Published online Apr 18, 2017. doi: 10.5312/wjo.v8.i4.342
Peer-review started: October 17, 2016
First decision: December 15, 2016
Revised: January 10, 2017
Accepted: February 8, 2017
Article in press: February 13, 2017
Published online: April 18, 2017
To assess serum levels of RANK-ligand (RANKL) and osteoprotegerin (OPG) as biomarkers for periprosthetic joint infection (PJI) and compare their accuracy with standard tests.
One hundred and twenty patients presenting with a painful total knee or hip arthroplasty with indication for surgical revision were included in this prospective clinical trial. Based on standard diagnostics (joint aspirate, microbiological, and histological samples) and Musculoskeletal Infection Society consensus classification, patients were categorized into PJI, aseptic loosening, and control groups. Implant loosening was assessed radiographically and intraoperatively. Preoperative serum samples were collected and analyzed for RANKL, OPG, calcium, phosphate, alkaline phosphatase (AP), and the bone-specific subform of AP (bAP). Statistical analysis was carried out, testing for significant differences between the three groups and between stable and loose implants.
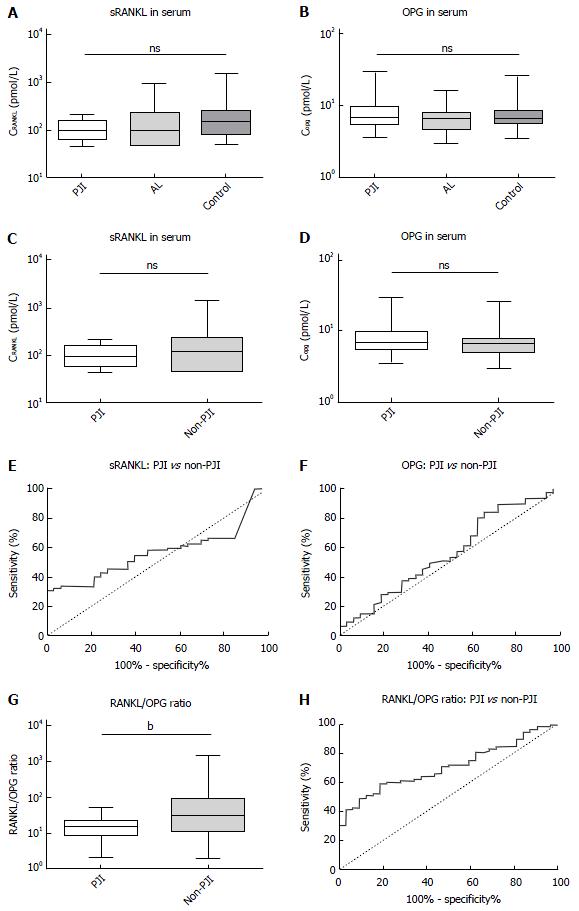
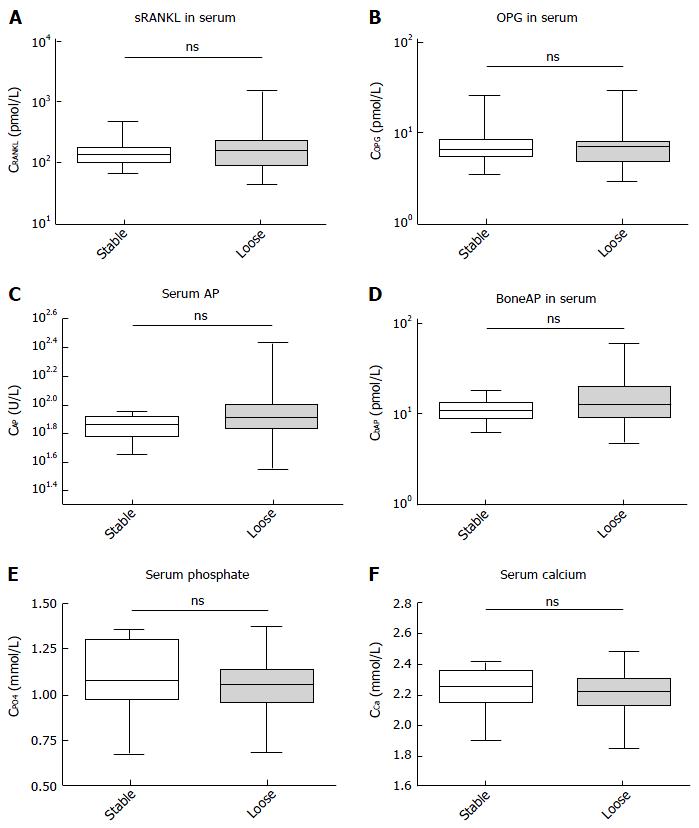
All three groups were identical in regards to age, gender, and joint distribution. No statistically significant differences in the serum concentration of RANKL (P = 0.16) and OPG (P = 0.45) were found between aseptic loosening and PJI, with a trend towards lower RANKL concentrations and higher OPG concentrations in the PJI group. The RANKL/OPG ratio was significant for the comparison between PJI and non-PJI (P = 0.005). A ratio > 60 ruled out PJI in all cases (specificity: 100%, 95%CI: 89, 11% to 100.0%) but only 30% of non-PJI patients crossed this threshold. The positive predictive value remained poor at any cut-off. In the differentiation between stable and loose implants, none of the parameters measured (calcium, phosphate, AP, and bAP) showed a significant difference, and only AP and bAP measurements showed a tendency towards higher values in the loosened group (with P = 0.09 for AP and P = 0.19 for bAP).
