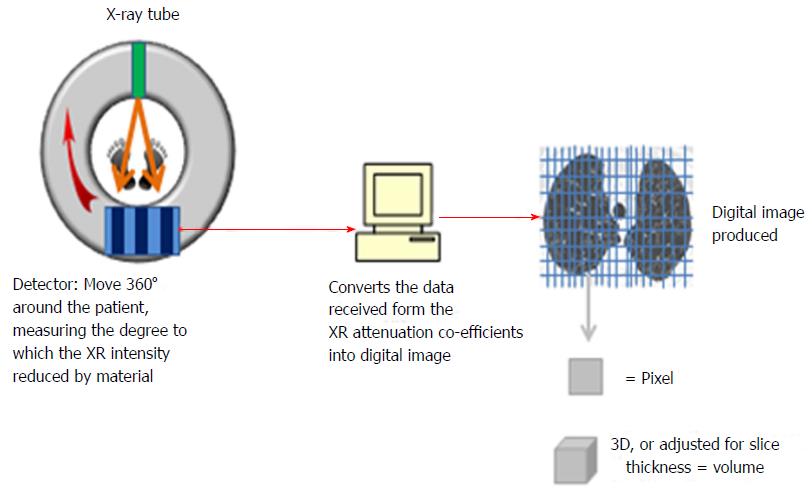
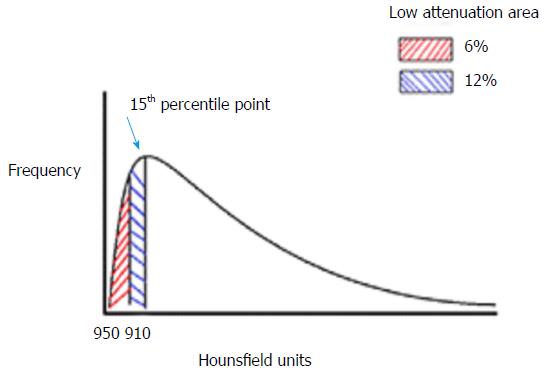
CT densitometry is the method of quantifying the severity of emphysema using dedicated software. Figure 1 demonstrates how the CT images are digitally produced. X-rays are emitted and passed through the subject and received by detectors that calculate how much the intensity has been reduced by the tissue. These attenuation co-efficients are then converted into a digital image in the form of a matrix consisting of many small data sets. Each small square in the matrix is a pixel, and in 3D with volume adjustment is a voxel. Each pixel is assigned a value in hounsfield units (HU) from -1000 representing the least possible density/attenuation, i.e., air and 1000 representing the highest, i.e., solids. These pixels or voxels can be plotted on a histogram as shown in Figure 2. There are two ways of reading the severity from this histogram. The first is the value of where the 15th percentile point lies on the curve (Perc15) and is the most preferred value in trials quoting density, as it is most accurate and sensitive to change[17,26-28]. The second method is to calculate the percentage under the curve that represents the low attenuation area for a selected threshold, e.g., -910HU or -950HU. These and other values are used in studies quoting density, and Table 3 demonstrates trials that have sought to ascertain the most valid method in both AATD and COPD.
| Condition | Type of study | 910 | 950 | Perc15 | Conclusion of superior measure | Ref. |
| Alpha one | RCT | x | x | 950 | Parr et al[29] | |
| RCT | x | x | 950 and Perc15 | Parr et al[30] | ||
| RCT | x | x | x | Perc15 | Parr et al[26] | |
| Review | x | x | x | Perc15 | Hogg et al[28] | |
| Chronic obstructive pulmonary disease | RCT | x | x | x | Perc15 | Shaker et al[31] |
| Review | x | x | Perc15 | Dirksen et al[27] | ||
| RCT | x | x | 950 | Chong et al[32] |
Pathological correlations: The ability of density analysis to accurately assess the degree of emphysema has been validated on pathological studies. Müller et al[33] in 1988 showed a strong correlation between density mask results and an assigned emphysema pathology score (1 to 100) in 28 patients who had undergone lobar resection for a lung tumour (r = 0.83, P < 0.001). In a larger group of patients who had undergone resection for similar reasons, Gould et al[34] also demonstrated a strong correlation between emphysema measures quantitatively on imaging and that on resected specimens (r = 0.77)[35,36].
Clinical correlations: Numerous studies have shown significant correlations between CT measures of emphysema (Perc15 and %LAA 950) and FEV1 and DLCO[37-40], as well as measures of exercise tolerance, e.g., MRC grade and 6 min walk distance (6MWD)[41-45]. There are also significant correlations with frequency of exacerbations and ultimately mortality[19,41,46-48]. In the NELSON trial (Dutch and Belgium Lung Cancer Screening Trial), Mohamed Hoesein et al[35,36] have shown smokers who normal lung function demonstrated evidence of emphysema on CT concluding that CT is a more sensitive in detecting emphysema than PFTs. However, the R2 value between CT density and FEV1 even when adjusted for other variables remains 0.3-0.68 indicating that the parenchymal disease detected by CT density only contributes for 30% to 68% of the total variation[18,49-51]. Therefore other factors including small airways disease must additionally contribute to the altered lung function seen.
Quantification: Luminal area (LA) and the wall area (WA) (expressed as a percentage (%WA = WA/LA + WA*100)[52] can be derived from CT measurements, as well as bronchial wall thickness (BWT) as the square root of WA adjusted for the internal perimeter[53,54] (Figure 3). Airway measurements are often based on the full width at half maximum principle[55,56]. However, this method is known to overestimate the value of wall thickness and various algorithms for quantification are modifications are of this[57,58].
Validation: Nakano et al[52,55] demonstrated on histology slices that those airways with an internal diameter of greater than 0.75 cm could accurately predict the dimensions of small airways with an internal diameter of 1.27 mm (r = 0.57, P < 0.01) and in particular measurements from the right S1 segmental bronchus. Airway wall thickening as measured by CT is related to obstructive spirometry[59-62], and chronic sputum production is associated with increased likelihood of an exacerbation leading to a hospital admission[63], and death from a pulmonary infection[64]. Chronic bronchitis (cough and sputum production for at least > 3 mo in 2 consecutive years)[5] has a greater mean %WA and internal perimeter, and is associated with higher exacerbation and mortality rates[53,65,66].
CT quantification variability: The potential pitfall of CT analysis is that the various components must all be equal in order to compare like for like. These factors include using the same software programme[67], the same reconstruction algorithm[68-70], appropriately calibrating the scanner[26,29] and adjusting for volume[27,32,71]. If CT density logistics are standardised, then scans may be compared longitudinally to measure treatment effect, and combined from different centres. A detailed review of CT noise reduction by Dirksen et al[27] 2008 recommended using a soft reconstruction algorithm, with a slice thickness of 3-5 mm, at a low radiation dose using a phantom. As for volume adjustments, there is no general consensus as to which method is preferable, though physiologically adjustment using the patient’s own volume measurements seems more intuitive.
Trials: CT has been used as an alternative outcome measure in therapeutic trials for patients with emphysema. When performing power calculations in the EXACTLE study using CT density as a measure of response to alpha one augmentation therapy, the author’s calculated 494 patients would need to be recruited in each treatment arm for 3 years using FEV1 as the primary outcome measure[72]. In the RAPID trial however, they calculated 180 patients distributed over the two treatment arms would provide a power of at least 80% using two sided P value of 0.05[73].
