Published online Oct 18, 2018. doi: 10.5312/wjo.v9.i10.190
Peer-review started: May 7, 2018
First decision: June 14, 2018
Revised: June 20, 2018
Accepted: June 28, 2018
Article in press: June 28, 2018
Published online: October 18, 2018
Processing time: 165 Days and 16.4 Hours
To evaluate the effects of sodium alendronate on bone repair in fractures created in appendicular bones.
Wistar rats (n = 36) were allocated into three distinct groups: group C (control), group B1 (received 1 mg/kg of alendronate), and group B2 (received 3 mg/kg of alendronate). The rats underwent femoral transversal linear fracture surgery using stable internal fixation with a 2.0 mm plate and screw system. Each animal randomly received intraperitoneal applications of sodium alendronate at a dose corresponding to group B1 or B2 three times a week, while the control group received a 0.9% saline solution. Drug administration was performed until euthanasia at 45 d. The femurs were removed and each surgical piece was sent for radiographic, tomographic and microtomographic analysis. Data were submitted to descriptive and inferential statistical analysis (95% confidence interval).
Quantitative evaluations of bone neoformation did not show differences among the groups in the radiographic (P = 0.341), microtomographic (P = 0.581) and tomographic evaluations (P = 0.171). In the qualitative microtomographic analysis, a smaller distance was observed between the internal bone trabeculae in the groups that used alendronate (P = 0.05). On the other hand, group B2 had a higher amount of bone trabeculae per unit length when compared to the other groups (P = 0.04).
It is likely that the use of alendronate did not have a direct influence on the amount of bone neoformation, however it did influence the bone quality in a dose-dependent manner, ultimately affecting the distance and quantity of the trabeculae.
Core tip: Several studies have been carried out to determine both the influence of alendronate in bone repair and the appropriate dose of this drug to promote bone regeneration. In this research, 36 Wistar rats were allocated into three distinct groups that received applications of either alendronate at different doses or saline solution three times a week for 45 d. The rats underwent femoral fracture surgery with stable internal fixation. The imaginologic results suggested that the use of alendronate did not have a direct influence on the amount of bone neoformation, however it did influence bone quality in a dose-dependent manner.
- Citation: Weiss SG, Kuchar GO, Gerber JT, Tiboni F, Storrer CLM, Casagrande TC, Giovanini AF, Scariot R. Dose of alendronate directly increases trabeculae expansivity without altering bone volume in rat femurs. World J Orthop 2018; 9(10): 190-197
- URL: https://www.wjgnet.com/2218-5836/full/v9/i10/190.htm
- DOI: https://dx.doi.org/10.5312/wjo.v9.i10.190
The repair of transversal appendicular bones requires a complex process that involves several biological stages, including cell recruitment, proliferation and differentiation[1]. The literature has described both direct and indirect types of bone repair for these fractures. Direct bone healing is uncommon and is characterized by a healing area that lacks periosteal or endosteal callus formation. This effect occurs when the fracture area is rigidly fixed, causing direct remodeling of the lamellar bone, angiogenesis, and the formation of Haversian channels[2]. Indirect bone repair, the most common form of fracture healing, consists of endochondral and intramembranous bone healing and is characterized by the formation of a bone callus[3].
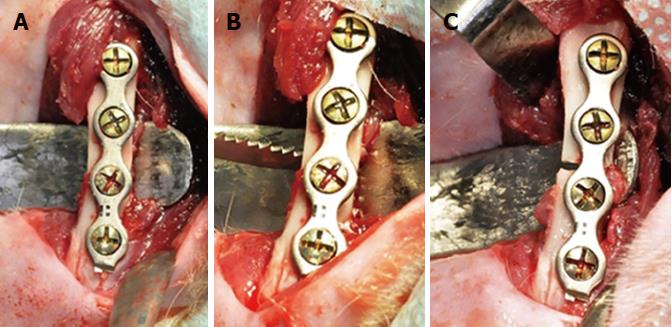
The establishment of a fracture pattern in an animal model requires both surgical and technical abilities, as well as accurate positioning and adequate bone fixation. The majority of these studies on rats are conducted with intramedullary pins, external fixators, pin-clips, etc[4]. The use of plates and screws for stable fixation in animal models is known to both favor and accelerate the repair process when compared to dispositive techniques that lack stable methods[5]. This is even the case when the bone repair is not considered direct. In this pilot study, we determined that the 2.0 system containing 4 hole plates presents better results for the bone repair of femur fractures when compared to other fixation systems.