Lower RANKL and higher OPG concentrations could be detected in PJI, without statistical significance.
Core tip: No statistically significant differences in the serum concentration of RANK-ligand (RANKL) and osteoprotegerin (OPG) were found between aseptic loosening and periprosthetic joint infection (PJI) with a certain trend of lower concentrations in the PJI group. Nevertheless, a RANKL/OPG ratio > 60 ruled out PJI in all cases. In the differentiation between a stable and loose implant the parameters measured showed no significant difference, which let to the conclusion that the sole use of these parameters for differentiating PJI and aseptic loosening cannot be recommended. RANK and OPG may have utility as a conformation test but are not an effective screening parameter for the discrimination of PJI and AL.
- Citation: Friedrich MJ, Wimmer MD, Schmolders J, Strauss AC, Ploeger MM, Kohlhof H, Wirtz DC, Gravius S, Randau TM. RANK-ligand and osteoprotegerin as biomarkers in the differentiation between periprosthetic joint infection and aseptic prosthesis loosening. World J Orthop 2017; 8(4): 342-349
- URL: https://www.wjgnet.com/2218-5836/full/v8/i4/342.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i4.342
Periprosthetic joint infection (PJI) after total joint replacement still remains one of the most serious complications and is a key challenge in orthopedic surgery. A precise and rapid diagnosis of implant failure is mandatory for treatment success. The differentiation between PJI and aseptic loosening can, in particular, be unyielding or controversial, and misdiagnosis can lead to serious and permanent impairment. As the treatment of PJI is completely different from the treatment of aseptic loosening, its correct and timely diagnosis is crucial for successful therapy and relies in part on the use of molecular markers. Nevertheless, establishing a definite diagnosis of PJI prior to surgical intervention is at times difficult. Numerous researchers have focused on the development of novel and more accurate molecular methods[1-5]. However, there is no diagnostic gold standard so far. Various definitions have been proposed and current recommendations are based on several prae-, intra-, and postoperative parameters[6,7].