CT has been used to measure response in both usual COPD and in Alpha 1 anti-trypsin deficiency and the summary detailing CT measure used, outcomes and the strengths and weaknesses of each study are presented in Table 4. Notably, in AATD the recent RAPID trial was the first RCT to demonstrate a significant improvement in lung density with alpha one augmentation therapy. Stockley et al[74] pooled the data from the two RCTs by Dirksen et al in 1999 and 2009 (EXACTLE), and with the increase in statistical power , augmentation therapy increased the lung density as measured by 2.997 g/L in comparison to the placebo arm (95%CI: 0.669 to 3.926, P = 0.006).
| Ref. | Study design | Pt N° | Duration | CT measure | Drug | Result |
| Usual COPD | ||||||
| Shaker et al[75] | RCT | 254 | 2-4 yr | Perc15 and -910HU | Budesonide or placebo | Annual fall in Perc15 ↑in the placebo arm vs budesonide (P = 0.09) Annual increase in -910HU↓in the budesonide arm (P = 0.02) |
| Hoshino et al[76] | RCT | 54 | 16 wk | %WA, LA, BWT | Tiotropium, Indacaterol or both | Combination therapy resulted in a ↓in %WA and wall thickness (P < 0.01) |
| Nordenmark et al[77] | RCT | 36 | 12 wk | BWT, air trapping index and %WA | Reversible neutrophil elastase inhibitor 60 mg BD | No difference |
| Shimizu et al[78] | Inter-ventional trial | 23 | 1 wk | Airway inner luminal area | SFC | Ct detected the significant change in airway inner luminal area r = 0.65, P < 0.001 |
| Alpha 1 Antitrypsin deficiency | ||||||
| Stolk et al[79] | RCT | 262 | 1 yr | Perc15 | Parlovarotene | No benefit on lung density |
| Mao et al[80] | RCT-pilot study | 20 | 9 mo | -910HU | ATRA | No benefit |
| Roth et al[81] | RCT feasibility study | 148 | 9 mo | -910HU | Patients received ATRA either LD, HD, 13-cRA or placebo | No definitive clinical benefits |
| Dirksen et al[82] | RCT | 32 | 3 yr | Perc15 | Alpha1-antitrysin | CT analysis showed a non-significant trend towards a favourable effect. CT lung density twice as sensitive as PFTs |
| Dirksen et al[72] (EXACTLE) | RCT | 77 | 2-2.5 yr | Perc15 | Prolastin | CT densitometry more sensitive measure for the detection of emphysema progression than PFTs or health status indices |
| Chapman et al[73] | RCT | 180 | 2 yr | Perc15 | Alpha 1 proteinase inhibitor | Annual rate of density decline at TLC ↓in treatment group (P = 0.03) |
Magnetic resonance imaging (MRI) measures the behaviour of protons once a strong magnetic force is applied. The lungs have therefore been notoriously difficult to image due to the abundance of air and low proton density. However, technology has advanced so that MRI may capture changes in a much shorter time window and use inhaled gases (oxygen and hyperpolarised helium/xenon) that alter the proton behaviour in different ways, so that disease and heterogeneity in the lung may be detected. The benefits of MRI over CT and PFTs are the ability to acquire functional information with regards to ventilation, perfusion and alveolar diffusion, and any regional differences. MRI therefore could offer an attractive solution to evaluating underlying pathology and targeting treatment.
Airways disease: MRI is already used to visualise airway changes in more detail in cystic fibrosis, e.g., inflammation, mucus plugging and bronchiectasis[83]. In this capacity, MRI is superior over CT with its ability to more accurately differentiate soft tissue, e.g., remodelling/inflammation[84,85]. The increased airway resistance seen in small airways disease in asthma has also been evaluated by MRI. Where bronchoconstriction has resolved clinically MRI assessment of ventilation demonstrated focal, fixed obstructive defects that may be reversible with targeted therapies, e.g., broncho-thermoplasty[86]. The ability of MRI to accurately measure the resultant degree of hyperinflation and air trapping has obvious potential clinical applications in COPD, e.g., endobronchial coils/LVRS.
Emphysema: The apparent diffusion co-efficient (ADC) measured in MRI is a reflection of the amount of measured molecular movement, with more movement in emphysema where there are larger air sacs and destroyed alveolar walls[87]. Therefore a high ADC indicates more severe emphysema and could be used either diagnostically or for assessment longitudinally. As there is increased interest in using CT density as a direct measure of parenchymal response to augmentation therapy in AATD, ADC would be another potential option of measuring alveolar changes.
Vascular remodelling secondary to hypoxic vasoconstriction is likely part of a more systemic process associated with COPD. Perfusion studies, i.e., dynamic contrast enhanced MRI may therefore act as another useful imaging biomarker to detect and prevent further disease[88]. For example, where there is a perfusion defect with preserved ventilation, then this maybe a target for bronchial dilators. Similarly where there is preserved perfusion, up to 20% have emphysematous regions which therefore may act as a map for targeted interventional therapies, e.g., Bronchoscopic Lung Volume Reduction Surgery (BVRS)[89]. Jobst et al[90] showed the association between oxygen enhanced MRI and contrast enhanced MRI r value is 0.52 therefore there is a link but there are other factors in play such that one is not a surrogate for the other. A summary of how MRI can help phenotype COPD is given in Table 5.
| Phenotype | MRI modality | Findings | Suggested treatments |
| Airways disease | Hyperpolarised MRI | Detailed anatomical information of airway inflammation, oedema and mucus plugging[84,85] | Nebulised antibiotics Chest clearance techniques[83] |
| Regional information re. lung volumes, e.g., focal bronchoconstriction | Broncho-thermoplasty[91] BVRS | ||
| Emphysema | Hyperpolarised MRI | Global high ADC[87] | Early disease detection |
| Low PaO2[92] | Future alpha one augmentation therapy1 | ||
| Oxygen enhanced MRI | ↑↓Relative enhancement signal[93,94] | Targets for resection | |
| Early emphysema detection | |||
| Dynamic contrast MRI | Global microvascular reduction blood flow[95] | Lifestyle moderation | |
| Focal defects, small pulmonary emboli | Anticoagulation | ||
| Increased pulmonary pressure | Treat as pulmonary hypertension |
Clinical validation: MRI findings from the various modalities have been correlated with lung function and CT density in numerous studies (Table 6), R values for FEV1 ranging from 0.61-0.72 and 0.45-0.9 for DLCO.
Pathological validation: One of the pathological hallmarks of emphysema is the destruction of alveolar walls and dilatation of respiratory bronchioles[103,104]. Histologically this may be measured by the surface area to volume ratio (SA/V) and this was compared with MRI findings in five patients who had undergone bilateral lung transplant for end-stage COPD. Using He-MRI and measuring the ADC, the correlation between histology and MRI findings was very strong (r = 0.96)[105]. Morino et al[106] in an animal model measured the correlation between dynamic contrast MRI and alveolar enlargement as defined by the mean linear intercept (Lm) and this demonstrated a slightly weaker correlation though still significant (r = -0.77, P < 0.001).