Pharmacological agents that may modulate bone formation and bone remodeling are widely used and developed for the treatment of osteoporosis and other disorders involving bone fragility[6]. Bisphosphonates are a class of drugs that may act on bone remodeling by reducing bone resorption in a dose-dependent manner, mainly by inhibiting recruitment and promoting apoptosis of osteoclasts, while also stimulating osteoblastic activity. Bisphosphonates are available both orally (alendronate, ibandronate and risedronate) and intravenously (ibandronate and zoledronate). Among these, sodium alendronate (part of the second generation Bisphosphonate class) causes fewer side effects than the first generation class and is the most widely used antiresorptive drug[7].
There are some studies that have investigated the positive effects of sodium alendronate in bone repair[8-10]. Based on the mechanism of action of this drug, it is hypothesized that alendronate, when applied at the appropriate dose following fracture fixation, accelerates the bone repair process and thus makes prognoses more favorable.
The animal protocol was designed to minimize pain and discomfort to the animals. The experiments were carried out in the Vivarium, in the Imaging Laboratory at Positivo University, and in the Laboratory of Analysis of Minerals and Rocks at Federal University Paraná following approval by the Ethics Committee on the Use of Animals (ECUA 320). This study followed the guidelines of ARRIVE (Animal Research: Reporting in Vivo Experiment). Throughout the experiment, the ambient light, temperature and humidity of each room were controlled by a digital panel in order to maintain a photoperiod of 12 h, a temperature range of 18 °C - 22 °C, and 65% humidity. The animals were euthanized on the 45th day.
Thirty-six 4 - 5 mo old Wistar rats weighing approximately 500 g were randomly divided equally into three groups: Group C (control), group B1 (received 1 mg/kg of alendronate) and group B2 (received 3 mg/kg). Following fracture, the intraperitoneal application of sodium alendronate was administered three times per week, and the control group concomitantly received applications of 0.9% saline solution until the time of euthanasia. Intraperitoneal applications were performed on the opposite side of the fracture.
During all surgical procedures, aseptic criteria were maintained. The rats were sedated for one minute via inhalation with isoflurane (Cristália, Itapira, SP, Brazil) and anesthetized with 10% ketamine hydrochloride (Vetbrands, Paulínia, SP, Brazil) and 2% xylasin hydrochloride (Vetbrands, Paulínia, SP, Brazil) by intraperitoneal injection. After anesthesia, the rats were placed in the left lateral decubitus position, and a right femur trichotomy was then performed with vigorous antisepsis using iodopovidone. A straight incision was made approximately 5 cm along the long axis of the femur with blade number 15C, with the aid of blunt scissors, and the tissue was divulsed into muscular planes. After incision and detachment of the periosteum with a scalpel, the surface of the femur could be accessed.
Before proceeding with the osteotomy, it was necessary to complete the positioning, drilling and adaptation of the 2.0 mm 4 hole titanium plate system with 4 mm-long screws (Orthoface, Curitiba-PR, Brazil) in order to avoid poor positioning of the bone segments. The fracture was then made using a reciprocating saw (Figure 1). Abundant lavage of the wound was done using saline solution. The suture was performed in planes with isolated stitches, using Vicryl-0® thread (Ethicon, Johnson and Johnson, São José dos Campos, SP, Brazil) for the muscular plane, and nylon 4-0 (Ethicon, Johnson and Johnson , São José dos Campos, SP, Brazil) for the skin. Analgesia, inflammation and infection were controlled throughout the postoperative period.
Immediately following the surgeries, intraperitoneal applications were initiated, which lasted until euthanasia. Three weekly applications were carried out. The control group (C) received 0.9% physiological saline solutions, while the animals in groups B1 and B2 received a 1 mg/kg and 3 mg/kg dose of alendronate, respectively.
At 45 d, the animals were euthanized, and then the right femurs, plates and screws were removed. The specimens were stored separately in pots containing 10% formaldehyde. For euthanasia, the rats were exposed to overdoses of isoflurane for about 10 min. The specimens were then sent for analysis. The statistical methods used in this study were reviewed by Rafaela Scariot, Professor of Biostatistics in the Masters Program in Dentistry at Positivo University.