Previous studies have suggested osteoprotegerin (OPG) and receptor activator of nuclear factor-ĸB ligand (RANK Ligand, RANKL) as markers of periprosthetic osteolysis[8,9]. RANKL and its receptor RANK and OPG play an important role in osteoclastogenesis as final effectors of bone resorption. RANKL, which expresses on the surface of osteoblast, stromal cells and activated T-lymphocytes, binds to RANK on osteoclastic precursors cells or mature osteoclasts, and thereby promotes osteoclastogenesis and bone resorption. OPG, which is expressed by osteoblasts and stromal cells, strongly inhibits bone resorption by binding to its ligand RANKL, and thereby preventing it from binding to its receptor, RANK. The RANKL/RANK/OPG system regulates the formation of multinucleated osteoclasts from their precursors as well as their activation and survival in normal bone remodeling[10,11]. Therefore, the balance between OPG and RANKL is essential to regulate bone remodeling, by controlling the activation state of RANK on osteoclasts[12].
In cases of aseptic loosening, it has been demonstrated that the accumulation of wear debris around the joint leads to an activation of mononuclear cells and T-lymphocytes, resulting in a multinuclear cell giant cell reaction. This causes an osteoclast activation and bone resorption[13]. Periprosthetic membranes retrieved from patients with aseptic loosening contain fibroblasts, macrophages, and T lymphocytes[14], as well as osteoclasts and multinucleated foreign body giant cells[15]. This periprosthetic tissue produces a variety of factors including tumor necrosis factor (TNF), interleukin-1 (IL-1), IL-6 and other peptides that stimulate osteoclasts through the induction of RANKL[16,17]. TNF in turn directly stimulates the production of RANKL by stromal cells, T-lymphocytes, and endothelial cells. Indirect stimulus of RANKL expression works the TNF-induced up regulation of prostaglandins, IL-1 or IL-17, resulting in an advanced expression of RANKL as well. The dominant form of this response is due to innate reactivity to implant debris through danger associated molecular pattern signaling and inflammatory responses[18].
Correspondingly, in the pathogen-associated molecular patterns in PJI, bacterial toxins and parts of the pathogen’s cell membrane seem to induce infiltration with mainly neutrophil granulocytes and macrophages. Though the trigger is a different one, the final result with bone loosening and prosthesis failure is the same. So far, there are no investigations concerning the exact role of interleukins and RANKL/OPG signaling in PJI-associated prosthesis failure. The role of the RANKL/RANK/OPG system has not yet been examined in the differentiation between PJI and aseptic prosthesis loosening. In this study, therefore, we defined the sensitivity, specificity, and accuracy of RANKL and OPG in patients with PJI vs aseptic loosening and compared these results to current standards of diagnostic testing. Total joint replacement without signs of PJI or aseptic loosening severed as the control group. Furthermore, we tested whether there is a difference between loosened and stable implants in the serum levels of these and other parameters.
Our hypothesis was that the measured serum levels of RANKL and OPG correlate positively: (1) with the presence of PJI; and (2) with implant loosening. Secondly, we investigated if the serum levels of calcium (Ca2+), phosphate (PO4), and alkaline phosphatase (AP) would be different in stable or loosened implants.
This prospective study was approved by the local Institutional Review Board and Ethics committee with informed consent obtained in compliance with the declaration of Helsinki prior to being enrolled in the study. Between 2010 and 2011 we included 120 consecutive patients presenting with a painful total hip or total knee arthroplasty undergoing revision arthroplasty surgery for (1) PJI; (2) aseptic failure (AL); or (3) aseptic revision causes without PJI or aseptic loosening. Any patient scheduled to undergo revision surgery of a hip or knee arthroplasty were included. After signing of informed consent, all patients underwent standardized diagnostics as outlined in literature[19]. Preoperative serum samples were collected and joint aspiration was performed under strictly aseptic conditions for cell count, cell differentiation, and microbiological analysis.
