Published online Jul 28, 2016. doi: 10.5320/wjr.v6.i2.63
Peer-review started: November 23, 2015
First decision: December 28, 2015
Revised: February 2, 2016
Accepted: March 22, 2016
Article in press: March 23, 2016
Published online: July 28, 2016
Bronchial hyperresponsiveness (BHR) is an important but not asthma-specific characteristic and can be assessed by direct and indirect methods, based on the stimulus causing airway obstruction. BHR has been proposed as a prognostic marker of asthma severity and persistence, and may also be used to control pharmacological management of asthma. The most recent data on the prevalence and development of BHR in childhood and its predictive value for subsequent asthma development in late adolescence and adulthood is discussed in this review. According to the BHR-related scientific articles written in the English language and indexed in the publicly searchable PubMed database, the prevalence of BHR varies based upon the methods used to assess it and the population examined. In general, however, BHR prevalence is reduced as children grow older, in both healthy and asthmatic populations. While asthma can be predicted by BHR, the predictive value is limited. Reduced lung function, allergic sensitization, female sex, and early respiratory illness have been identified as risk factors for BHR. The collective studies further indicate that BHR is a dynamic feature related to asthma, but asymptomatic BHR is also common. Ultimately, the prevalence of BHR varies depending on the population, the environment, and the evaluation methods used. While both the methacholine challenge and the exercise test may predict asthma in adolescence or early adulthood, the predictive value is higher for the methacholine challenge compared to the exercise test. The collective data presented in the present study demonstrate how BHR develops through childhood and its relation to bronchial asthma.
Core tip: Bronchial hyperresponsiveness (BHR) is a dynamic feature related to asthma and is also common among children without an asthma diagnosis. In children, BHR may be predictive of asthma development. This review article summarizes the current literature on the prevalence of BHR, highlighting the reported evidence elucidating its risk factors and predictive value for asthma in children.
- Citation: Riiser A. Bronchial hyperresponsiveness in childhood: A narrative review. World J Respirol 2016; 6(2): 63-68
- URL: https://www.wjgnet.com/2218-6255/full/v6/i2/63.htm
- DOI: https://dx.doi.org/10.5320/wjr.v6.i2.63
In 1859, Sir Henry Hyde Salter was the first to describe bronchial sensibility[1], a phenomenon currently known by its descriptive name “bronchial hyperresponsiveness” (BHR). BHR is an abnormal reaction that occurs under otherwise normal physiologic conditions, in which airway obstruction is induced by a non-allergic stimulus. The clinical characterization of this event as abnormal arises from systematic comparison of bronchial responses in people with BHR to those in healthy individuals when the same stimuli and measurement methods are used[2]. An identified trigger of BHR, eosinophilic pulmonary inflammation, is often associated with asthma. Central to this shared pathology is the cellular T helper type 2 (Th2) response that is hallmarked by infiltration of CD4+ Th2 cells, natural killer T cells, eosinophils, neutrophils, and mast cells, as well as structural and functional defects in airway epithelium and airway remodeling[3]. Thus, assessment of BHR involves comparison of lung function before and after stimulus, using such methods as exercise test (in which the complete stimulus-exercise is given on a single occasion) or the methacholine challenge (in which the stimulus is administered in increasing doses or concentrations and response-lung function is measured after each to calculate dose response).
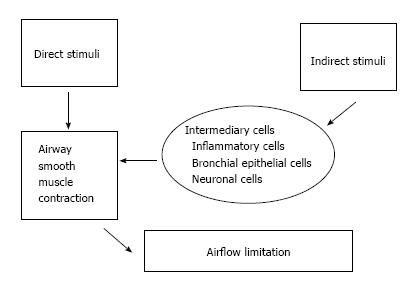
The various stimuli for bronchial constriction act either directly or indirectly. The former act directly on the smooth muscles around the airways, stimulating contractility, while the latter instead stimulate the release of inflammatory mediators from basophilic and eosinophilic granulocytes[2,4] (Figure 1). These different types of stimuli reflect different underlying mechanisms in the airways, likely explaining the sometimes limited correlation between BHR defined by different bronchial challenges[2]. Historically, the most common constrictor agents used in these challenge studies have been histamine and methacholine, which show close correlation between the severities of bronchial constriction induced by equal dosages. In the last decades, however, the experimental use of histamine has declined due to the associated local side effects, particularly with high doses[5], and methacholine challenge has emerged as the test of choice for many clinicians[6] and researchers. The exercise test was standardized in the 1970s and quickly became a popular method to investigate BHR, especially in children[7]. Since then, other indirect stimuli that have been commonly used in testing include hypertonic saline and eucapnic hyperventilation (EVH). The collective studies have shown that severity of bronchial constriction induced by either exercise or EVH is dependent upon climatic conditions, such as temperature and humidity of the inhaled air, with colder and dryer air having been shown to induce greater bronchial constriction[8,9].