Oxygen enhanced MRI: Proton MRI measures the longitudinal and transverse relaxation times (T1 and T2 respectively) after the strong magnetic force has been applied[85]. Oxygen molecules shorten the T1 relaxation time, and mapping the degree of change can depict the heterogeneity of ventilation within the lungs[107]. The mean wash in time maps of oxygen created significantly correlates to FEV1 and FEV/FVC ratio (-0.74 for both) demonstrating its strong relationship to current measures of ventilation[93]. The degree of altered signal change as depicted by the mean relative enhancement signal has a stronger correlation with gas transfer (r = 0.83)[94] and therefore as well as acting as a map of ventilation, oxygen enhanced MRI may also reflect alveolar-capillary gas transfer 4214[93]. O2 MRI has also been demonstrated to be able to separate emphysematous patients from asymptomatic smokers[92].
Benefits of offering oxygen enhanced MRI particularly over other inhaled gases acting as a contrast is that it may technically be implemented at most centres without the need for specialist equipment but would require specialist software[85]. There is no breath holding manoeuvres required which is preferable in COPD patients, the signal artefacts are relatively low as is the overall cost. However, the scanning time is considerably longer (30 min vs 5 min) and the repeatability has not yet been confirmed[108].
ADC: Using spin technology to hyperpolarise inhaled gases through polarised laser light, the signal enhancement is amplified and then measured[107]. The larger the range of movement of the gas particles, the higher the ADC. Therefore in emphysematous alveoli where there is destruction of attachments, there will be more movement, and a higher ADC[87]. For this reason ADC can give information about alveolar anatomy unlike HRCT. ADC correlates with lung function, and is sensitive at detecting differences between emphysematous and non-emphysematous patients[109].
PaO2: Based on the rate of polarised helium decay in relation to regional oxygen concentration, and the diffusion across alveolar membranes, the alveolar partial pressure of PaO2 can be calculated[87,110]. This can detect changes in asymptomatic current smokers, as well as correlating with lung function, SGRQ and 6MWD[92].
Helium ventilation MRI: Following a breath hold, the thoracic volume can be calculated together with He ventilated images in order to calculate the percentage ventilated volume and ventilation defect volume% (VDV%)[85,111]. This was able to discriminate between healthy smokers and those with COPD in a 2015 trial, but there was no significant correlation with spirometry[111].
The main drawbacks of hyperpolarised helium MRI are that hyperpolarised helium is in limited supply and expensive. The technique requires specialist centres with appropriately trained radiologists[85], and patients are required to breath hold for around 20 s, which is very challenging for patients with COPD. However, hyperpolarised MRI has no radiation dose and gives high spatial resolution. It provides detailed regional information about gas exchange and ventilation, and its repeatability has been established[108].
Perfusion: Detecting early changes in the vascularity of patients at risk of developing emphysema could potentially act as another early biomarker of disease. Dynamic Contrast Enhanced MRI involves injecting contrast and measuring the amount of time taken for the contrast to pass through the pulmonary circulation, i.e., the longer the time taken, the more flow restriction there must be. Transit time of blood through the pulmonary circulation is notoriously rapid, though MRI with ultra-fast capabilities is able to capture this[112,113]. Not only is this technique feasible it also correlates to clinical parameters. Hueper et al[95] demonstrated this is possible on a microvascular scale, and demonstrated evidence of disease in patients with COPD in areas of lung not emphysematous on CT.
Trials: Multiple studies have demonstrated that MRI correlates more strongly with PFTs than CT does (Table 7). However at this early stage it still remains unclear if MRI is more sensitive, as the literature is not as advanced.
| Ref. | Year | Pt No. | Variables | Results |
| Ley et al[96] | 2004 | 13 | ADC and EI vs FEV1 | ADC vs FEV1, R = 0.7 EI vs FEV1, R = 0.5 MLD vs FEV1, R = 0.4 |
| Ohno et al[93] | 2008 | 71 | O2 enhanced MRI (mean wash in time and relative enhancement ratio), CT defined lung volumes vs lung function | Mean wash in time vs FEV1, r = -0.74 Relative Enhancement Ratio vs KCO, r = 0.66 CT lung volume vs FEV1, r = 0.61 CT lung volume vs KCO, r = 0.56 |
| Van Beek et al[98] | 2009 | 94 | ADC and MLD vs FEV1/FVC and DLCO | ADC vs Fev1/fvc, r = 0.5 MLD vs Fev1/fvc, r = 0.52 ADC vs DLCO, r = 0.59 MLD vs DLCO, r = 0.29 |
| Diaz et al[38] | 2009 | 27 | ADC and EI vs FEV1 and DLCO | ADC vs FEV1, r = 0.67 EI vs FEV1, r = 0.55 ADC vs DLCO, r = -0.82 Perc15 vs DLCO, r = 0.6 |
| Quirk et al[114] | 2011 | 30 | Hyperpolarised He vs CT density in at risk smokers | Lung morphometry vs %LAA 950: Significant difference seen in those still smoke, not on CT |
| Xia et al[101] | 2014 | 55 | +ve rate of Perfusion defects vs CT changes | Early COPD: MRI detected 8/8, vs CT 3/8 P = 0.003 Mod. COPD: MRI detected 9/9, vs CT 7/9 P = 0.47 |
| Hueper et al[95] | 2015 | 144 | DCE-MRI vs CT density | PMBF vs %LAA 950: Evidence of non-linearity, P = 0.015 |
Nuclear imaging techniques provide useful information regarding ventilation and perfusion which can be used for assessing emphysematous lungs and regional contributions. There is no significant scope for information regarding soft tissue and fine anatomical measurements, and therefore whilst can measure the severity of emphysema to a certain degree, it is not able to phenotype in the same way as CT/MRI.
Positron emission tomography (PET) measures gamma rays emitted from molecules labelled with radioisotopes, and an image of where the molecules concentrated is created. Most commonly PET is used in oncology to look for the extent and spread of malignant disease by using labelled glucose, and determining metabolically active sites. There has been increased recognition of the role of increased neutrophil activity in COPD. 18-FDG has been used as a surrogate marker of neutrophilic inflammation in order to ascertain if it could be a useful biomarker[115]. The authors found uptake was significantly higher in the upper zones in those with COPD compared with healthy controls (P = 0.009) and correlated with lung function. They additionally tried to use PET-CT as an outcome measure for augmentation therapy in patients with AATD but found no significant difference in readings before and after treatment.
Vidal Melo et al[116] labelled and injected nitrogen (13-NN-labelled saline) in 15 patients with COPD. Nitrogen has very low solubility in blood and therefore in the lungs diffuses rapidly in to alveolar space[117]. PET scanning with this method exploits these features of nitrogen so that areas where there is high concentration of nitrogen in the lung initially must be well perfused. Furthermore, once the patient breathes, nitrogen is washed out and therefore areas with retained nitrogen are less well ventilated.