White blood cell count was determined from the blood samples, and serum samples were analyzed for C-reactive protein (CRP) (Dimension Vista, Siemens Medical Solutions Diagnostics GmbH, Eschborn, Germany), RANKL, and OPG (Sandwich ELISA, Fa. BioVendor GmbH, Heidelberg, Germany); Serum Ca2+, serum PO4, AP and the bone-specific subform of the AP (bAP) were also analyzed in serum (Immunolite, Siemens, Eschborn, Germany). Ratio of RANKL/OPG was calculated from the determined values.
Intraoperatively, tissue specimens were taken for microbiological and histological analysis[20], and the intraoperative aspect was recorded. Assessment of relevant implant instability is a routine for the experienced arthroplasty surgeon and part of many revision algorithms. If in the surgeon’s view at least one implant component with bony contact could be removed with ease after debridement, the implant was considered “loosened”[19]. Also, radiographic signs of loosening were taken into account where implant migration or dislocation could clearly be seen preoperatively.
Depending on the results of the laboratory diagnostics, including serum CRP as well as cell count and differentiation of the aspirate, microbiologic assessment of aspirate and intraoperative cultures, as well as histopathology of the intraoperative samples, the diagnosis of PJI was considered proven following the criteria according to the Musculoskeletal Infection Society (MSIS) consensus paper by Parvizi et al[7], independent of the implant being loose or stable. Those who did not meet the criteria for a diagnosis of PJI and required a revision due to loosening were assigned as aseptic loosening (AL) group. Those without signs of PJI or loosening were assigned as controls (control group). For subanalysis of loosening, the PJI group was divided for those presenting with a macroscopically loosened implant vs those with stable implants. Demographic data (age, sex, body mass index, type of prosthesis [total hip arthroplasty (THA)/total knee arthroplasty (TKA)] were collected for comparative analysis.
Data were collected in Microsoft Excel (Microsoft Corporation, Richmond, United States), and statistical analysis was carried out using GraphPad Prism 5.04 (GraphPad Software, La Jolla, CA, United States), testing for statistical significance between the three groups with Kruskal-Wallis-ANOVA without assuming normal distribution and with Dunn’s post-hoc test. To test for significance between PJI vs non-PJI or stable vs loose, Mann-Whitney t-tests were used, and Receiver-Operator-Characteristic (ROC) curves were calculated to assess the discriminatory strength on the basis of the area under the curve (AUC) and to determine optimal cut-off. Nonparametric Correlation (Spearman) was calculated between selected parameters. According to the “Standards for Reporting of Diagnostic Accuracy”, probabilistic measures, such as sensitivity, specificity, likelihood ratios, and their confidence limits for individual values and combinations were calculated[21]. For calculating the geometric coefficient of variation (GCV), data was log-transformed and coefficient of variation calculated from the transformed data set.
One hundred and twenty patients were enrolled into our prospective cohort study. In all groups, there were no differences with regard to age, gender, or joint distribution. In the PJI group (26 THA, 54%) and in the aseptic loosening group (35 THA, 69%), more THA were recruited, while the control group included more TKA (13 TKA, 62%). The patient demographics and details are given in Table 1. In our collective, 31 out of 48 patients (64%) in the PJI group had consistent findings in two or more positive microbiology cultures, matching the “major” MSIS criterion for microbiology; another five patients had one positive culture, and the remaining 12 patients were “culture negative” PJIs.