Many individual factors, including age[10] and asthma[11,12], may influence the result of a bronchial challenge. Children with severe asthma have been shown to have more severe methacholine-stimulated BHR than children with mild to moderate asthma[12]. Moreover, children with persistent asthma have significantly greater prevalence of exercise induced bronchial constriction (EIB) than children with intermittent asthma[11]. Airborne particles (i.e., allergens, air pollution and chemical irritants) may increase BHR over a period of weeks and months after exposure, as shown in a study of school-age children following infancy exposure[13]. BHR was also shown to be temporarily increased in children with recent airway infection[14,15].
There appears to be crossover between BHR and asthma, both clinically and physiologically. For example, BHR can be successfully treated with anti-asthmatic medications, such as inhaled corticosteroids[16] and β-agonists[17], but use of BHR as the diagnostic factor for asthma can lead to a false positive test result[15]. The pathogenesis of BHR, and its molecular underpinnings, remain to be fully elucidated but are of interest to both clinicians and researchers. Thus, the aim of the present study was to review the English language literature in the PubMed database and present a useful review on the prevalence and development of BHR in children, in addition to the predictive value of childhood BHR for development of asthma in late adolescence and adulthood.
Obtaining the accurate rate of BHR prevalence is complicated by the varied stimuli, exposure/administration methods, and cut-off values used to define BHR (Table 1). In a birth cohort from New Zealand, BHR (defined therein as the concentration causing a 20% reduction in forced expiratory volume in one second (FEV1) (PC20): ≤ 8 mg/mL) was assessed at age 9 and then again at age 21. The BHR prevalence dropped from 16% at the age of first assessment to 7% 12 years later[18]. A similar reduction in prevalence from childhood to adolescence was found in a Norwegian birth cohort (ECA) assessed at age 10 and again at age 16; specifically, the proportion of children with mild to severe BHR (defined therein as the dose causing a 20% reduction in FEV1 (PD20): ≤ 8 μmol) dropped from 33% to 15% 6 years later. These cohorts from such geographically and socially disparate countries both had an approximately 50% reduction of childhood BHR during the years corresponding to puberty. The ECA study further divided the BHR cases according to severity, and found that 57% of the overall cohort had the same degree of BHR from age 10 to age 16. However, among those whose degree of BHR changed over the 6 year period, 82% changed to a category with a lower degree of BHR[19].
| Stimulus | Definition | Cut-off point | Ref. |
| Methacholine PD20 | Severe BHR | PD20: ≤ 1 μmol | [19,31] |
| Mild to severe BHR | PD20: ≤ 8 μmol | [19,31] | |
| Any BHR | PD20: ≤ 16 μmol | [19] | |
| BHR | PD20: < 3.91 μmol | [20] | |
| Methacholine PC20 | BHR | PC20: ≤ 8 mg/mL | [18] |
| BHR | PC20: < 2 mg/mL for children between 6 to 9 yr | [22] | |
| BHR | PC20: < 4 mg/mL for children between 10 to 14 yr | [22] | |
| Exercise | BHR | > 15% fall in FEV1 after exercise | [21] [30] |
| EIB | > 10% fall in FEV1 after running | [25] | |
| AHR | A fall in FEV1 of 1.96 standard deviations more than in healthy children | [26] | |
| BHR | ≥ 8.6% fall in FEV1 after exercise | [32] |
Besides the two studies mentioned above, an Australian study demonstrated a consistent BHR prevalence of 20% in children between the ages of 8 and 11 for the years of 1992 and 2002[20]. Additionally, that study demonstrated a reduction in asthma prevalence, from 38% to 31%, over the same decade[20]. In an Estonian study, BHR was assessed by methacholine challenge in 1106 children in two different cities. The prevalence of BHR was found to be 32% in the less industrialized town of Tartu compared to 19% in Tallinn, a coastal town with more air pollution[21]. Cross-sectional studies of children with asthma in Chile and Germany showed BHR prevalence ranging from 15% (when BHR was assessed by cold air) to 70% (when measured following methacholine stimulation)[22,23]. The same study from Germany[23], but using a longitudinal approach, showed the same reduction in BHR occurring around puberty that is similar to the trend seen in the general population[18,19].