Using this method, the labelled radioisotope emits one rather than two gamma rays during the decay process, and for this reason has less radiation but subsequently less resolution. Labelled agents are inhaled (e.g., xenon) and injected (e.g., technetium DTPA) and the contributions of ventilation/perfusion ascertained. The merits of both tests are summarised in Table 8. The clinical application of single photon emission CT in COPD are largely sub-divided into pre-operative assessment for those considered for lung volume reduction surgery (including bullectomy), and for the early detection of emphysema.
| Modality | Advantages | Disadvantages |
| PET | Increased resolution | Cyclotron and radiopharmaceutical preparation Rapid repeat testing not possible[87] |
| SPECT | Lower cost More widely available. Dynamic SPECT give time course of ventilation | Lower spatial and contrast resolution |
Assessing V/Q mismatch can give functional information about regions of inadequate ventilation not visible on CT, and is cheaper and more convenient than MRI. Suga et al[118] demonstrated its usefulness particularly in the pre-operative assessment for bullectomy, and the valuable information gained regarding function of lung tissue within and surrounding the bullous before it is resected. A retrospective analysis was performed on patients who had undergone endobronchial valve placement (EBVs) and perfusion as measured by perfusion scintigraphy. They found that those with lower baseline local perfusion benefitted from EBV placement independent of the lobe, summarising that assessing a patients perfusion pre-operatively may be a method of calculating predicted benefit[119]. Finally, Sudoh et al[120] compared PET/CT to PPO segment counting in predicting post-operative outcomes but found no superiority.
The pathobiological theory that COPD is a systemic disorder with ongoing inflammation and microvascular changes is exploited in assessment of V/Q mismatch. Changes in perfusion may well precede visible changes on CT and certainly lung function, and has therefore potential to diagnose and initiate treatment earlier if required[121,122].
A summary of correlations between SPECT and various other clinical measures is shown in Table 9. There is moderate-strong correlation with FEV1 but less so with gas transfer and MRI (0.45-0.67)[123]. With regards to sensitivity and specificity for emphysema diagnosis, MRI would seem superior to perfusion scintigraphy[124]. There is a very small amount of work regarding pathological validation and nuclear imaging, but so far these are animal models only[125,126].
OCT works through a bronchoscope and using near infra-red rays instead of soundwaves (used in ultrasound), can give extremely precise image of the airway. Using two light beams with one shone onto a mirror to act as a standard measure, the other beam is directed into the tissue and the pattern and the amount that is reflected back is interpreted as an image[129]. It can visualise around 2-3 mm and gives almost a histological view of the airway wall[130]. Unlike ultrasound which requires a water medium and direct contact to operate, the OCT probe doesn’t need to be pressed against the airway wall. Better than CT or MRI, OCT can give a clear view of the airway wall components, i.e., the submucosa, the smooth muscle, and cartilage[131]. In asthma and COPD where there is ongoing inflammation and subsequent airway remodelling, OCT would serve a purpose to view the causes of airway wall thickening and intra-luminal narrowing. The technology is already used in ophthalmology and cardiology, but in respiratory despite having promising capacity, it is still in its research phase.
OCT can only image as far as the device carrying it (usually a bronchoscope) can go. Therefore this technology is limited to the airways and not the parenchyma. However, through creating a pleural window, and miniaturised devices within a 30 gauge needle, the probe can be inserted through the chest wall[132]. The potential for phenotyping patients in COPD could be assessing the amount of active inflammation, airway remodelling/fibrosis to assess why there are regional problems with sputum production or bronchiectasis. Those in favour of OCT have optimistic views that assessing airway pathology would make way for targeted therapeutic interventions (Table 10). OCT is in its infancy however, and more trials are needed.
| Condition | OCT method | Findings | Suggested treatments |
| Chronic bronchitis | Endoscopic | Increased volume of submucosal glands; central airway inflammation[133-135] | Investigations directed towards asthma overlap syndrome; targeted inhaled steroids |
| Emphysema | Anatomical OCT | Can visualise collapsibility dynamically[136] | Bronchodilators; smoking cessation |
There have been two studies that have compared OCT to FEV1, both from the same group in 2008 and then 2014[137]. They find the correlation in these two studies between FEV1 and OCT to be strong (-0.75 and -0.78 respectively) though the 2014 study only found a significant correlation in the male subjects. The slope of the line plotted between OCT and FEV vs CT and FEV1 was steeper, and therefore the authors concluded OCT’s potential superiority over CT for assessing small airways disease.
Tsuboi took 7 human lungs immediately resected for lung cancer, and placed the OCT camera down. They showed that the images of the airway and of the alveolus taken from OCT matched though seen on histology, i.e., definition between submucosa, smooth muscle and cartilage, and then the structure of the alveoli and its adjacent bronchial wall. In a small number of subjects, no statistical analyses were performed but the results are visually convincing[131,138].
Quantitative imaging techniques provide sensitive, repeatable and accurate information in COPD patients, and are likely to be used increasingly for both diagnosis and measuring the response to treatment. There are differences in the application of each modality and common pitfalls to be recognised, and standardising each of them is necessary before they can become a bigger player in clinical practice.
Manuscript source: Invited manuscript
Specialty type: Respiratory system
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Andell P, Cho W, Meteran H, Pavasini R S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Uppaluri R, Mitsa T, Sonka M, Hoffman EA, McLennan G. Quantification of pulmonary emphysema from lung computed tomography images. Am J Respir Crit Care Med. 1997;156:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 204] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | da Silva SM, Paschoal IA, De Capitani EM, Moreira MM, Palhares LC, Pereira MC. COPD phenotypes on computed tomography and its correlation with selected lung function variables in severe patients. Int J Chron Obstruct Pulmon Dis. 2016;11:503-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Subramanian DR, Gupta S, Burggraf D, Vom Silberberg SJ, Heimbeck I, Heiss-Neumann MS, Haeussinger K, Newby C, Hargadon B, Raj V. Emphysema- and airway-dominant COPD phenotypes defined by standardised quantitative computed tomography. Eur Respir J. 2016;48:92-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Westwood M, Bourbeau J, Jones PW, Cerulli A, Capkun-Niggli G, Worthy G. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res. 2011;12:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256-1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3133] [Cited by in F6Publishing: 3702] [Article Influence: 161.0] [Reference Citation Analysis (0)] |