In order to evaluate the postoperative recovery as well as the positioning of the bone segments, the animals were submitted to digital radiography at days 7 and 45. The time-point of 7 d served only as a radiographic follow-up to observe the control and evaluate the plaque adaptation, while the time-point of 45 d was used to evaluate bone repair. The animals were sedated within 7 d and their femurs were positioned on a digital sensor (Kodak RVG 5100, Carestream Dental, Rochester, NY, United States) for radiographic imaging with an exposure time of 1.0 sec. The images were then processed and evaluated using Dental Imaging software (version 6.12.17.0 - A, Carestream Dental, Rochester, NY, United States). By the time that radiographic evaluation was taken at day 45, the animals had already been euthanized.
For evaluation of bone neoformation using the Image J program (version 1.49t National Institute of Health-NIH Bethesda, MD, United States), the examiner had previously been trained. The fracture’s region of interest was then established. To define the region of interest, a 10 mm line was drawn, 5 mms before the fracture line and 5 mms posterior to it. From this line, a rectangle was then created using the Selection Brush tool to delineate the edges surrounding the bone callus and therein obtain the total area value. Outside of the fracture region, a rectangle was also constructed to obtain the total bone area so that bone formation could later be compared with individual femur thickness. The area value in the fracture region was subtracted from the total bone area without the fracture. From this value, the excess bone value was calculated.
The 36 specimens were sent to a dental tomography center, which used the same tomography device calibrated at 120 kVp and 36.12 mAs (i-CAT® CONE BEAM 3-D, Kavo Kerr, Joinville-SC, Brazil), to construct images using an exposure time of 40 s with 0.25 Voxel. From this scan, 216 tomographic sections with a 250 μm pixel size were obtained for each set of five samples. In order to decrease the number of intakes, the femurs to be evaluated by tomography were grouped into an acrylic base that accommodated up to five femurs. These were placed vertically and, with the help of utility wax, attached to the base. After acquisition of the tomographic sections, the images were analyzed using I-CAT Vision software.
The longitudinal distance of the defect, in which the amount of bone was generated laterally in the sample, was evaluated. The distance between the contact points of the femurs and the femoral diameter were also measured without considering the effects of formation and the formed bone callus. The I-CAT Vision software helped facilitate the analysis of distances by allowing the examiner to view samples in two distinct ways: through the Implant Screen, which provides an analysis of multiple tomographic sections within a selected region of interest, or the MPR Screen, which displays three views of the obtained femurs. In the MPR Screen, it was possible to analyze distances by using the axial, coronal and sagittal views of the samples, and also by using regions of interest that delineated the femurs individually. This screen was therefore used for analysis. In addition to the advantage of easily performing distance analysis, the brightness and contrast scales could also be altered so that only the femur, which is more radiopaque, is detected. This makes it possible to identify the diameter of the femur. Despite using the diameter for analysis, the femur is not exactly cylindrical, so the distance acquired corresponds to the greater distance of the femur and the longitudinal dimension of the defect. This sample movement was possible with the “Explore” function in the software. Once the region of interest, which corresponded to a single femur, was selected by using the rulers shown on the sides of each image of the figure, the “Explore” function of the software was performed. In the coronal image, the formation of a circle indicating the presence of a diameter was observed. Using the cursor, we could rotate the inscribed sample in any direction within the plane. The distance measurements were done by selecting the “Distance” software tool and dragging the cursor.
The computerized microtomography analysis was performed using a Skyscan computed micro-CT model 1172 (Bruker Skyscan, Luxembourg, Belgium), with power and current adjusted to 90 kV and 112 μA, respectively. The pixel size was 12.8 μm, and a multichannel acquisition camera with a resolution of 2000 x 1336 pixels was used to detect signal. No filters were used to correct the energy of the X-ray beam. The portion was rotated 180º with a rotation step of 0.4º. A projection image of the part was obtained for each rotation step. The exposure time of the sample within the X-ray beam was 1.1 s per rotation step, and the total acquisition time of the projection images was 30 min. After acquisition, projection images were processed using NRecon software (Bruker, Luxembourg, Belgium). The software reconstructed the projection images into tomographic sections using the Feldkamp algorithm. After obtaining the tomographic sections, the measurements of the three-dimensional space, trabecular formation and bone volume were evaluated by the software, which included the CT Analyzer (v.1.16.1.0 +; Bruker Skyscan, Kontich, Belgium) and the Dataviewer (v.1.5.2.4; Bruker Skyscan, Kontich, Belgium). In the CT Analyser software, it is possible to separate different mineral densities from the contrast difference shown in the tomographic section slices. The contrast in the tomographic section image comes from the different radiopacities of the materials in the sample, according to the interaction of these phases with the X-ray beam. This makes it possible to separate the neoformed bone from both the autogenous bone and cartilaginous material, each of which have different radiopacities.