| Group | Total (n) | Mean age (± SD) | Sex (W:M) | Joint (hip:knee) |
| PJI | 48 | 69.5 yr | 27 female | 22 TKA |
| (± 12.1 yr) | 21 male | 26 THA | ||
| Aseptic loosening | 51 | 68 yr | 33 female | 16 TKA |
| (± 11.1 yr) | 18 male | 35 THA | ||
| Control | 21 | 64.05 yr | 13 female | 13 TKA |
| (± 11.9 yr) | 8 male | 8 THA | ||
| All | 120 | 67.94 | 73 female | 51 TKA |
| (± 11.7) | 47 male | 69 THA | ||
| P | 0.2686 | 0.8611 | 0.1110 |
Statistical analysis was completed to compare the means of laboratory values between the three groups. The results are summarized in Figure 1A and B. We found no significant differences in the mean values of PJI, AL, or the control group in the serum concentration of RANKL (P = 0.16) or OPG (P = 0.45) with a certain trend of lower RANKL concentrations and higher OPG concentrations in the PJI group. The “geometric” coefficients of variation were within a tolerable range. For RANKL, we calculated GCV as 10.65% (PJI), 18.6% (AL), and 15.85% (Control), for OPG we calculated GCV as 21.46% (OPG), 19.33% (AL) and 19.65% (Control). To assess discriminatory strength of these parameters, we pooled the AL and control group into a larger non-PJI group and calculated ROC with AUC, and a non-parametric t-test (Figure 1C-F). Neither RANKL nor OPG showed a significant difference (P = 0.26 for RANKL and P = 0.3 for OPG), and discriminatory strength was poor (AUC: 0.57 ± 0.05 for RANKL and 0.56 ± 0.06 for OPG). Since the aforementioned trend was still visible, we calculated sensitivity and specificity for different cut-offs and found the best, yet still poor likelihood ratio to detect a PJI for RANKL at < 188.9 pmol/L [sensitivity: 93.94%, 95% confidence interval (95%CI): 79,77% to 99.26%; specificity: 32.47%, 95%CI: 22.23% to 44.10%, likelihood ratio: 1.39], and for OPG at > 9.38 pmol/L (sensitivity: 28.13%, 95%CI: 13.75% to 46.75%; specificity: 89.33%, 95%CI: 80.06% to 95.28%, likelihood ratio: 2.64).
To determine if the parameters were independent of each other, we calculated the Spearman correlation, which showed an r of 0.01 (95%CI: 0.18 to 0.21, P = 0.88) stating that there was neither a positive nor a negative correlation between OPG and RANKL. We therefore calculated the RANKL/OPG ratio as an additional parameter, to make use of possible synergistic effects. Though this parameter also remained without statistical significance between all three groups (P = 0.1), the comparison between PJI and non-PJI (Figure 1G and H) was significant (with P = 0.005) and the discriminatory strength was much enhanced (AUC: 0.7 ± 0.05). A ratio > 60 ruled out PJI in all cases (specificity: 100%, 95%CI: 89.11% to 100.0%) but only 30% of non-PJI patients crossed this threshold (95%CI: 21.67% to 40.29%), while the positive predictive value remained poor at any cut-off.
Both groups, PJI and non-PJI included patients where parts of the prosthesis were loosened. We therefore assessed whether or not any of the parameters would correlate with the bony integration and a stable interface of the prosthesis. None of the parameters measured showed a significant difference in this analysis (Figure 2), and only the AP and bAP measurements showed a tendency towards higher values in the loosened group (with P = 0.09 for AP and P = 0.19 for bAP). No other trends were visible, and no further statistics were calculated.
The accurate diagnosis of PJI is difficult, as the clinical symptoms often resemble those of aseptic loosening, with nonspecific pain and swelling of the joint. Though both entities share a common final pathway, leading to osteolysis and implant failure, their exact pathomechanisms remain unclear. Analyzing the available evidence and existing published data on the definition of PJI, a workgroup convened by the MSIS presented a summary of recommendations concerning a new definition for PJI[7]. These recommendations are based on clinical findings, laboratory parameters, sterile joint aspiration for synovial leucocyte count, and microbiological analysis as well as tissue sampling for histopathology. Nevertheless, because of the inconsistent data, even the MSIS cannot provide general recommendations in interpretation of single aspects (e.g., different cut-off values of CRP or leukocyte count in synovial tests). Consequently, there is a need for further research and development into new methods aimed at improving diagnostic accuracy and speed of detection.