| 6. | Nakano Y, Müller NL, King GG, Niimi A, Kalloger SE, Mishima M, Paré PD. Quantitative assessment of airway remodeling using high-resolution CT. Chest. 2002;122:271S-275S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, Criner GJ, Kim V, Bowler RP, Hanania NA. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261:274-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 8. | Kitaguchi Y, Fujimoto K, Kubo K, Honda T. Characteristics of COPD phenotypes classified according to the findings of HRCT. Respir Med. 2006;100:1742-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Fujimoto K, Kitaguchi Y, Kubo K, Honda T. Clinical analysis of chronic obstructive pulmonary disease phenotypes classified using high-resolution computed tomography. Respirology. 2006;11:731-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Pistolesi M, Camiciottoli G, Paoletti M, Marmai C, Lavorini F, Meoni E, Marchesi C, Giuntini C. Identification of a predominant COPD phenotype in clinical practice. Respir Med. 2008;102:367-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | National Institute for Health and Care Excellence (NICE). Chronic obstructive pulmonary disease in over 16s: diagnosis and management. NICE. 2010;Abstract. [PubMed] [Cited in This Article: ] |
| 12. | Brown WM. Treating COPD with PDE 4 inhibitors. Int J Chron Obstruct Pulmon Dis. 2007;2:517-533. [PubMed] [Cited in This Article: ] |
| 13. | Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, Rabe KF. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 394] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 14. | Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 534] [Cited by in F6Publishing: 520] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 15. | Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;CD009764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Nakano Y, Sakai H, Muro S, Hirai T, Oku Y, Nishimura K, Mishima M. Comparison of low attenuation areas on computed tomographic scans between inner and outer segments of the lung in patients with chronic obstructive pulmonary disease: incidence and contribution to lung function. Thorax. 1999;54:384-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Parr DG, Stoel BC, Stolk J, Stockley RA. Pattern of emphysema distribution in alpha1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med. 2004;170:1172-1178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Castaldi PJ, San José Estépar R, Mendoza CS, Hersh CP, Laird N, Crapo JD, Lynch DA, Silverman EK, Washko GR. Distinct quantitative computed tomography emphysema patterns are associated with physiology and function in smokers. Am J Respir Crit Care Med. 2013;188:1083-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Dawkins P, Wood A, Nightingale P, Stockley R. Mortality in alpha-1-antitrypsin deficiency in the United Kingdom. Respir Med. 2009;103:1540-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, DeCamp MM, Benditt J, Sciurba F, Make B. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;12:1326-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 315] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 21. | Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059-2073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1262] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 22. | Gierada DS. Radiologic assessment of emphysema for lung volume reduction surgery. Semin Thorac Cardiovasc Surg. 2002;14:381-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Sverzellati N, Chetta A, Calabrò E, Carbognani P, Internullo E, Olivieri D, Zompatori M. Reliability of quantitative computed tomography to predict postoperative lung function in patients with chronic obstructive pulmonary disease having a lobectomy. J Comput Assist Tomogr. 2005;29:819-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Gierada DS, Yusen RD, Villanueva IA, Pilgram TK, Slone RM, Lefrak SS, Cooper JD. Patient selection for lung volume reduction surgery: An objective model based on prior clinical decisions and quantitative CT analysis. Chest. 2000;117:991-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Flaherty KR, Kazerooni EA, Curtis JL, Iannettoni M, Lange L, Schork MA, Martinez FJ. Short-term and long-term outcomes after bilateral lung volume reduction surgery : prediction by quantitative CT. Chest. 2001;119:1337-1346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Parr DG, Sevenoaks M, Deng CQ, Stoel BC, Stockley RA. Detection of emphysema progression in alpha 1-antitrypsin deficiency using CT densitometry; Methodological advances. Respiratory Research. 2008;9:21. [Cited in This Article: ] |
| 27. | Dirksen A. Monitoring the progress of emphysema by repeat computed tomography scans with focus on noise reduction. Proc Am Thorac Soc. 2008;5:925-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Newell JD, Hogg JC, Snider GL. Report of a workshop: quantitative computed tomography scanning in longitudinal studies of emphysema. Eur Respir J. 2004;23:769-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Parr DG, Stoel BC, Stolk J, Nightingale PG, Stockley RA. Influence of calibration on densitometric studies of emphysema progression using computed tomography. Am J Respir Crit Care Med. 2004;170:883-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Parr DG, Stoel BC, Stolk J, Stockley RA. Validation of computed tomographic lung densitometry for monitoring emphysema in alpha1-antitrypsin deficiency. Thorax. 2006;61:485-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Shaker SB, Dirksen A, Laursen LC, Skovgaard LT, Holstein-Rathlou NH. Volume adjustment of lung density by computed tomography scans in patients with emphysema. Acta Radiol. 2004;45:417-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Chong D, Brown MS, Kim HJ, van Rikxoort EM, Guzman L, McNitt-Gray MF, Khatonabadi M, Galperin-Aizenberg M, Coy H, Yang K. Reproducibility of volume and densitometric measures of emphysema on repeat computed tomography with an interval of 1 week. Eur Radiol. 2012;22:287-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 516] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 34. | Gould GA, MacNee W, McLean A, Warren PM, Redpath A, Best JJ, Lamb D, Flenley DC. CT measurements of lung density in life can quantitate distal airspace enlargement--an essential defining feature of human emphysema. Am Rev Respir Dis. 1988;137:380-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 216] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Mohamed Hoesein FA, Hoop B, Zanen P, Gietema H, Kruitwagen CL, Ginneken B, Isgum I, Mol C, Klaveren RJ, Dijkstra AE. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax [Internet]. 2011;9:782. [Cited in This Article: ] |
| 36. | Mohamed Hoesein FA, Schmidt M, Mets OM, Gietema HA, Lammers JWJ, Zanen P, De Koning HJ, Van Der Aalst C, Oudkerk M, Vliegenthart R. Discriminating dominant computed tomography phenotypes in smokers without or with mild COPD. Respir Med. 2014;108:136-143. [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Akira M, Toyokawa K, Inoue Y, Arai T. Quantitative CT in chronic obstructive pulmonary disease: inspiratory and expiratory assessment. AJR Am J Roentgenol. 2009;192:267-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Diaz S, Casselbrant I, Piitulainen E, Magnusson P, Peterson B, Wollmer P, Leander P, Ekberg O, Akeson P. Validity of apparent diffusion coefficient hyperpolarized 3He-MRI using MSCT and pulmonary function tests as references. Eur J Radiol. 2009;71:257-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Shaker SB, Stavngaard T, Hestad M, Bach KS, Tonnesen P, Dirksen A. The extent of emphysema in patients with COPD. Clin Respir J. 2009;3:15-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Shaker SB, Maltbaek N, Brand P, Haeussermann S, Dirksen A. Quantitative computed tomography and aerosol morphometry in COPD and alpha1-antitrypsin deficiency. Eur Respir J. 2005;25:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, Hirai T, Niimi A, Nishimura K, Chin K. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138:635-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 42. | Grydeland TB, Dirksen A, Coxson HO, Eagan TM, Thorsen E, Pillai SG, Sharma S, Eide GE, Gulsvik A, Bakke PS. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181:353-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 43. | Camiciottoli G, Bartolucci M, Maluccio NM, Moroni C, Mascalchi M, Giuntini C, Pistolesi M. Spirometrically gated high-resolution CT findings in COPD: lung attenuation vs lung function and dyspnea severity. Chest. 2006;129:558-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Díaz AA, Pinto-Plata V, Hernández C, Peña J, Ramos C, Díaz JC, Klaassen J, Patino CM, Saldías F, Díaz O. Emphysema and DLCO predict a clinically important difference for 6MWD decline in COPD. Respir Med. 2015;109:882-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Diaz AA, Bartholmai B, San José Estépar R, Ross J, Matsuoka S, Yamashiro T, Hatabu H, Reilly JJ, Silverman EK, Washko GR. Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respir Med. 2010;104:1145-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | McAllister DA, Ahmed FS, Austin JH, Henschke CI, Keller BM, Lemeshow A, Reeves AP, Mesia-Vela S, Pearson GD, Shiau MC. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PLoS One. 2014;9:e93221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Cheng T, Wan HY, Cheng QJ, Guo Y, Qian YR, Fan L, Feng Y, Song YY, Zhou M, Li QY. Obvious emphysema on computed tomography during an acute exacerbation of chronic obstructive pulmonary disease predicts a poor prognosis. Intern Med J. 2015;45:517-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, DeCamp MM, Benditt J, Sciurba F, Make B. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173:1326-1334. [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 315] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 49. | Kim SS, Seo JB, Lee HY, Nevrekar DV, Forssen AV, Crapo JD, Schroeder JD, Lynch DA. Chronic obstructive pulmonary disease: lobe-based visual assessment of volumetric CT by Using standard images--comparison with quantitative CT and pulmonary function test in the COPDGene study. Radiology. 2013;266:626-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Hong Y, Chae EJ, Seo JB, Lee JH, Kim EK, Lee YK, Kim TH, Kim WJ, Lee JH, Lee SM. Contributors of the severity of airflow limitation in COPD patients. Tuberculosis and Respiratory Diseases. 2012;8. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Aziz ZA, Wells AU, Desai SR, Ellis SM, Walker AE, MacDonald S, Hansell DM. Functional impairment in emphysema: contribution of airway abnormalities and distribution of parenchymal disease. AJR Am J Roentgenol. 2005;185:1509-1515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Paré PD. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 296] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 53. | Choi SH, Lee HY, Lee KS, Chung MP, Kwon OJ, Han J, Kim N, Seo JB. The value of CT for disease detection and prognosis determination in combined pulmonary fibrosis and emphysema (CPFE). PLoS One. 2014;9:e107476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Patel BD, Coxson HO, Pillai SG, Agustí AG, Calverley PM, Donner CF, Make BJ, Müller NL, Rennard SI, Vestbo J. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:500-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 55. | Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Paré PD, Hogg JC. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162:1102-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 465] [Cited by in F6Publishing: 489] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 56. | Nakano Y, Whittall KP, Kalloger SE, Coxson HO, Flint J, Pare PD, English JC, editors . Development and validation of human airway analysis algorithm using multidetector row CT. 2002. Proc Spie. 2002;4683:460-469. [Cited in This Article: ] |
| 57. | Saba OI, Hoffman EA, Reinhardt JM. Maximizing quantitative accuracy of lung airway lumen and wall measures obtained from X-ray CT imaging. J Appl Physiol (1985). 2003;95:1063-1075. [PubMed] [Cited in This Article: ] |
| 58. | Achenbach T, Weinheimer O, Dueber C, Heussel CP. Influence of pixel size on quantification of airway wall thickness in computed tomography. J Comput Assist Tomogr. 2009;33:725-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Achenbach T, Weinheimer O, Biedermann A, Schmitt S, Freudenstein D, Goutham E, Kunz RP, Buhl R, Dueber C, Heussel CP. MDCT assessment of airway wall thickness in COPD patients using a new method: correlations with pulmonary function tests. Eur Radiol. 2008;18:2731-2738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Arakawa H, Fujimoto K, Fukushima Y, Kaji Y. Thin-section CT imaging that correlates with pulmonary function tests in obstructive airway disease. Eur J Radiol. 2011;80:e157-e163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Deveci F, Murat A, Turgut T, Altuntaş E, Muz MH. Airway wall thickness in patients with COPD and healthy current smokers and healthy non-smokers: assessment with high resolution computed tomographic scanning. Respiration. 2004;71:602-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Mohamed Hoesein FA, de Jong PA, Lammers JW, Mali WP, Schmidt M, de Koning HJ, van der Aalst C, Oudkerk M, Vliegenthart R, Groen HJ. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J. 2015;45:644-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Burgel PR, Nesme-Meyer P, Chanez P, Caillaud D, Carré P, Perez T, Roche N. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135:975-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 64. | Prescott E, Lange P, Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J. 1995;8:1333-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 178] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 65. | Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:228-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 287] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 66. | Kim V, Han MK, Vance GB, Make BJ, Newell JD, Hokanson JE, Hersh CP, Stinson D, Silverman EK, Criner GJ. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140:626-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 67. | Wielpütz MO, Bardarova D, Weinheimer O, Kauczor HU, Eichinger M, Jobst BJ, Eberhardt R, Koenigkam-Santos M, Puderbach M, Heussel CP. Variation of densitometry on computed tomography in COPD--influence of different software tools. PLoS One. 2014;9:e112898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Shaker SB, Dirksen A, Laursen LC, Maltbaek N, Christensen L, Sander U, Seersholm N, Skovgaard LT, Nielsen L, Kok-Jensen A. Short-term reproducibility of computed tomography-based lung density measurements in alpha-1 antitrypsin deficiency and smokers with emphysema. Acta Radiol. 2004;45:424-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Kemerink GJ, Kruize HH, Lamers RJ, van Engelshoven JM. Density resolution in quantitative computed tomography of foam and lung. Med Phys. 1996;23:1697-1708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Gierada DS, Bierhals AJ, Choong CK, Bartel ST, Ritter JH, Das NA, Hong C, Pilgram TK, Bae KT, Whiting BR. Effects of CT section thickness and reconstruction kernel on emphysema quantification relationship to the magnitude of the CT emphysema index. Acad Radiol. 2010;17:146-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 71. | Stoel BC, Putter H, Bakker ME, Dirksen A, Stockley RA, Piitulainen E, Russi EW, Parr D, Shaker SB, Reiber JH. Volume correction in computed tomography densitometry for follow-up studies on pulmonary emphysema. Proc Am Thorac Soc. 2008;5:919-924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Dirksen A, Piitulainen E, Parr DG, Deng C, Wencker M, Shaker SB, Stockley RA. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J. 2009;6:1345-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 73. | Chapman KR, Burdon JG, Piitulainen E, Sandhaus RA, Seersholm N, Stocks JM, Stoel BC, Huang L, Yao Z, Edelman JM. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:360-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 341] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 74. | Stockley RA, Parr DG, Piitulainen E, Stolk J, Stoel BC, Dirksen A. Therapeutic efficacy of α-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res. 2010;11:136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 75. | Shaker SB, Dirksen A, Ulrik CS, Hestad M, Stavngaard T, Laursen LC, Maltbaek N, Clementsen P, Skjaerbaek N, Nielsen L. The effect of inhaled corticosteroids on the development of emphysema in smokers assessed by annual computed tomography. COPD. 2009;2:104-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Hoshino M, Ohtawa J. Computed tomography assessment of airway dimensions with combined tiotropium and indacaterol therapy in COPD patients. Respirology. 