For EIB, the magnitude of bronchial constriction has been shown to be dependent on the intensity of the stimulating exercise[24]. In the ECA study, 9% of the 10-year-old experienced a ≥ 10% reduction in FEV1 after 6 min of treadmill running, with the children having been encouraged to achieve a heart rate of 200 beats/min in the last 4 min of the exercise test[25]. An Australian study of children between the ages of 8 and 11 similarly found an EIB prevalence of 20%, with the children having performed a 6 min running test outdoors and the criterion for a positive EIB test result being a fall in FEV1 of 1.96 standard deviations more than in healthy children[26]. Other studies of EIB in asthmatic children have reported prevalence rates from 40% to 90%[11,27], and the broad range may be due to the different protocols and different definitions of EIB used. A cross-sectional study in 1973 and 1988 from Wales found a similar incidence of EIB in 12-year-old (7% and 8%, respectively), although the rates of asthma prevalence increased from 4% to 9% during this same period[28].
The International Study of Asthma and Allergy in Childhood assessed BHR with hypertonic saline in 6826 schoolchildren in 16 different countries. The national prevalences of BHR varied from 2.1% in Albania to 47.8% in India[29]. Given the wide variation in BHR prevalence, a comparative assessment of EIB in a country with high prevalence and in a country with low prevalence was performed using the definition of BHR as a fall in FEV1 of ≥ 15 % after exercise. The data indicated that seven times as many children had EIB in the United Kingdom compared to Albania[30]. In the previously discussed Estonian study, when BHR was assessed by methacholine challenge the children in Tallinn showed significantly less BHR compared to the children in Tartu (6% vs 18%)[21], which agrees with the former study’s finding of less BHR in a less developed area.
The ECA study investigated whether BHR measured by methacholine challenge or by exercise test at age 10 could predict whether the participant had developed asthma by 6 years later. The study results suggested that BHR (PD20: ≤ 8 μmol) is a weak predictor for asthma, but the ability to predict asthma was significantly improved when clinical characteristics were included in the prediction model. The cut-off value with the best sensitivity and specificity for asthma 6 years later was PD20 of ≤ 7 μmol[31].
The risk of having asthma 6 years after BHR was 6.8 times greater if a child had a severe case of BHR (vs no BHR). The corresponding values were 3.5 times greater for mild to severe BHR (PD20: ≤ 8 μmol) and 2.8 times greater for any BHR (PD20: ≤ 16 μmol). If the subjects had EIB, on the other hand, the risk for asthma 6 years later was 2.6 times greater compared to not having EIB. Only 51% of the subjects with PD20 of ≤ 8 μmol and the same proportion of subjects with a positive exercise test experienced asthma symptoms[31], confirming previous reports of asymptomatic BHR as a common phenomenon[32,33].
To my knowledge, no other studies have examined the predictive value of BHR for subsequent asthma. However, the risk estimates for subsequent asthma reported from the ECA study are in line with those reported previously. A study from Australia showed that children with a positive BHR response to methacholine challenge had 5.1 times greater risk of developing asthma symptoms in early adulthood[20]. Sears et al[18] reported that the risk of wheezing in young adults was 4.2 times greater if the subjects had at least one positive BHR response to methacholine challenge in childhood adolescence. A study from Denmark showed that children with a positive EIB response to exercise test at 10 years of age had 2.3 times increased risk of experiencing the asthma symptom of wheezing during young adulthood[32]. Indeed, the association between BHR and subsequent asthma may begin before birth. Bisgaard et al[34] reported a significant association between positive BHR response to methacholine in newborns and development of asthma at 7 years of age in a population of children with asthmatic mothers and, therefore, at high risk of asthma. That study also demonstrated that neonates with BHR had reduced lung function in addition to the asthma 7 years later and that the airflow deficit progressed independently of allergic sensitization or atopic dermatitis.
There is considerable uncertainty about which BHR test is optimal for subsequent asthma prediction. In the ECA study, severe BHR at 10 years of age had the highest positive predictive value (0.49) for asthma 6 years later, compared with EIB and other methacholine cut-off values defining BHR. The negative predictive value was similar (0.89 to 0.92) for all definitions of BHR and EIB[31]. The receiver operating characteristic (ROC) curve is plotted as an XY diagram, with sensitivity and 1 - specificity representing different cut-off values; the area under the ROC curve provides information about the suitability of a test.