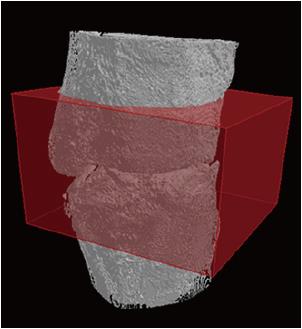
Quantitative analysis begins with the binarization process of the sample’s different phases. This binarization process corresponds to a range of gray tones to which each radiopaque material fits. The range of gray tones used to determine neoformed bone volume corresponds to 50 - 105 on a total dimensionless scale from 0 - 255. The determination of this volume occurs through the delimitation of a region of interest into a prismatic format (12 mm x 11 mm x 6.3 mm). This involves the whole region of the defect and bone callus, which is in the center of the defect (Figure 2). Thus, bone formation was investigated both in the defect region and in the volume of bone callus formed. This created region was used for all pieces. From this procedure, the analysis of initial bone, neoformed bone and total surface area of bone formation, including the bone callus (lateral available area of the femoral bone), were obtained. The measurements of the relationship between the new bone formation and the femoral surface area were obtained by determining the difference in thickness between samples.
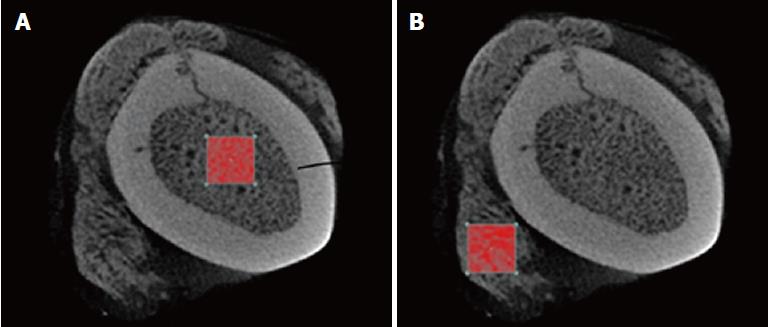
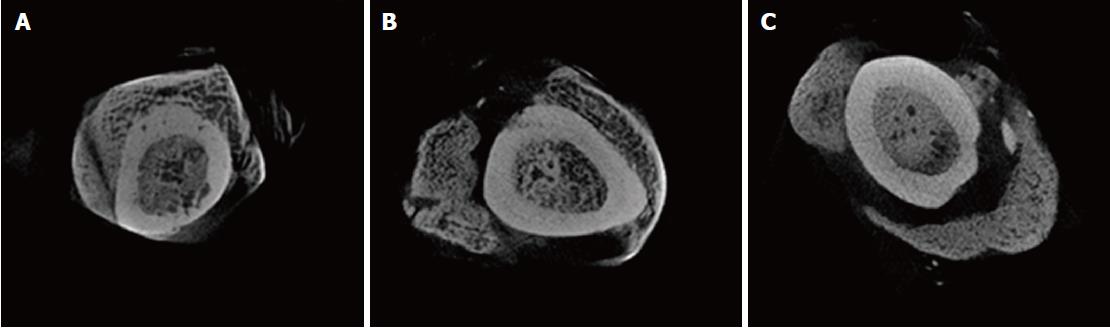
To analyze the trabeculae that was internally generated in both the bone and external region, a region of interest was made both inside and outside of the femur bone (Figure 3) by selection an area that had the maximum density of trabecular bone within the bone callus. Calculations using CTAn software were made of the following: the mean trabecular thickness (Tb.Th) of the internal and external regions, the trabecular linear density (Tb.N), which measures the average number of trabeculae per unit length, and the average distance between the internal and external trabeculae (Tb.Sp)[11,12].
The results were subjected to descriptive and statistical analysis. Statistical evaluation was performed using a frequency data specific test called the Statistical Package for Social Science program (SPSS, version 24.0; SPSS Inc., Chicago, IL – United States) using a 95% confidence interval. The values obtained were subjected to a normality test (Shapiro-Wilk), and parametric variables were described using mean and standard deviation. Nonparametric variables were described as minimum, median and maximum. For comparison between groups, the ANOVA and Kruskal-Wallis tests were used, according to the normality of the variable. When there was a statistical difference detected between the groups, a Tukey test was performed for parametric samples. For nonparametric samples, comparisons were performed in two groups using the Mann-Whitney test.
Bone formation, which was evaluated by radiographic analysis, was higher in group B2 (91.683 ± 35.657 mm²), followed by the control group (65.57 ± 32.642 mm²) and group B1 (62.670 ± 45.578 mm²). The measurements obtained (bone surplus) showed no difference between the groups (P = 0.341).
Tomographic evaluation of the samples did not show quantitative differences in bone neoformation among the groups (One-way ANOVA test; P = 0.171). In Group C, the measurement between the defect and femur ratio (mm³/mm²) was 1.76 ± 0.56. The measurements in group B1 and B2 were 1.59± 0.31 and 1.44 ± 0.21, respectively.
Considering the relationship between the new bone formation and the femur surface area, the B2 group [5.87 (2.10 – 14.60) mm³/mm²] presented greater bone formation when compared with the B1 group [4.88 (2.30–16.02) mm³/mm²] and C group [5.65 (3.64 – 12.40) mm³/mm²]; however, there was no difference among the groups (Kruskall-wallis test/P = 0.581).
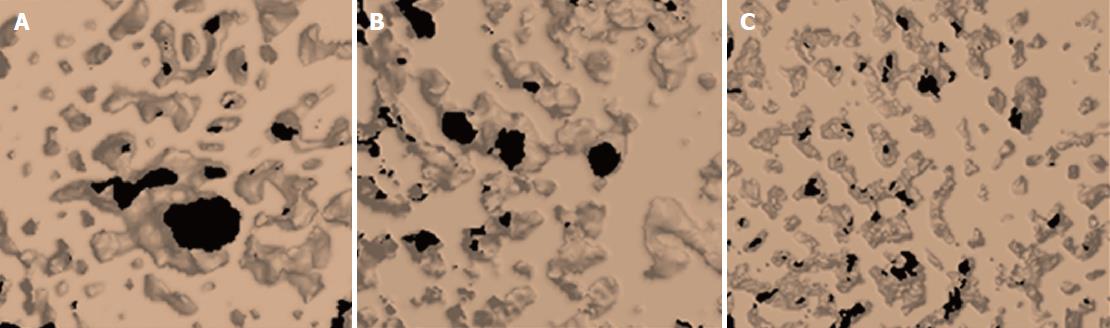
In qualitative microtomographic analysis, it was possible to observe that there was a difference in the number of trabeculae per unit length (Tb.N) between groups (P = 0.05). Group B2, when compared with both groups, had higher linear density.
There was also a significant difference between groups in the spacing of the internal bone trabeculae, showing that the Tb.Sp is lower in the control group vs. the B2 group (P = 0.04, Figures 4 and 5). The data for Tb.N, Tb.Th and Tb.Sp can be found in Table 1.
| Group C | Group B1 | Group B2 | P | |
| Tb.Th | 0.11 (0.09-0.14) | 0.09 (0.07-0.16) | 0.14 (0.09-0.16) | 0.07 |
| Tb.N | 5.26 (3.39-6.24)1 | 5.45 (1.42-5.87)2 | 5.94 (2.49-6.40)1,2 | 0.05 |
| Tb.Sp | 0.10 (0.06-0.16) | 0.09 (0.05-0.35) | 0.06 (0.05-0.21) | 0.07 |
| Tb.Th | 0.09 (0.07-0.13) | 0.10 (0.06-0.23) | 0.12 (0.06-0.15) | 0.06 |
| Tb.N | 4.60 (0.13-6.15) | 4.78 (0.15-6.90) | 6.15 (0.22-7.22) | 0.13 |
| Tb.Sp | 0.20 (0.08-0.50)3,4 | 0.09 (0.05-0.44)3 | 0.07 (0.05-0.42)4 | 0.04 |
The aim of this study was to use image analysis to evaluate the evolution of appendicular femur repair when fixed with plates and treated with varying alendronate concentrations. It is known that during the early stages of bone healing, a less rigid mechanical environment results in a prolonged phase of chondral bone regeneration, whereas the intramembranous ossification process appears to be independent of mechanical stability[13]. In direct osseous repair, there is no formation of a bone callus. Therefore, it is possible to predict that when using fixation with plates and screws, direct healing occurs since there is no movement of the bone preserves. However, in animal models, this is not true; indirect healing occurs instead because the animal is not immobilized and the plates are not designed for animals, thus generating micromovement in the region. During early fracture healing, mechanical stimulation appears to enhance callus formation, however the extent of callus formation does not correspond to rigidity[14].