Several studies have attempted to assess the clinical relevance of RANKL and OPG levels in a variety of human diseases characterized by local or systemic changes in bone remodeling[8,17,22,23]. The essential role of the OPG/RANK/RANKL pathway in regulating bone remodeling around orthopedic implants is well recognized, but the clinical usefulness of circulating OPG and RANKL levels in the differentiation between PJI and aseptic loosening is unknown.
Our hypothesis was therefore that the measured serum levels of RANKL and OPG correlate positively: (1) with the presence of PJI; and (2) with implant loosening. Secondly, we investigated if the serum levels of calcium, phosphate, and alkaline phosphatase would be different in stable or loosened implants.
According to the results, we had to discard our above mentioned hypotheses, as we found no significant differences in the mean values of circulating RANKL and OPG in PJI vs AL or control groups, but found a certain trend of lower RANKL concentrations and higher OPG concentrations in the PJI group.
Granchi et al[8] tried to evaluate whether serum levels of OPG and RANKL could be different in patients with aseptic loosening compared to patients with stable implants. While the serum levels of RANKL and the OPG-to-RANKL ratio showed no significant changes with the clinical condition or status of the implant, an increased serum level of OPG provides good diagnostic accuracy in detecting implant failure due to aseptic loosening with a sensitivity of 92%, a specificity of 75%, and a positive likelihood ratio of 7.1[8]. These findings are in accordance with the results of He et al[24] who analyzed multiple biomarkers for the detection of aseptic loosening in total hip arthroplasty. They found elevated plasma levels of OPG in failed THA and an increase of OPG plasma level from stable healthy patients to early aseptic loosening to late aseptic loosening, stating that OPG may reflect a protective mechanism of the skeleton to increased bone resorption thereby inhibiting osteoclast formation and bone resorbing activity in aseptic loosening. These findings are in contrast to our observations, as we could not see any significant differences of OPG and RANKL plasma levels in the subanalyses between stable and loose implants. Only the AP and bAP measurements showed a tendency towards higher values in the loosened group. On the other hand, we successfully evaluated and confirmed that the RANKL/OPG ratio as an additional parameter may help in the differentiation between aseptic loosening and PJI as a ratio > 60 ruled out PJI in all cases. These results suggest that osteolysis inside the periprosthetic interface of artificial joints is not associated with a significant systemic elevation of the RANKL/RANK/OPG system.
The current paradigm to explain aseptic loosening involves an inflammatory response to wear debris particles produced by prosthetic implants. These particles are phagocytosed by macrophages adjacent to the implant resulting in cell activation and the release of cytokines as well as in a localized inflammatory response. By examination of periprosthetic tissues of 59 patients undergoing hip replacement revision for aseptic loosening Veigl et al[25] could show that RANKL is present only in tissues with a large amount of wear debris and predominantly in cases involving loosened cemented implants. Gehrke et al[9] examined the presence and distribution of RANKL, RANK and OPG in the periprosthetic interface in cases of septic and aseptic loosening by immunohistochemistry and immunoblotting. They could show a different histopathologic pattern as well as a difference in grade of inflammatory infiltrate. The inflamed periprosthetic tissue produces a variety of factors including TNFα, IL-1, IL-6 and prostaglandin stimulating osteoclast to resorb bone through the induction of RANKL. However, none of these cytokines represents a final common pathway for the process of particle-induced osteoclast differentiation and maturation. While many of these biomarkers are established in the differentiation between aseptic loosening and PJI, to the best of our knowledge, the role of the RANKL/RANK/OPG system has not yet been examined.
We acknowledge that our study has limitations. It must be considered that group definition is difficult in revision arthroplasty. The MSIS has defined a “gold standard“ in PJI diagnostics. But they also acknowledge that infection may be present even without major or minor criteria being fulfilled. We therefore cannot guarantee that patients with low-grade infections and low virulence would not be misclassified into the “aseptic loosening” or control group. Also, the sample size is low for a study investigating arthroplasties. The inhomogenity of the patients investigated is both a weakness and strength of the paper. We did not exclude patients with systemic or inflammatory diseases that may also interfere with the parameters investigated. Patients with PJI are complex and difficult to compare, but this represents day-to-day clinical experience. Eventually, new biomarkers and a further modification of the therapy algorithm may become necessary.
Periprosthetic joint infection (PJI) after total joint replacement still remains one of the most serious complications and is a key challenge in orthopedic surgery. A precise and rapid diagnosis of implant failure is mandatory for treatment success. Especially the differentiation between PJI and aseptic loosening can be unyielding or controversial and misdiagnosis can lead to serious and permanent impairment. As the treatment of PJI is completely different to the treatment of aseptic loosening the correct and timely diagnosis is crucial for successful therapy and relies in part on the use of molecular markers. Nevertheless, establishing a definite diagnosis of PJI prior to surgical intervention is at times difficult.
Numerous researchers have focused on the development of novel and more accurate molecular methods. However, there is no diagnostic gold standard so far. Several studies have attempted to assess the clinical relevance of RANK-ligand (RANKL) and osteoprotegerin (OPG) levels in a variety of human diseases characterized by local or systemic changes in bone remodeling. The essential role of the OPG/RANK/RANKL pathway in regulating bone remodeling around orthopedic implants is well recognized, but the clinical usefulness of circulating OPG and RANKL levels in the differentiation between PJI and aseptic loosening is unknown.
No statistically significant differences in the serum concentration of RANKL and OPG were found between aseptic loosening and PJI, with a trend towards lower RANKL concentrations and higher OPG concentrations in the PJI group.
The sole use of these parameters for differentiating PJI and aseptic loosening cannot be recommended, but they may have utility as a conformation test.
Receptor activator of nuclear factor-ΚB (RANK) ligand (RANKL), its receptor RANK and OPG play an important role in osteoclastogenesis as final effectors of bone resorption. RANKL, which expresses on the surface of osteoblast, stromal cells and activated T-lymphocytes, binds to RANK on osteoclastic precursors cells or mature osteoclasts, and thereby promotes osteoclastogenesis and bone resorption. While OPG, which is expressed by osteoblasts and stromal cells, strongly inhibits bone resorption by binding to its ligand RANKL and thereby preventing it from binding to its receptor, RANK. The RANKL/RANK/OPG system regulates the formation of multinucleated osteoclasts from their precursors as well as their activation and survival in normal bone remodeling. Therefore, the balance between OPG and RANKL is essential to regulate bone remodeling, by controlling the activation state of RANK on osteoclasts.
A good study with a well stated hypothesis and methodology.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P- Reviewer: Cui Q, Hooper GJ, Nishio K, Ohishi T S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Gollwitzer H, Dombrowski Y, Prodinger PM, Peric M, Summer B, Hapfelmeier A, Saldamli B, Pankow F, von Eisenhart-Rothe R, Imhoff AB. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95:644-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472:3254-3262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 261] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 3. | Gravius S, Randau TM, Casadonte R, Kriegsmann M, Friedrich MJ, Kriegsmann J. Investigation of neutrophilic peptides in periprosthetic tissue by matrix-assisted laser desorption ionisation time-of-flight imaging mass spectrometry. Int Orthop. 2015;39:559-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Friedrich MJ, Randau TM, Wimmer MD, Reichert B, Kuberra D, Stoffel-Wagner B, Wirtz DC, Gravius S. Lipopolysaccharide-binding protein: a valuable biomarker in the differentiation between periprosthetic joint infection and aseptic loosening? Int Orthop. 2014;38:2201-2207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Randau TM, Friedrich MJ, Wimmer MD, Reichert B, Kuberra D, Stoffel-Wagner B, Limmer A, Wirtz DC, Gravius S. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9:e89045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-1654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2193] [Cited by in F6Publishing: 2026] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 7. | Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992-2994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1212] [Cited by in F6Publishing: 1317] [Article Influence: 101.3] [Reference Citation Analysis (1)] |
| 8. | Granchi D, Pellacani A, Spina M, Cenni E, Savarino LM, Baldini N, Giunti A. Serum levels of osteoprotegerin and receptor activator of nuclear factor-kappaB ligand as markers of periprosthetic osteolysis. J Bone Joint Surg Am. 2006;88:1501-1509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Gehrke T, Sers C, Morawietz L, Fernahl G, Neidel J, Frommelt L, Krenn V. Receptor activator of nuclear factor kappaB ligand is expressed in resident and inflammatory cells in aseptic and septic prosthesis loosening. Scand J Rheumatol. 2003;32:287-294. [PubMed] [Cited in This Article: ] |
| 10. | Boyce BF, Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007;5:98-104. [PubMed] [Cited in This Article: ] |
| 11. | Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9 Suppl 1:S1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 551] [Cited by in F6Publishing: 577] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 12. | Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4529] [Cited by in F6Publishing: 4539] [Article Influence: 216.1] [Reference Citation Analysis (0)] |
| 13. | Charnley J. Long-term results of low-friction arthroplasty. Hip. 1982;42-49. [PubMed] [Cited in This Article: ] |
| 14. | Goldring SR, Schiller AL, Roelke M, Rourke CM, O’Neil DA, Harris WH. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am. 1983;65:575-584. [PubMed] [Cited in This Article: ] |
| 15. | Gravius S, Mumme T, Delank KS, Eckardt A, Maus U, Andereya S, Hansen T. [Immunohistochemical analysis of periprosthetic osteolysis in aseptic loosening of hip arthroplasty]. Z Orthop Unfall. 2007;145:169-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Chiba J, Rubash HE, Kim KJ, Iwaki Y. The characterization of cytokines in the interface tissue obtained from failed cementless total hip arthroplasty with and without femoral osteolysis. Clin Orthop Relat Res. 1994;304-312. [PubMed] [Cited in This Article: ] |
| 17. | Ritchlin CT, Schwarz EM, O’Keefe RJ, Looney RJ. RANK, RANKL and OPG in inflammatory arthritis and periprosthetic osteolysis. J Musculoskelet Neuronal Interact. 2004;4:276-284. [PubMed] [Cited in This Article: ] |
| 18. | Landgraeber S, Jäger M, Jacobs JJ, Hallab NJ. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediators Inflamm. 2014;2014:185150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Wimmer MD, Randau TM, Petersdorf S, Pagenstert GI, Weißkopf M, Wirtz DC, Gravius S. Evaluation of an interdisciplinary therapy algorithm in patients with prosthetic joint infections. Int Orthop. 2013;37:2271-2278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Krenn V, Morawietz L, Kienapfel H, Ascherl R, Matziolis G, Hassenpflug J, Thomsen M, Thomas P, Huber M, Schuh C. [Revised consensus classification. Histopathological classification of diseases associated with joint endoprostheses]. Z Rheumatol. 2013;72:383-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med. 2003;138:40-44. [PubMed] [Cited in This Article: ] |
| 22. | Hofbauer LC, Schoppet M. Serum measurement of osteoprotegerin--clinical relevance and potential applications. Eur J Endocrinol. 2001;145:681-683. [PubMed] [Cited in This Article: ] |
| 23. | Martin TJ, Sims NA. RANKL/OPG; Critical role in bone physiology. Rev Endocr Metab Disord. 2015;16:131-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 371] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 24. | He T, Wu W, Huang Y, Zhang X, Tang T, Dai K. Multiple biomarkers analysis for the early detection of prosthetic aseptic loosening of hip arthroplasty. Int Orthop. 2013;37:1025-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Veigl D, Niederlová J, Krystůfková O. Periprosthetic osteolysis and its association with RANKL expression. Physiol Res. 2007;56:455-462. [PubMed] [Cited in This Article: ] |