In the ECA study, PD20 had a larger area under the curve than the exercise-induced reduction in FEV1 (0.69, 95%CI: 0.62-0.75 vs 0.60, 95%CI: 0.53-0.67) when the predictive ability of BHR at 10-year-old for asthma development 6 years later was tested[31]. In cross-sectional studies, the methacholine challenge is reported to be more sensitive than either a free running (exercise) test[35] or a sport-specific exercise test[36] for identifying active asthma. In contrast, Haby et al[26] reported that BHR assessment by the exercise test and histamine challenge had similar sensitivity and specificity for identifying asthma in randomly selected schoolchildren. In general, however, neither the methacholine challenge nor the exercise test is ideal for predicting asthma; based on the area under the curve, the methacholine challenge may be better than the EIB test if the aim is to predict future asthma in children.
If one wants to identify children with future asthma with the greatest possible certainty, a high positive predictive value is desirable, and one should, therefore, select severe BHR as a criterion for a positive test. However, if the aim is to identify as many children as possible who will develop asthma within a 6-year future range, higher cut-off values will be more appropriate. In many cases, it may be equally relevant to predict which children will not develop asthma in that 6-year range, in which case the mild to severe BHR criterion is appropriate.
The “optimal” cut-off value which gave the highest sensitivity and specificity for asthma 6 years later was a 6% reduction in FEV1 related to EIB[31]. However, this level of reduction is close to the normal variation between FEV1 measurements in children[8], precluding its recommendation as the cut-off value for defining the EIB. A reduction in FEV1 of ≥ 10% is reportedly the best cut-off value to discriminate between asthmatic and normal response to an exercise test on a treadmill[37] and is the cut-off value recommended in the American and European guidelines[38,39].
The ECA study identified airway obstruction measured by FEV1 or FEV1/forced vital capacity (FVC) at 10 years of age as risk factors for BHR at both 10-year-old and 16-year-old. Additionally, the study identified 16-year-old with airway obstruction as being at higher risk for BHR. In multivariate analysis, BHR, female sex, and reduced FEV1/FVC at 10 years of age were significant risk factors for developing BHR by 16 years of age. In bivariate analysis, allergic sensitization was a risk factor for later BHR, but the significant association was lost when the analysis was controlled for sex, airway obstruction, and previous BHR[19]. Both a cross-sectional study[40] and a longitudinal study[41] have revealed associations between BHR and allergic sensitization in general and dust mites in particular. A study from New South Wales by Peat et al[42] reported that female sex was a minor risk factor for BHR and that allergic sensitization assessed by skin prick test was the most important risk factor for BHR in school children. However, that study design did not include control for obstructive lung function. Through the ECA study, Håland et al[43] identified reduced lung function at birth as a risk factor for BHR 10 years later, and Hovland et al[44] identified recurrent bronchial obstructions before the age of 2 as a risk for developing BHR by either age 10 or age 16. These findings were supported by those of Peat et al[42], in which early respiratory illness was identified as a risk factor for future BHR. Yavuz et al[45] identified nocturnal cough, exercise-induced cough, eosinophilia, and borderline bronchodilator response as significant risk factors for BHR in children with asthma like symptoms but without obstructive patterns or evidence of reversibility demonstrated by spirometry. Thus, the collective body of evidence suggests that reduced lung function at any age, early respiratory illness, female sex, and allergic sensitization are common risk factors for BHR. However, the effect of allergic sensitization may not be significant after controlling for obstructive lung function, at least in some populations.
The prevalence of BHR varies in different populations and according to results from different assessment methods. BHR rates decrease throughout childhood and adolescence and BHR is common in both children with and without asthma. The presence of BHR may predict future asthma both in asthmatic and non-asthmatic children, and asthma can be predicted by methods using both direct and indirect stimuli to assess BHR. Although its predictive values are modest, BHR in childhood increases the likelihood of subsequent asthma; generally, the predictive value is increased with increased severity of BHR. The methacholine challenge seems to be more appropriate than an exercise test if the goal is to predict future asthma.
P- Reviewer: Huang CJ, Rovina N S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Salter H. On Asthma: its pathology & treatment. London: J Churchill, 1859. . [Cited in This Article: ] |
| 2. | Van Schoor J, Pauwels R, Joos G. Indirect bronchial hyper-responsiveness: the coming of age of a specific group of bronchial challenges. Clin Exp Allergy. 2005;35:250-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Holgate ST, Davies DE. Rethinking the pathogenesis of asthma. Immunity. 2009;31:362-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Pauwels R, Joos G, Van der Straeten M. Bronchial hyperresponsiveness is not bronchial hyperresponsiveness is not bronchial asthma. Clin Allergy. 1988;18:317-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 126] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Higgins BG, Britton JR, Chinn S, Jones TD, Vathenen AS, Burney PG, Tattersfield AE. Comparison of histamine and methacholine for use in bronchial challenge tests in community studies. Thorax. 1988;43:605-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 957] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 7. | Silverman M, Anderson SD. Standardization of exercise tests in asthmatic children. Arch Dis Child. 1972;47:882-889. [PubMed] [Cited in This Article: ] |
| 8. | Carlsen KH, Engh G, Mørk M, Schrøder E. Cold air inhalation and exercise-induced bronchoconstriction in relationship to metacholine bronchial responsiveness: different patterns in asthmatic children and children with other chronic lung diseases. Respir Med. 1998;92:308-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Noviski N, Bar-Yishay E, Gur I, Godfrey S. Exercise intensity determines and climatic conditions modify the severity of exercise-induced asthma. Am Rev Respir Dis. 1987;136:592-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Hopp RJ, Bewtra A, Nair NM, Townley RG. The effect of age on methacholine response. J Allergy Clin Immunol. 1985;76:609-613. [PubMed] [Cited in This Article: ] |
| 11. | Cabral AL, Conceição GM, Fonseca-Guedes CH, Martins MA. Exercise-induced bronchospasm in children: effects of asthma severity. Am J Respir Crit Care Med. 1999;159:1819-1823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Lang AM, Konradsen J, Carlsen KH, Sachs-Olsen C, Mowinckel P, Hedlin G, Lødrup Carlsen KC. Identifying problematic severe asthma in the individual child--does lung function matter? Acta Paediatr. 2010;99:404-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Søyseth V, Kongerud J, Haarr D, Strand O, Bolle R, Boe J. Relation of exposure to airway irritants in infancy to prevalence of bronchial hyper-responsiveness in schoolchildren. Lancet. 1995;345:217-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Cheung D, Dick EC, Timmers MC, de Klerk EP, Spaan WJ, Sterk PJ. Rhinovirus inhalation causes long-lasting excessive airway narrowing in response to methacholine in asthmatic subjects in vivo. Am J Respir Crit Care Med. 1995;152:1490-1496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 97] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Jayet PY, Schindler C, Künzli N, Zellweger JP, Brändli O, Perruchoud AP, Keller R, Schwartz J, Ackermann-Liebrich U, Leuenberger P. Reference values for methacholine reactivity (SAPALDIA study). Respir Res. 2005;6:131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Sont JK, Willems LN, Bel EH, van Krieken JH, Vandenbroucke JP, Sterk PJ. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. The AMPUL Study Group. Am J Respir Crit Care Med. 1999;159:1043-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 618] [Cited by in F6Publishing: 595] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 17. | Rabe KF, Jörres R, Nowak D, Behr N, Magnussen H. Comparison of the effects of salmeterol and formoterol on airway tone and responsiveness over 24 hours in bronchial asthma. Am Rev Respir Dis. 1993;147:1436-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 119] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414-1422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 945] [Cited by in F6Publishing: 859] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 19. | Riiser A, Hovland V, Mowinckel P, Carlsen KH, Carlsen KL. Bronchial hyperresponsiveness decreases through childhood. Respir Med. 2012;106:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Toelle BG, Ng K, Belousova E, Salome CM, Peat JK, Marks GB. Prevalence of asthma and allergy in schoolchildren in Belmont, Australia: three cross sectional surveys over 20 years. BMJ. 2004;328:386-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Vasar M, Bråbäck L, Julge K, Knutsson A, Riikjärv MA, Björkstén B. Prevalence of bronchial hyperreactivity as determined by several methods among Estonian schoolchildren. Pediatr Allergy Immunol. 1996;7:141-146. [PubMed] [Cited in This Article: ] |
| 22. | Castro-Rodriguez JA, Navarrete-Contreras P, Holmgren L, Sanchez I, Caussade S. Bronchial hyperreactivity to methacholine in atopic versus nonatopic asthmatic schoolchildren and preschoolers. J Asthma. 2010;47:929-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Nicolai T, Illi S, Tenbörg J, Kiess W, v Mutius E. Puberty and prognosis of asthma and bronchial hyper-reactivity. Pediatr Allergy Immunol. 2001;12:142-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Carlsen KH, Engh G, Mørk M. Exercise-induced bronchoconstriction depends on exercise load. Respir Med. 2000;94:750-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Lødrup Carlsen KC, Håland G, Devulapalli CS, Munthe-Kaas M, Pettersen M, Granum B, Løvik M, Carlsen KH. Asthma in every fifth child in Oslo, Norway: a 10-year follow up of a birth cohort study. Allergy. 2006;61:454-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Haby MM, Peat JK, Mellis CM, Anderson SD, Woolcock AJ. An exercise challenge for epidemiological studies of childhood asthma: validity and repeatability. Eur Respir J. 1995;8:729-736. [PubMed] [Cited in This Article: ] |
| 27. | Sano F, Solé D, Naspitz CK. Prevalence and characteristics of exercise-induced asthma in children. Pediatr Allergy Immunol. 1998;9:181-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Burr ML, Butland BK, King S, Vaughan-Williams E. Changes in asthma prevalence: two surveys 15 years apart. Arch Dis Child. 1989;64:1452-1456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 537] [Cited by in F6Publishing: 538] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 29. | Büchele G, Genuneit J, Weinmayr G, Björkstén B, Gehring U, von Mutius E, Priftanji A, Stein RT, Addo-Yobo EO, Priftis KN. International variations in bronchial responsiveness in children: findings from ISAAC phase two. Pediatr Pulmonol. 2010;45:796-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Priftanji A, Strachan D, Burr M, Sinamati J, Shkurti A, Grabocka E, Kaur B, Fitzpatrick S. Asthma and allergy in Albania and the UK. Lancet. 2001;358:1426-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Riiser A, Hovland V, Carlsen KH, Mowinckel P, Lødrup Carlsen KC. Does bronchial hyperresponsiveness in childhood predict active asthma in adolescence? Am J Respir Crit Care Med. 2012;186:493-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Rasmussen F, Lambrechtsen J, Siersted HC, Hansen HS, Hansen NC. Asymptomatic bronchial hyperresponsiveness to exercise in childhood and the development of asthma related symptoms in young adulthood: the Odense Schoolchild Study. Thorax. 1999;54:587-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | van den Nieuwenhof L, Schermer T, Heijdra Y, Bottema B, Akkermans R, Folgering H, van Weel C. Are asymptomatic airway hyperresponsiveness and allergy risk factors for asthma? A longitudinal study. Eur Respir J. 2008;32:70-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Bisgaard H, Jensen SM, Bønnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185:1183-1189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 35. | Remes ST, Pekkanen J, Remes K, Salonen RO, Korppi M. In search of childhood asthma: questionnaire, tests of bronchial hyperresponsiveness, and clinical evaluation. Thorax. 2002;57:120-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Stensrud T, Mykland KV, Gabrielsen K, Carlsen KH. Bronchial hyperresponsiveness in skiers: field test versus methacholine provocation? Med Sci Sports Exerc. 2007;39:1681-1686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Kattan M, Keens TG, Mellis CM, Levison H. The response to exercise in normal and asthmatic children. J Pediatr. 1978;92:718-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 108] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1440] [Cited by in F6Publishing: 1462] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 39. | Sterk PJ, Fabbri LM, Quanjer PH, Cockcroft DW, O’Byrne PM, Anderson SD, Juniper EF, Malo JL. Airway responsiveness. Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:53-83. [PubMed] [Cited in This Article: ] |
| 40. | Ulrik CS, Backer V, Hesse B, Dirksen A. Risk factors for development of asthma in children and adolescents: findings from a longitudinal population study. Respir Med. 1996;90:623-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Ulrik CS, Backer V. Longitudinal determinants of bronchial responsiveness to inhaled histamine. Chest. 1998;113:973-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Peat JK, Britton WJ, Salome CM, Woolcock AJ. Bronchial hyperresponsiveness in two populations of Australian schoolchildren. III. Effect of exposure to environmental allergens. Clin Allergy. 1987;17:291-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Håland G, Carlsen KC, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, Carlsen KH. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682-1689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 44. | Hovland V, Riiser A, Mowinckel P, Carlsen KH, Lødrup Carlsen KC. The significance of early recurrent wheeze for asthma outcomes in late childhood. Eur Respir J. 2013;41:838-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Yavuz ST, Civelek E, Tuncer A, Sahiner UM, Sekerel BE. Predictive factors for airway hyperresponsiveness in children with respiratory symptoms. Ann Allergy Asthma Immunol. 2011;106:365-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |