Copyright
©The Author(s) 2015.
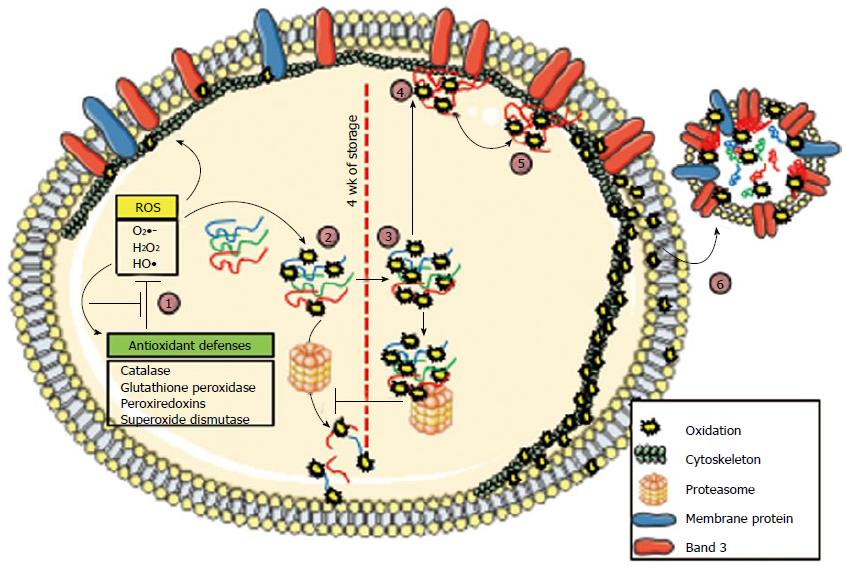
Figure 1 Representation of oxidative pathways involved in red blood cell storage.
The model shows an red blood cell (RBC), divided in the middle (dashed line) to show features of early (left half) and late stages (right half) of oxidative damage. Membrane-bound and cytosolic components are indicated in the legend. A microvesicle is shown outside the RBC (right). Progressive oxidation steps are indicated as follows: Step 1: (Left of midline) RBC antioxidant defenses prevent most oxidative damage by reducing reactive oxygen species (ROS), which converts ROS to less-reactive intermediates, or by becoming oxidized by ROS, and then recycling through a restorative mechanism. After prolonged storage, these defenses are overwhelmed by oxidative stress and protein oxidation, which occurs in both the cytosol and the plasma membrane; Step 2: As oxidized proteins unfold, they expose hydrophobic moieties that are recognized by 20S proteasome complexes, which perform proteolysis. Antioxidant defense proteins are also oxidized, and they become susceptible to proteolysis; the lack of defense leads to the overoxidation of RBC content (right of midline); Step 3: At around 4 wk of storage, overoxidized proteins undergo crosslinking, which prevents further degradation; thus, partially-oxidized proteins and damaged proteins accumulate; Step 4: Oxidized hemoglobin aggregates into hemichromes, which bind to the membrane-bound band 3 protein; this binding modifies band 3 conformation, which potentially alters its association with the cytoskeleton, and the hemichromes displace the glycolytic enzymes bound to band 3; Step 5: Hemichrome autoxidation produces ROS, which oxidize cytoskeletal and membrane proteins; Step 6: The release of microvesicles enters an exponential phase, which allows the elimination of RBC aging markers, such as altered band 3 and externalized phosphatidylserine. Reprinted from ref. [65], with permission from Elsevier.
- Citation: Delobel J, Garraud O, Barelli S, Lefrère JJ, Prudent M, Lion N, Tissot JD. Storage lesion: History and perspectives. World J Hematol 2015; 4(4): 54-68
- URL: https://www.wjgnet.com/2218-6204/full/v4/i4/54.htm
- DOI: https://dx.doi.org/10.5315/wjh.v4.i4.54