Published online Mar 18, 2016. doi: 10.5312/wjo.v7.i3.149
Peer-review started: July 3, 2015
First decision: October 13, 2015
Revised: December 1, 2015
Accepted: December 29, 2015
Article in press: January 4, 2016
Published online: March 18, 2016
Articular cartilage repair techniques are challenging. Human embryonic stem cells and induced pluripotent stem cells (iPSCs) theoretically provide an unlimited number of specialized cells which could be used in articular cartilage repair. However thus far chondrocytes from iPSCs have been created primarily by viral transfection and with the use of cocultured feeder cells. In addition chondrocytes derived from iPSCs have usually been formed in condensed cell bodies (resembling embryoid bodies) that then require dissolution with consequent substantial loss of cell viability and phenotype. All of these current techniques used to derive chondrocytes from iPSCs are problematic but solutions to these problems are on the horizon. These solutions will make iPSCs a viable alternative for articular cartilage repair in the near future.
Core tip: Herein we review the challenges in articular cartilage repair. Further we explain that induced pluripotent stem cells (iPSCs) represent an exciting theoretically limitless source of autologous cells for articular cartilage repair. We also discuss a novel systematic approach to optimally derive articular chondrocytes from iPSCs.
- Citation: Lietman SA. Induced pluripotent stem cells in cartilage repair. World J Orthop 2016; 7(3): 149-155
- URL: https://www.wjgnet.com/2218-5836/full/v7/i3/149.htm
- DOI: https://dx.doi.org/10.5312/wjo.v7.i3.149
Nearly 1 in 2 people develop symptomatic knee osteoarthritis (OA) by age 85 years, two in three people who are obese develop symptomatic knee OA in their lifetime[1], and 1 in 4 people develop painful hip arthritis in their lifetime[2]. Over 30 million Americans suffer from arthritis and other rheumatic conditions that affect joint and connective tissue; and by 2030 nearly 25% of the American population is expected to be affected by such conditions[3]. Joint Replacement.
Perhaps as a result or as a testament to the inability of articular cartilage to heal, knee replacement is now the most common elective surgery in the United States (Figure 1). Knee replacement though is not appropriate for young patients as it only lasts for an average of 15 years[4] so alternative cellular treatments for osteoarthritis have been sought.
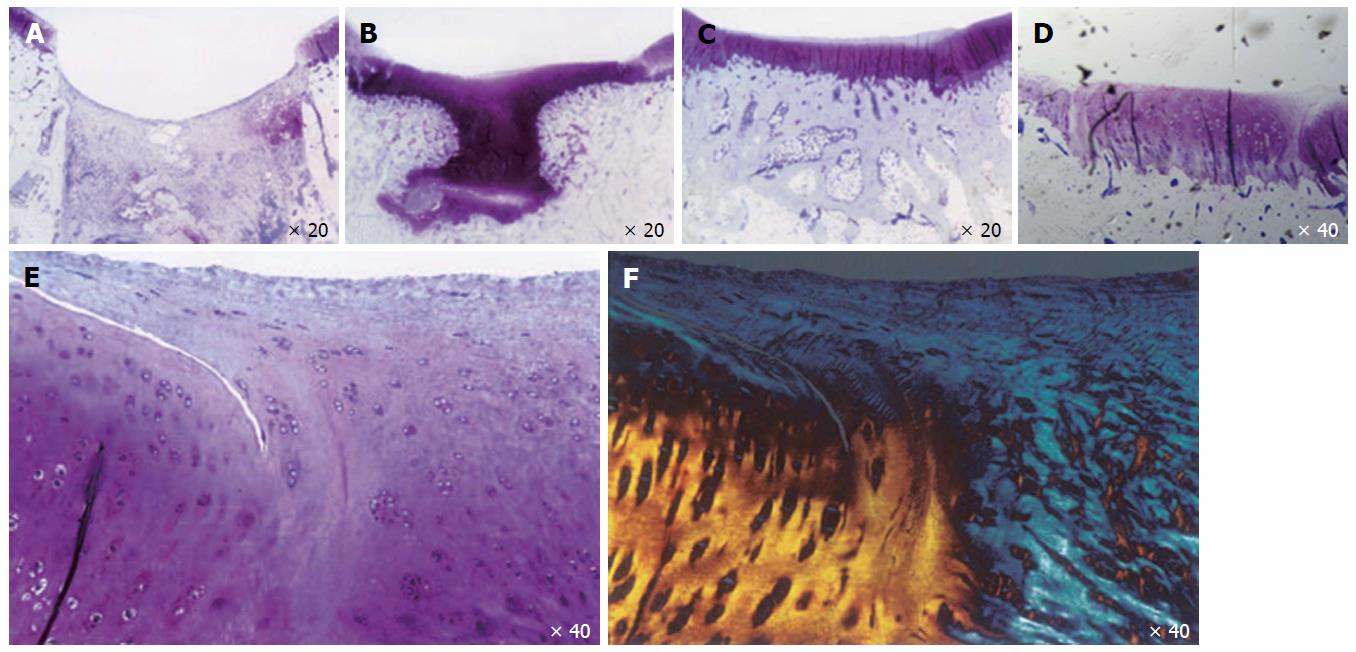
Articular cartilage is made up of cells (5%) with extracellular matrix and water (95%)[5]. Articular chondrocytes express high levels of COL2A1, SOX9 and AGGRECAN[6]. Endogenous attempts at cartilage repair are ineffective in composition (primarily creating fibrocartilage with type I rather than type II collagen) and the reparative tissue does not provide durable healing to the adjacent normal cartilage Figure 1[6]. During embryonic cartilage formation, mesenchymal condensation is the prerequisite for the induction of chondrogenesis. Initiation of limb development starts with the lateral plate mesodermal cells, which proliferate, aggregate and form mesenchymal condensations[7]. These primordial cells differentiate into chondrocytes and form cartilage anlagen[7-10].
One major limitation when studying primary chondrocytes in culture is their loss of phenotype[11]. Research in cell-based cartilage tissue engineering has focused on identifying a cell source suitable for regenerating cartilage. Mesenchymal stem cells (MSCs) would seem to be well suited for tissue engineering and are multipotent cells able to differentiate into chondrocytes, osteoblasts, adipocytes and myocytes[12-15]. However, even though MSCs can be easily obtained from bone marrow, fat and skin, these primary cells have limited proliferation capacity when cultured in vitro and relatively low numbers of MSCs are capable of chondrocyte differentiation[16-21]. Autologous chondrocytes and MSCs have still been used in regeneration of articular cartilage[22-24]. However there are limitations in terms of the ability of adult differentiated chondrocytes to heal a cartilage defect, the numbers of cells that can be obtained using these autologous cells due to their obscurity, and due to the limited maintenance of their phenotype with cell division[16]. The only exception to the inability of a cartilage defect to heal effectively and seamlessly appears to be in a fetal lamb model in which partial thickness articular cartilage defects did heal to subsequently normal appearing cartilage[25].
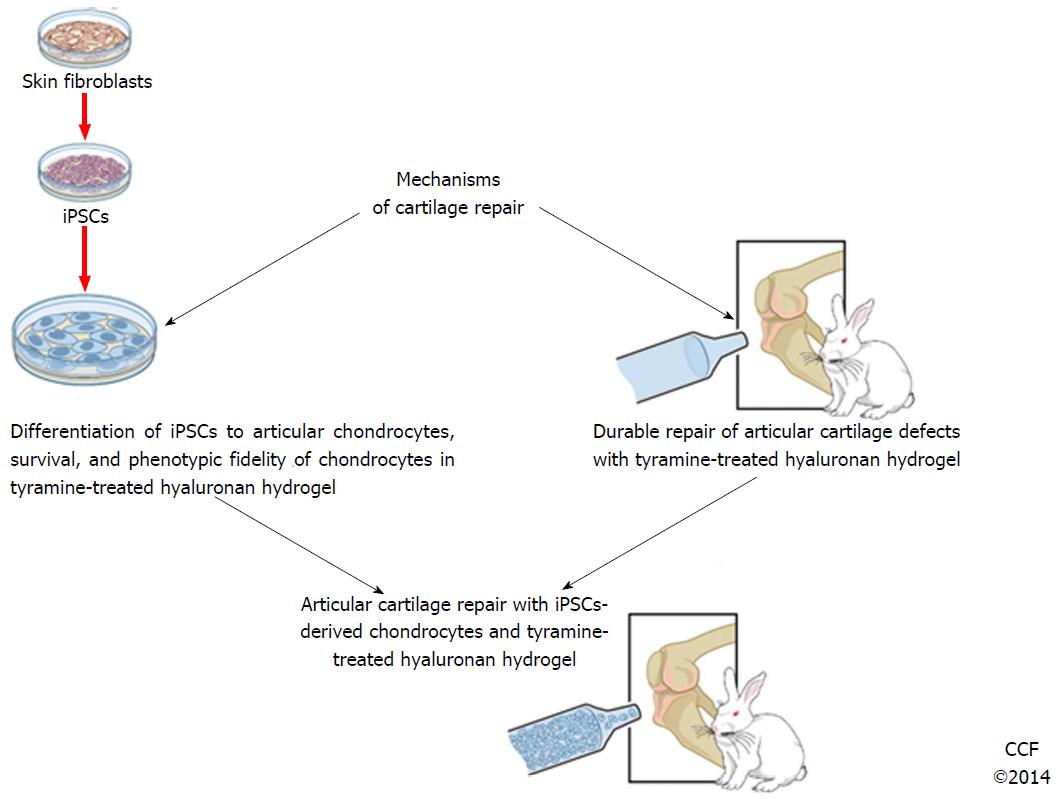
As a result our group and others have become interested in the use of induced pluripotent stem cells (iPSCs) that can be derived from a patient skin biopsy, transformed into iPSCs and then into articular chondrocytes with theoretically large numbers of cells without the concerns of disease transmission from allogeneic cell transfer. In this review we will discuss the current status and recent progress in the development of articular chondrocytes from iPSCs.
Many attempts have been made in the last decade to obtain various MSCs, derived from iPSCs, in ample quantity and high purity after differentiation in vitro[26-33]; and the International Society for Cellular Therapy has defined three primary criteria for cells to meet the definition of MSCs. First, MSCs must be plastic-adherent when maintained in standard culture conditions. Second, MSCs must express CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14, CD11b, CD79alpha or CD19 and HLA-DR surface molecules. Third, MSCs must be able to differentiate into osteoblasts, adipocytes and chondrogenic cells in vitro[34]. In the past, undifferentiated iPSCs have contaminated the differentiated population of MSCs, and they can contribute to teratoma tumor formation; and a uniformly differentiated cell population is necessary for clinical use[35]. iPSCs were developed by Yamanaka by taking differentiated cells and reprogramming them to an embryonic-like state by transfer of nuclear contents into oocytes or by fusion with cells. Specifically he demonstrated induction of pluripotent stem cells from mouse adult fibroblasts by introducing four factors, Oct3/4, Sox2, c-Myc, and Klf4, under ES cell culture conditions[36,37]. These cells, which his group designated iPSCs, exhibit the morphology and growth properties of ES cells and express ES cell marker genes. Subcutaneous transplantation of these iPSCs into nude mice resulted in tumors containing a variety of tissues from all three germ layers. Their work demonstrated that pluripotent stem cells could be directly generated from fibroblast cultures by the addition of only a few defined factors[38].
The fibroblasts used to derive iPSCs can be obtained from a skin punch biopsy done in clinic at the time of patient presentation. iPSCs have the potential to self-renew and differentiate into many adult cell types[39] and represent a theoretically nearly unlimited supply of cells for studying normal cell function and modeling of disease[16,17,27,31,40]. More recent publications have proven the beneficial effect of cells derived from stem cells[41,42]. Stem cell derived cardiomyocytes improve myocardial performance in animal models[42]; and stem cells derived from neuroprogenitor cells lead to regeneration of functional neurons in in vivo models[4,43]. Stem cells derived from retinal epithelial cells improve vision in rodents and humans[33,44]. iPSCs, also potentially provide cell sources for the development of regenerative therapy in articular cartilage repair[45-48]. The chondrogenic cells derived from iPSCs are similar to the fetal lamb chondrocytes, (effectively able to repair cartilage) based on their rapid proliferation and ability to make healthy appearing tissue[38,47-51]. iPSCs can also be manipulated to correct genetic defects, a very important consideration for genetically inherited diseases, including RA. Genetic manipulations could indeed allow de novo produced articular cells to be resistant to inflammatory stimuli and to produce tissues insensitive to degrading enzymes. Based on these considerations and evidence that human iPSCs can be directed to undergo differentiation into various cell types, iPSCs are currently the best option to develop strategies for tissue repair in articular cartilage.
iPSCs can be derived from a small skin biopsy done with minimal intervention before orthopaedic surgery and can be amplified into virtually limitless amounts of homogeneous cell populations. iPSCs could thus be better than other cell sources to create highly reproducible orthopaedic biologic implants such as for articular cartilage (requiring large amounts of cells). Interestingly, iPSCs apparently produce differentiated cells that exhibit young rather than adult properties, including faster proliferation and creation of healthier, longer-lasting reparative tissues such as the cartilage repair observed in the fetal lamb[25,36,47-50,52-55].
Recent reports have demonstrated the ability to induce differentiation of iPSCs into different lineages (similar to embryogenesis) by using small molecules, cytokines and overexpression of transgenes[40,45,56-62]. There are several existing protocols for generating mesenchymal progenitors or MSCs from ESCs and iPSCs that utilize embryoid bodies and/or co-culture with primary cells[26,29,30,40,46]. These protocols are important steps in developing the use of iPSCs for articular cartilage repair but they have limitations in terms of using either an embryoid body stage or feeder cells which lead to cell heterogeneity or the sue of serum which decreases reproducibility.
Two large groups have had a specific interest in chondrogenic differentiation from iPSCs. Tim Hardingham’s group has developed techniques using a number of growth factors to differentiate iPSCs to impressive chondrogenic cells with feeder cells and use fibrin as a control group which we believe actually inhibits in vivo cartilage repair[63,64]. Craft et al[65] developed a protocol with an embryoid body stage with healing in an in vivo model with impressive cartilage formation without an adequate control group. Recently, a third group made chondrogenic cells without the use of feeder cells and do not use an embryoid body stage but at the end of their protocol it is not clear why the cells are in suspension, moreover their toluidine blue staining is not similar to that of the adjacent articular cartilage indicating a difference in the sulfated glycosaminoglycans[30,51,66-69].
Chondrogenic differentiation from iPSCs has been demonstrated by monolayer cell culture and in coculture experiments with primary chondrocytes in 3D culture systems such as condensed cell bodies and pellet cultures, but the necessity of coculture conditions increases the chance of contamination of differentiated cells with feeders or other undesired cells[6,28,70].
A strategy for large-scale production of chondrogenic cells from human ESCs and iPSCs in vitro without the use of serum or feeder cells and without the necessity of a condensed cell body step. To aid in the development of an optimal protocol and to avoid the use of feeder cells, serum and the formation of embryoid bodies we plan to use a Quality-by-Design (QbD)-based method similar to that used in the pharmaceutical industry. Specifically the FDA recommends using QbD-based methods to develop new drugs and cell-based treatments for patients[71]. QbD is a systematic approach that utilizes experimental design and statistical methods in order to gain an in-depth understanding of the effects of input parameters and obtain optimal results and quality[72]. We have begun to apply QbD by implementing the Design-of-Experiment theory and by combining it with Multivariate Data Analysis will more thoroughly and systematically optimize protocols for chondrocyte differentiation from iPSCs.
One of the main challenges in using iPSCs for either therapeutic applications or in vitro modeling is the difficulty in achieving uniform differentiation of the desired cell type. One cause for a lack of uniform differentiation is the use of serum in the differentiation process of cells, which is imprecise due to batch variability and the presence of undefined extracellular factors within serum. The other primary cause for heterogeneity is the use of feeder cells or an embryoid body stage.
Coculture of MSCs with primary chondrocytes to get chondrogenic differentiation has been used to avoid the inconsistent differentiation of primary MSCs in a cartilage regeneration model[73-75]. However coculture is problematic as there are contamination issues when the desired cells need to be separated from the feeder cells as mentioned above[30].
Thus current issues which need to be addressed to further the use of iPSCs in articular cartilage repair and are critically important in cartilage regeneration in an articular cartilage repair model are: (1) Chondrogenic potential and fidelity of the cells; (2) Long term survival of the cells in the repair tissue; (3) Healing to the adjacent endogenous “normal” cartilage in comparison to an adequate untreated control group; and (4) Contamination with (a) undifferentiated cells that form teratomas with embryoid body formation or (b) with feeder cells used in coculture (Figure 2). Despite these hurdles our group and others have preliminary solutions to these issues. Our group believes that a more systematic approach similar to that used in the pharmaceutical industry could add important information to optimize chondrocyte generation from iPSCs with QbD techniques. We predict that the use of iPSCs clinically for cartilage repair holds the most promise to provide a biologic solution for cartilage damage in the near future and that we and others will be able to optimize protocols applicable for clinical use in cartilage repair in the near future.
P- Reviewer: Danisovic L, Musumeci G S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207-1213. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Murphy LB, Helmick CG, Schwartz TA, Renner JB, Tudor G, Koch GG, Dragomir AD, Kalsbeek WD, Luta G, Jordan JM. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage. 2010;18:1372-1379. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15-25. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Avaliani N, Sørensen AT, Ledri M, Bengzon J, Koch P, Brüstle O, Deisseroth K, Andersson M, Kokaia M. Optogenetics reveal delayed afferent synaptogenesis on grafted human-induced pluripotent stem cell-derived neural progenitors. Stem Cells. 2014;32:3088-3098. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Poole AR, Kobayashi M, Yasuda T, Laverty S, Mwale F, Kojima T, Sakai T, Wahl C, El-Maadawy S, Webb G. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann Rheum Dis. 2002;61 Suppl 2:ii78-ii81. [PubMed] [Cited in This Article: ] |
| 6. | Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, Guilak F. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:19172-19177. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Decker RS, Koyama E, Enomoto-Iwamoto M, Maye P, Rowe D, Zhu S, Schultz PG, Pacifici M. Mouse limb skeletal growth and synovial joint development are coordinately enhanced by Kartogenin. Dev Biol. 2014;395:255-267. [PubMed] [Cited in This Article: ] |
| 8. | Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA. 2005;102:14665-14670. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Koga T, Matsui Y, Asagiri M, Kodama T, de Crombrugghe B, Nakashima K, Takayanagi H. NFAT and Osterix cooperatively regulate bone formation. Nat Med. 2005;11:880-885. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332-336. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Benya PD, Padilla SR, Nimni ME. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15:1313-1321. [PubMed] [Cited in This Article: ] |
| 12. | Prockop DJ. Further proof of the plasticity of adult stem cells and their role in tissue repair. J Cell Biol. 2003;160:807-809. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Sekiya I, Larson BL, Vuoristo JT, Cui JG, Prockop DJ. Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J Bone Miner Res. 2004;19:256-264. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | de Peppo GM, Vunjak-Novakovic G, Marolt D. Cultivation of human bone-like tissue from pluripotent stem cell-derived osteogenic progenitors in perfusion bioreactors. Methods Mol Biol. 2014;1202:173-184. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Bhumiratana S, Eton RE, Oungoulian SR, Wan LQ, Ateshian GA, Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci USA. 2014;111:6940-6945. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Ahfeldt T, Schinzel RT, Lee YK, Hendrickson D, Kaplan A, Lum DH, Camahort R, Xia F, Shay J, Rhee EP. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol. 2012;14:209-219. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Barberi T, Studer L. Mesenchymal cells. Methods Enzymol. 2006;418:194-208. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675-682. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91-116. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, Nöth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163-173. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Brittberg M, Lindahl A, Homminga G, Nilsson A, Isaksson O, Peterson L. A critical analysis of cartilage repair. Acta Orthop Scand. 1997;68:186-191. [PubMed] [Cited in This Article: ] |
| 23. | Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895. [PubMed] [Cited in This Article: ] |
| 24. | Brittberg M, Nilsson A, Lindahl A, Ohlsson C, Peterson L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res. 1996;270-283. [PubMed] [Cited in This Article: ] |
| 25. | Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80:4-10. [PubMed] [Cited in This Article: ] |
| 26. | Chen YS, Pelekanos RA, Ellis RL, Horne R, Wolvetang EJ, Fisk NM. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Transl Med. 2012;1:83-95. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, Marolt D. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110:8680-8685. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Guzzo RM, Gibson J, Xu RH, Lee FY, Drissi H. Efficient differentiation of human iPSC-derived mesenchymal stem cells to chondroprogenitor cells. J Cell Biochem. 2013;114:480-490. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Koyama N, Miura M, Nakao K, Kondo E, Fujii T, Taura D, Kanamoto N, Sone M, Yasoda A, Arai H. Human induced pluripotent stem cells differentiated into chondrogenic lineage via generation of mesenchymal progenitor cells. Stem Cells Dev. 2013;22:102-113. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Nejadnik H, Diecke S, Lenkov OD, Chapelin F, Donig J, Tong X, Derugin N, Chan RC, Gaur A, Yang F. Improved approach for chondrogenic differentiation of human induced pluripotent stem cells. Stem Cell Rev. 2015;11:242-253. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Samuel R, Daheron L, Liao S, Vardam T, Kamoun WS, Batista A, Buecker C, Schäfer R, Han X, Au P. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110:12774-12779. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Whitworth DJ, Frith JE, Frith TJ, Ovchinnikov DA, Cooper-White JJ, Wolvetang EJ. Derivation of mesenchymal stromal cells from canine induced pluripotent stem cells by inhibition of the TGFβ/activin signaling pathway. Stem Cells Dev. 2014;23:3021-3033. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, Buchholz DE, Ahmado A, Semo M, Smart MJ. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [PubMed] [Cited in This Article: ] |
| 35. | Kuroda T, Yasuda S, Kusakawa S, Hirata N, Kanda Y, Suzuki K, Takahashi M, Nishikawa S, Kawamata S, Sato Y. Highly sensitive in vitro methods for detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human iPS cells. PLoS One. 2012;7:e37342. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081-3089. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Koyanagi-Aoi M, Ohnuki M, Takahashi K, Okita K, Noma H, Sawamura Y, Teramoto I, Narita M, Sato Y, Ichisaka T. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110:20569-20574. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [PubMed] [Cited in This Article: ] |
| 39. | Inoue H, Nagata N, Kurokawa H, Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33:409-417. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Taura D, Noguchi M, Sone M, Hosoda K, Mori E, Okada Y, Takahashi K, Homma K, Oyamada N, Inuzuka M. Adipogenic differentiation of human induced pluripotent stem cells: comparison with that of human embryonic stem cells. FEBS Lett. 2009;583:1029-1033. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428-439. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322-325. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y, Van Camp N, Perrier AL, Hantraye P, Björklund A. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15:653-665. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509-516. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Inui A, Iwakura T, Reddi AH. Human stem cells and articular cartilage regeneration. Cells. 2012;1:994-1009. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Gong G, Ferrari D, Dealy CN, Kosher RA. Direct and progressive differentiation of human embryonic stem cells into the chondrogenic lineage. J Cell Physiol. 2010;224:664-671. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55-70. [PubMed] [Cited in This Article: ] |
| 48. | Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177-1181. [PubMed] [Cited in This Article: ] |
| 49. | Sommer AG, Rozelle SS, Sullivan S, Mills JA, Park SM, Smith BW, Iyer AM, French DL, Kotton DN, Gadue P. Generation of human induced pluripotent stem cells from peripheral blood using the STEMCCA lentiviral vector. J Vis Exp. 2012;pii:4327. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Yamanaka S, Li J, Kania G, Elliott S, Wersto RP, Van Eyk J, Wobus AM, Boheler KR. Pluripotency of embryonic stem cells. Cell Tissue Res. 2008;331:5-22. [PubMed] [Cited in This Article: ] |
| 51. | Yamashita A, Morioka M, Yahara Y, Okada M, Kobayashi T, Kuriyama S, Matsuda S, Tsumaki N. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Reports. 2015;4:404-418. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | Buganim Y, Markoulaki S, van Wietmarschen N, Hoke H, Wu T, Ganz K, Akhtar-Zaidi B, He Y, Abraham BJ, Porubsky D. The developmental potential of iPSCs is greatly influenced by reprogramming factor selection. Cell Stem Cell. 2014;15:295-309. [PubMed] [DOI] [Cited in This Article: ] |
| 53. | Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424-429. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29:731-734. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Soldner F, Laganière J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe LI, Myers RH, Lindquist S. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146:318-331. [PubMed] [DOI] [Cited in This Article: ] |
| 56. | Alfred R, Taiani JT, Krawetz RJ, Yamashita A, Rancourt DE, Kallos MS. Large-scale production of murine embryonic stem cell-derived osteoblasts and chondrocytes on microcarriers in serum-free media. Biomaterials. 2011;32:6006-6016. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Noguchi M, Hosoda K, Nakane M, Mori E, Nakao K, Taura D, Yamamoto Y, Kusakabe T, Sone M, Sakurai H. In vitro characterization and engraftment of adipocytes derived from human induced pluripotent stem cells and embryonic stem cells. Stem Cells Dev. 2013;22:2895-2905. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Guzzo RM, Scanlon V, Sanjay A, Xu RH, Drissi H. Establishment of human cell type-specific iPS cells with enhanced chondrogenic potential. Stem Cell Rev. 2014;10:820-829. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Okada M, Ikegawa S, Morioka M, Yamashita A, Saito A, Sawai H, Murotsuki J, Ohashi H, Okamoto T, Nishimura G. Modeling type II collagenopathy skeletal dysplasia by directed conversion and induced pluripotent stem cells. Hum Mol Genet. 2015;24:299-313. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Outani H, Okada M, Yamashita A, Nakagawa K, Yoshikawa H, Tsumaki N. Direct induction of chondrogenic cells from human dermal fibroblast culture by defined factors. PLoS One. 2013;8:e77365. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Tsumaki N, Okada M, Yamashita A. iPS cell technologies and cartilage regeneration. Bone. 2015;70:48-54. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Uto S, Nishizawa S, Takasawa Y, Asawa Y, Fujihara Y, Takato T, Hoshi K. Bone and cartilage repair by transplantation of induced pluripotent stem cells in murine joint defect model. Biomed Res. 2013;34:281-288. [PubMed] [Cited in This Article: ] |
| 63. | Cheng A, Kapacee Z, Peng J, Lu S, Lucas RJ, Hardingham TE, Kimber SJ. Cartilage repair using human embryonic stem cell-derived chondroprogenitors. Stem Cells Transl Med. 2014;3:1287-1294. [PubMed] [DOI] [Cited in This Article: ] |
| 64. | Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE, Kimber SJ. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187-1194. [PubMed] [DOI] [Cited in This Article: ] |
| 65. | Craft AM, Rockel JS, Nartiss Y, Kandel RA, Alman BA, Keller GM. Generation of articular chondrocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33:638-645. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | Mobasheri A, Csaki C, Clutterbuck AL, Rahmanzadeh M, Shakibaei M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histol Histopathol. 2009;24:347-366. [PubMed] [Cited in This Article: ] |
| 67. | Mobasheri A, Kalamegam G, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78:188-198. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Guilak F, Estes BT, Diekman BO, Moutos FT, Gimble JM. 2010 Nicolas Andry Award: Multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop Relat Res. 2010;468:2530-2540. [PubMed] [DOI] [Cited in This Article: ] |
| 69. | Cheng A, Hardingham TE, Kimber SJ. Generating cartilage repair from pluripotent stem cells. Tissue Eng Part B Rev. 2014;20:257-266. [PubMed] [DOI] [Cited in This Article: ] |
| 70. | Craft AM, Ahmed N, Rockel JS, Baht GS, Alman BA, Kandel RA, Grigoriadis AE, Keller GM. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development. 2013;140:2597-2610. [PubMed] [DOI] [Cited in This Article: ] |
| 71. | Schachter B. Therapies of the state. Nat Biotechnol. 2014;32:736-741. [PubMed] [DOI] [Cited in This Article: ] |
| 72. | Mercier SM, Diepenbroek B, Wijffels RH, Streefland M. Multivariate PAT solutions for biopharmaceutical cultivation: current progress and limitations. Trends Biotechnol. 2014;32:329-336. [PubMed] [DOI] [Cited in This Article: ] |
| 73. | Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 2013;3:3553. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Qu C, Puttonen KA, Lindeberg H, Ruponen M, Hovatta O, Koistinaho J, Lammi MJ. Chondrogenic differentiation of human pluripotent stem cells in chondrocyte co-culture. Int J Biochem Cell Biol. 2013;45:1802-1812. [PubMed] [DOI] [Cited in This Article: ] |
| 75. | Bigdeli N, Karlsson C, Strehl R, Concaro S, Hyllner J, Lindahl A. Coculture of human embryonic stem cells and human articular chondrocytes results in significantly altered phenotype and improved chondrogenic differentiation. Stem Cells. 2009;27:1812-1821. [PubMed] [DOI] [Cited in This Article: ] |