2014;3:403-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Nordenmark LH, Taylor R, Jorup C. Feasibility of Computed Tomography in a Multicenter COPD Trial: A Study of the Effect of AZD9668 on Structural Airway Changes. Advances in Therapy. 2015;32:548-566. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Shimizu K, Makita H, Hasegawa M, Kimura H, Fuke S, Nagai K, Yoshida T, Suzuki M, Konno S, Ito YM. Regional bronchodilator response assessed by computed tomography in chronic obstructive pulmonary disease. Eur J Radiol. 2015;84:1196-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 79. | Stolk J, Stockley RA, Stoel BC, Cooper BG, Piitulainen E, Seersholm N, Chapman KR, Burdon JG, Decramer M, Abboud RT. Randomised controlled trial for emphysema with a selective agonist of the ?-type retinoic acid receptor. Eur Respir J. 2012;40:306-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 80. | Mao JT, Goldin JG, Dermand J, Ibrahim G, Brown MS, Emerick A, McNitt-Gray MF, Gjertson DW, Estrada F, Tashkin DP. A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med. 2002;165:718-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 81. | Roth MD, Connett JE, D’Armiento JM, Foronjy RF, Friedman PJ, Goldin JG, Louis TA, Mao JT, Muindi JR, O’Connor GT. Feasibility of retinoids for the treatment of emphysema study. Chest. 2006;130:1334-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 82. | Dirksen A, Dijkman JH, Madsen F, Stoel B, Hutchison DC, Ulrik CS, Skovgaard LT, Kok-Jensen A, Rudolphus A, Seersholm N. A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999;160:1468-1472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 322] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 83. | Mentore K, Froh DK, de Lange EE, Brookeman JR, Paget-Brown AO, Altes TA. Hyperpolarized HHe 3 MRI of the lung in cystic fibrosis: assessment at baseline and after bronchodilator and airway clearance treatment. Acad Radiol. 2005;12:1423-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 84. | Ley-Zaporozhan J, Ley S, Kauczor HU. Proton MRI in COPD. COPD. 2007;4:55-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Coxson HO, Mayo J, Lam S, Santyr G, Parraga G, Sin DD. New and current clinical imaging techniques to study chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:588-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 86. | Fain SB, Gonzalez-Fernandez G, Peterson ET, Evans MD, Sorkness RL, Jarjour NN, Busse WW, Kuhlman JE. Evaluation of structure-function relationships in asthma using multidetector CT and hyperpolarized He-3 MRI. Acad Radiol. 2008;15:753-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 87. | Milne S, King GG. Advanced imaging in COPD: insights into pulmonary pathophysiology. J Thorac Dis. 2014;6:1570-1585. [PubMed] [Cited in This Article: ] |
| 88. | Washko GR. The role and potential of imaging in COPD. Med Clin North Am. 2012;96:729-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Kauczor HU, Ley-Zaporozhan J, Ley S. Imaging of pulmonary pathologies: focus on magnetic resonance imaging. Proc Am Thorac Soc. 2009;6:458-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Jobst BJ, Triphan SM, Sedlaczek O, Anjorin A, Kauczor HU, Biederer J, Ley-Zaporozhan J, Ley S, Wielpütz MO. Functional lung MRI in chronic obstructive pulmonary disease: comparison of T1 mapping, oxygen-enhanced T1 mapping and dynamic contrast enhanced perfusion. PLoS One. 2015;10:e0121520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 91. | Andrychiewicz A, Gorka K, Reid M, Soja J, Sladek K, Szczeklik W. Modern methods for endoscopic treatment of obstructive pulmonary diseases. J Asthma. 2015;52:920-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 92. | Hamedani H, Kadlecek SJ, Ishii M, Xin Y, Emami K, Han B, Shaghaghi H, Gopstein D, Cereda M, Gefter WB. Alterations of regional alveolar oxygen tension in asymptomatic current smokers: assessment with hyperpolarized (3)He MR imaging. Radiology. 2015;274:585-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Ohno Y, Koyama H, Nogami M, Takenaka D, Matsumoto S, Obara M, Sugimura K. Dynamic oxygen-enhanced MRI versus quantitative CT: pulmonary functional loss assessment and clinical stage classification of smoking-related COPD. AJR Am J Roentgenol. 2008;190:W93-W99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 94. | Ohno Y, Hatabu H, Takenaka D, Van Cauteren M, Fujii M, Sugimura K. Dynamic oxygen-enhanced MRI reflects diffusing capacity of the lung. Magn Reson Med. 2002;47:1139-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 95. | Hueper K, Vogel-Claussen J, Parikh MA, Austin JH, Bluemke DA, Carr J, Choi J, Goldstein TA, Gomes AS, Hoffman EA. Pulmonary Microvascular Blood Flow in Mild Chronic Obstructive Pulmonary Disease and Emphysema. The MESA COPD Study. Am J Respir Crit Care Med. 2015;192:570-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 96. | Ley S, Zaporozhan J, Morbach A, Eberle B, Gast KK, Heussel CP, Biedermann A, Mayer E, Schmiedeskamp J, Stepniak A. Functional evaluation of emphysema using diffusion-weighted 3Helium-magnetic resonance imaging, high-resolution computed tomography, and lung function tests. Invest Radiol. 2004;39:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Swift AJ, Wild JM, Fichele S, Woodhouse N, Fleming S, Waterhouse J, Lawson RA, Paley MN, Van Beek EJ. Emphysematous changes and normal variation in smokers and COPD patients using diffusion 3He MRI. Eur J Radiol. 2005;54:352-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 98. | van Beek EJ, Dahmen AM, Stavngaard T, Gast KK, Heussel CP, Krummenauer F, Schmiedeskamp J, Wild JM, Søgaard LV, Morbach AE. Hyperpolarised 3He MRI versus HRCT in COPD and normal volunteers: PHIL trial. Eur Respir J. 2009;34:1311-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 99. | Fain SB, Panth SR, Evans MD, Wentland AL, Holmes JH, Korosec FR, O’Brien MJ, Fountaine H, Grist TM. Early emphysematous changes in asymptomatic smokers: detection with 3He MR imaging. Radiology. 2006;239:875-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 100. | Alvarez Diaz H, Aznar MU, Afonso Afonso FJ. Bone lesions simulating multiple myeloma: Unusual presentation of esophageal cancer. Eur J Intern Med. 2009;20:e14. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 101. | Xia Y, Guan Y, Fan L, Liu SY, Yu H, Zhao LM, Li B. Dynamic contrast enhanced magnetic resonance perfusion imaging in high-risk smokers and smoking-related COPD: correlations with pulmonary function tests and quantitative computed tomography. COPD. 2014;11:510-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Weijing M, Khadija S, Damien P, Sarah S, Harvey OC, David GM, Grace P. Conventional Pulmonary MRI And CT Of Bronchiectasis And Emphysema: Tissue Density Measurements And Relationship To Pulmonary Function Tests. A108 LUNG IMAGING: STATE OF PLAY ON STRUCTURE AND FUNCTION: American Thoracic Society 2014; A2399-A2399. [Cited in This Article: ] |
| 103. | Barzilai B, Waggoner AD, Spessert C, Picus D, Goodenberger D. Two-dimensional contrast echocardiography in the detection and follow-up of congenital pulmonary arteriovenous malformations. Am J Cardiol. 1991;68:1507-1510. [PubMed] [Cited in This Article: ] |
| 104. | Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc. 2008;5:475-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 105. | Woods JC, Choong CK, Yablonskiy DA, Bentley J, Wong J, Pierce JA, Cooper JD, Macklem PT, Conradi MS, Hogg JC. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med. 2006;56:1293-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 106. | Morino S, Toba T, Araki M, Azuma T, Tsutsumi S, Tao H, Nakamura T, Nagayasu T, Tagawa T. Noninvasive assessment of pulmonary emphysema using dynamic contrast-enhanced magnetic resonance imaging. Exp Lung Res. 2006;32: 55-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Sverzellati N, Molinari F, Pirronti T, Bonomo L, Spagnolo P, Zompatori M. New insights on COPD imaging via CT and MRI. Int J Chron Obstruct Pulmon Dis. 2007;2:301-312. [PubMed] [Cited in This Article: ] |
| 108. | Kruger SJ, Nagle SK, Couch MJ, Ohno Y, Albert M, Fain SB. Functional imaging of the lungs with gas agents. J Magn Reson Imaging. 2016;43:295-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 109. | Bink A, Hanisch G, Karg A, Vogel A, Katsaros K, Mayer E, Gast KK, Kauczor HU. Clinical aspects of the apparent diffusion coefficient in 3He MRI: results in healthy volunteers and patients after lung transplantation. J Magn Reson Imaging. 2007;25:1152-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 110. | Hamedani H, Kadlecek SJ, Ishii M, Emami K, Kuzma NN, Xin Y, Rossman M, Rizi RR. A variability study of regional alveolar oxygen tension measurement in humans using hyperpolarized 3He MRI. Magn Reson Med. 2013;70:1557-1566. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Woodhouse N, Wild JM, Paley MN, Fichele S, Said Z, Swift AJ, van Beek EJ. Combined helium-3/proton magnetic resonance imaging measurement of ventilated lung volumes in smokers compared to never-smokers. J Magn Reson Imaging. 2005;21:365-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 112. | Fink C, Puderbach M, Bock M, Lodemann KP, Zuna I, Schmähl A, Delorme S, Kauczor HU. Regional lung perfusion: assessment with partially parallel three-dimensional MR imaging. Radiology. 2004;231:175-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 113. | Ohno Y, Hatabu H, Murase K, Higashino T, Kawamitsu H, Watanabe H, Takenaka D, Fujii M, Sugimura K. Quantitative assessment of regional pulmonary perfusion in the entire lung using three-dimensional ultrafast dynamic contrast-enhanced magnetic resonance imaging: Preliminary experience in 40 subjects. J Magn Reson Imaging. 2004;20:353-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 114. | Quirk JD, Lutey BA, Gierada DS, Woods JC, Senior RM, Lefrak SS, Sukstanskii AL, Conradi MS, Yablonskiy DA. In vivo detection of acinar microstructural changes in early emphysema with (3)He lung morphometry. Radiology. 2011;260:866-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 115. | Subramanian DR, Jenkins L, Edgar R, Quraishi N, Stockley RA, Parr DG. Assessment of pulmonary neutrophilic inflammation in emphysema by quantitative positron emission tomography. Am J Respir Crit Care Med. 2012;186:1125-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 116. | Vidal Melo MF, Winkler T, Harris RS, Musch G, Greene RE, Venegas JG. Spatial heterogeneity of lung perfusion assessed with (13)N PET as a vascular biomarker in chronic obstructive pulmonary disease. J Nucl Med. 2010;51:57-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 117. | Musch G, Venegas JG. Positron emission tomography imaging of regional pulmonary perfusion and ventilation. Proc Am Thorac Soc. 2005;2:522-527, 508-509. [PubMed] [Cited in This Article: ] |
| 118. | Suga K, Iwanaga H, Tokuda O, Okada M, Matsunaga N. Intrabullous ventilation in pulmonary emphysema: assessment with dynamic xenon-133 gas SPECT. Nucl Med Commun. 2012;33:371-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 119. | Argula RG, Strange C, Ramakrishnan V, Goldin J. Baseline regional perfusion impacts exercise response to endobronchial valve therapy in advanced pulmonary emphysema. Chest. 2013;144:1578-1586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 120. | Sudoh M, Ueda K, Kaneda Y, Mitsutaka J, Li TS, Suga K, Kawakami Y, Hamano K. Breath-hold single-photon emission tomography and computed tomography for predicting residual pulmonary function in patients with lung cancer. J Thorac Cardiovasc Surg. 2006;131:994-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 121. | Bajc M, Markstad H, Jarenback L, Tufvesson E, Bjermer L, Jogi J. Grading obstructive lung disease using tomographic pulmonary scintigraphy in patients with chronic obstructive pulmonary disease (COPD) and long-term smokers. Ann Nucl Med. 2015;29:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 122. | Jögi J, Ekberg M, Jonson B, Bozovic G, Bajc M. Ventilation/perfusion SPECT in chronic obstructive pulmonary disease: an evaluation by reference to symptoms, spirometric lung function and emphysema, as assessed with HRCT. Eur J Nucl Med Mol Imaging. 2011;38:1344-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 123. | Sandek K, Bratel T, Lagerstrand L, Rosell H. Relationship between lung function, ventilation-perfusion inequality and extent of emphysema as assessed by high-resolution computed tomography. Respir Med. 2002;96:934-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 124. | Johkoh T, Müller NL, Kavanagh PV, Cartier Y, Mayo JR, Tomiyama N, Murakami T, Naito H, Nakamura H, Moriya H. Scintigraphic and MR perfusion imaging in preoperative evaluation for lung volume reduction surgery: pilot study results. Radiat Med. 2000;18:277-281. [PubMed] [Cited in This Article: ] |
| 125. | Jobse BN, Rhem RG, Wang IQ, Counter WB, Stämpfli MR, Labiris NR. Detection of lung dysfunction using ventilation and perfusion SPECT in a mouse model of chronic cigarette smoke exposure. J Nucl Med. 2013;54:616-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 126. | Jobse BN, McCurry CA, Morissette MC, Rhem RG, Stämpfli MR, Labiris NR. Impact of inflammation, emphysema, and smoking cessation on V/Q in mouse models of lung obstruction. Respir Res. 2014;15:42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 127. | Molinari F, Fink C, Risse F, Tuengerthal S, Bonomo L, Kauczor HU. Assessment of differential pulmonary blood flow using perfusion magnetic resonance imaging: comparison with radionuclide perfusion scintigraphy. Invest Radiol. 2006;41:624-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 128. | Stavngaard T, Søgaard LV, Mortensen J, Hanson LG, Schmiedeskamp J, Berthelsen AK, Dirksen A. Hyperpolarized 3He MRI and 81mKr SPECT in chronic obstructive pulmonary disease. Eur J Nucl Med Mol Imaging. 2005;32:448-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 129. | McLaughlin RA, Noble PB, Sampson DD. Optical coherence tomography in respiratory science and medicine: from airways to alveoli. Physiology (Bethesda). 2014;29:369-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 130. | Sainter AW, King TA, Dickinson MR. Effect of target biological tissue and choice of light source on penetration depth and resolution in optical coherence tomography. J Biomed Opt. 2004;9:193-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 131. | Tsuboi M, Hayashi A, Ikeda N, Honda H, Kato Y, Ichinose S, Kato H. Optical coherence tomography in the diagnosis of bronchial lesions. Lung Cancer. 2005;49:387-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 132. | Lorenser D, Yang X, Kirk RW, Quirk BC, McLaughlin RA, Sampson DD. Ultrathin side-viewing needle probe for optical coherence tomography. Opt Lett. 2011;36:3894-3896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 133. | Burton PA, Dixon MF. A comparison of changes in the mucous glands and goblet cells of nasal, sinus, and bronchial mucosa. Thorax. 1969;24: 180-185. [Cited in This Article: ] |
| 134. | Mullen JB, Wright JL, Wiggs BR, Pare PD, Hogg JC. Reassessment of inflammation of airways in chronic bronchitis. Br Med J (Clin Res Ed). 1985;291:1235-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 133] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 135. | James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J. 2007;30:134-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 136. | Coxson HO, Eastwood PR, Williamson JP, Sin DD. Phenotyping airway disease with optical coherence tomography. Respirology. 2011;16:34-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 137. | Coxson HO, Quiney B, Sin DD, Xing L, McWilliams AM, Mayo JR, Lam S. Airway wall thickness assessed using computed tomography and optical coherence tomography. Am J Respir Crit Care Med. 2008;177:1201-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 138. | Kirby M, Zhang W, Laratta PK, Sin DD, Lam S, Coxson HO, editors . Sex differences in chronic obstructive pulmonary disease evaluated using optical coherence tomography. Proc. SPIE 8927, Endoscopic Microscopy IX; and Optical Techniques in Pulmonary Medicine, 89270Z; 4 March. 2014;. [DOI] [Cited in This Article: ] |