Sodium alendronate is a drug that prevents bone resorption and may induce osteogenesis by inhibiting osteoclast activity. As a result, it is able to both maintain and promote callus formation in bone repair of fractures, as well as increase bone mineral density in the fracture region[15]. In the present study, we demonstrated that alendronate at concentrations of 1 mg/kg and 3 mg/kg did not alter the amount of bone neoformation according to imaging analysis. Remarkably, bone neoformation was equal among all treatment groups.
Under qualitative microtomographic analysis, we observed that bone repair was more effective in the groups that received sodium alendronate applications, especially in the group with the highest dosage. This was visualized through the greater number of trabeculae and smaller spacing between the trabeculae in the 3 mg/kg group. Since alendronate promotes osteoblasts and mesenchymal cell osteoblastogenesis, while also inhibiting osteoclastic activity[6,16], this may suggest that the amount and arrangement of bone trabeculae is directly linked to the dosage and administration of alendronate. This study suggests that the higher the dose, the larger the expansion of mineral-like matrices, while spacing among these areas (chondroid or osteoid matrix) remains lower.
One hypothesis that should be considered and may explain the results found here is the likely action of TGFβ1, which was previously shown to increase upon alendronate administration[17]. It is noteworthy that this cytokine is an important growth factor that may contribute to mineral expansion. In the endosteum area, bone matrix deposition is common and independent of the chondroid area. Thus, alendronate could be responsible for the increase of this cytokine that may, in turn, increase BMP2 expression. This was observed in a recent study where specimens receiving alendronate showed a significant increase of this protein, which improved bone deposition in rabbit calvarias[18].
On the other hand, the same situation may be extrapolated for the periosteal area. Regarding this peculiar topography, chondrocyte expansion may be an effect that is strictly associated with functional endogenous TGFβ signaling. In addition to this effect, TGFβ1 also induces the differentiation of hypertrophic cartilage, which is required for calcium deposition and ossification in this topography. To reach this conclusion, the authors of this study induced the inhibition of specific TGFβ receptors. They therein verified that TGFβ suppression culminated in the inhibition of cartilaginous growth and chondroid differentiation, while also inhibiting both the medullary area and hematopoiesis[19].
Thus, all these hypotheses are possible explanations of our results. We observed an important growth in minerals through accurate imaging analysis, independent of whether the matrix was chondroid or osteoid.
Sodium alendronate at concentrations of 1 mg/kg and 3 mg/kg, when assessed by imaging tests, did not alter the amount of bone neoformation.
Sodium alendronate interferes with the quality of bone neoformation in the context of the quantity and disposition of bone trabeculae. The higher the dose of alendronate, the greater the number of trabeculae and the smaller the spaces among them.
Bisphosphonates are potent inhibitors of bone resorption. Sodium alendronate is the most used drug of this class, and may act on bone remodeling by reducing bone resorption in a dose-dependent manner. Its mechanism of action works primarily by both inhibiting the recruitment and promoting the apoptosis of osteoclasts, while simultaneously stimulating osteoblastic activity.
Despite what is currently know about alendronate-induced bone repair alterations, the literature has not yet fully elucidated the appropriate dose required to achieve better bone regeneration, nor the effects of this drug when using fixation methods.
To evaluate the dose-dependent effects of sodium alendronate on bone repair in treated femur fractures by using stable internal fixation and imaging tests (radiography, tomography and microtomography).
Wistar rats were separated into three distinct groups that received applications of either saline solution (control) or different doses of alendronate. The rats then underwent femoral transversal linear fracture surgery using stable internal fixation. Drug administration lasted 45 d. The femurs were sent for radiographic, tomographic and microtomographic analysis in order to evaluate bone quantity and quality.
Results did not reveal differences in bone quantity by radiographic, tomographic and microtomography analysis. However, when analyzing bone quality, it was evident that alendronate affected the distance and quantity of trabeculae in a dose-dependent manner, thus promoting better bone regeneration.
Our research results reveal that sodium alendronate, at concentrations of 1 - 3 mg/kg when assessed by imaging tests, does not alter the amount of bone neoformation. Nevertheless, it does interfere with the quality of bone neoformation when considering the quantity and disposition of bone trabeculae. The higher the dose of alendronate, the greater the number of trabeculae and the smaller the spaces among them.
More research using this method of fixation and sodium alendronate are required and may relate, for example, to the mechanical force of the specimens. It is also important to compare the effects of alendronate with different markers. We suggest that follow-up studies use a dose of 1 mg/kg alendronate, since we have demonstrated here that it successfully promotes bone regeneration.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Anand A, Elgafy H, Li JM S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Song H
| 1. | Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19:S4-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1113] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 3. | Green E, Lubahn JD, Evans J. Risk factors, treatment, and outcomes associated with nonunion of the midshaft humerus fracture. J Surg Orthop Adv. 2005;14:64-72. [PubMed] |
| 4. | Holstein JH, Garcia P, Histing T, Kristen A, Scheuer C, Menger MD, Pohlemann T. Advances in the establishment of defined mouse models for the study of fracture healing and bone regeneration. J Orthop Trauma. 2009;23:S31-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Neagu TP, Ţigliş M, Popp CG, Jecan CR. Histological assessment of fracture healing after reduction of the rat femur using two different osteosynthesis methods. Rom J Morphol Embryol. 2016;57:1051-1056. [PubMed] |
| 6. | Fu L, Tang T, Miao Y, Zhang S, Qu Z, Dai K. Stimulation of osteogenic differentiation and inhibition of adipogenic differentiation in bone marrow stromal cells by alendronate via ERK and JNK activation. Bone. 2008;43:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Maeda SS, Lazaretti-Castro M. An overview on the treatment of postmenopausal osteoporosis. Arq Bras Endocrinol Metabol. 2014;58:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Fu LJ, Tang TT, Hao YQ, Dai KR. Long-term effects of alendronate on fracture healing and bone remodeling of femoral shaft in ovariectomized rats. Acta Pharmacol Sin. 2013;34:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Oliveira D, Hassumi JS, Gomes-Ferreira PH, Polo TO, Ferreira GR, Faverani LP, Okamoto R. Short term sodium alendronate administration improves the peri-implant bone quality in osteoporotic animals. J Appl Oral Sci. 2017;25:42-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Sandberg O, Bernhardsson M, Aspenberg P. Earlier effect of alendronate in mouse metaphyseal versus diaphyseal bone healing. J Orthop Res. 2017;35:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Hildebrand T, Rüegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc. 1997;185: 67-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1303] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 12. | Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2930] [Cited by in RCA: 3346] [Article Influence: 223.1] [Reference Citation Analysis (0)] |
| 13. | Epari DR, Schell H, Bail HJ, Duda GN. Instability prolongs the chondral phase during bone healing in sheep. Bone. 2006;38:864-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Jagodzinski M, Krettek C. Effect of mechanical stability on fracture healing--an update. Injury. 2007;38 Suppl 1:S3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Aonuma H, Miyakoshi N, Kasukawa Y, Kamo K, Sasaki H, Tsuchie H, Segawa T, Shimada Y. Effects of combined therapy of alendronate and low-intensity pulsed ultrasound on metaphyseal bone repair after osteotomy in the proximal tibia of aged rats. J Bone Miner Metab. 2014;32:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Giuliani N, Pedrazzoni M, Negri G, Passeri G, Impicciatore M, Girasole G. Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone. 1998;22:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 197] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Giovanini AF, Göhringer I, Tavella R, Linzmeyer MC, Priesnitz TF, Bonetto LM, Resende RG, Scariot R, Zielak JC. Intermittent administration of PTH induces the expression of osteocalcin and BMP-2 on choroid plexus cells associated with suppression of sclerostin, TGF-β1, and Na+-K+ATPase. Endocrine. 2018;59:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Göhringer I, Muller CLS, Cunha EJ, Passoni GNS, Vieira JS, Zielak JC, Scariot R, Deliberador TM, Giovanini AF. Would Be Prophylactic Administrations of Low Concentration of Alendronate an Alternative for Improving the Craniofacial Bone Repair? A Preliminary Study Focused in the Period of Cellular Differentiation and Tissue Organization. J Craniofac Surg. 2017;28:1869-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Tekari A, Luginbuehl R, Hofstetter W, Egli RJ. Transforming growth factor beta signaling is essential for the autonomous formation of cartilage-like tissue by expanded chondrocytes. PLoS One. 2015;10:e0120857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |