Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.259
Revised: August 1, 2013
Accepted: September 4, 2013
Published online: October 18, 2013
AIM: To determine the feasibility and potential role of combining radiostereometric analysis (RSA), gait analysis and activity monitoring in the follow-up of fracture patients.
METHODS: Two patients with similar 41B3 tibial plateau fractures were treated by open reduction internal fixation augmented with impaction bone grafting and were instructed to partial weight bear to 10 kg for the first six postoperative weeks. Fracture reduction and fixation were assessed by postoperative computer tomographic (CT) scanning. Both patients had tantalum markers inserted intra-operatively to monitor their fracture stability during healing using RSA and differentially loaded RSA (DLRSA) at 6 and 12 wk postoperatively. Gait analyses were performed at 1, 2, 6, and 12 wk postoperatively. Activity monitors were worn for 4 wk between the 2 and 6 wk appointments. In addition to gait analysis, knee function was assessed using the patient reported Lysholm scores, and doctor reported knee range of motion and stability, at 6 and 12 wk postoperatively.
RESULTS: There were no complications. CT demonstrated that both fractures were reduced anatomically. Gait analysis indicated that Patient 1 bore weight to 60% of body weight at 2 wk postoperative and 100% at 6 wk. Patient 2 bore weight at 10% of body weight to 6 wk and had very low joint contact forces to that time. At 12 wk however, there was no difference between the gait patterns in the two patients. Patient 1 increased activities of moderate-vigorous intensity from 20 to 60 min/d between 2 and 6 postoperative weeks, whereas Patient 2 remained more stable at 20-30 min/d. The Lysholm scores were similar for both patients and did not improve between 6 and 12 wk postoperatively. DLRSA examination at 12 wk showed that both patients were comfortable to weight bear to 80 kg and under this weight the fractures displaced less than 0.4 mm. RSA measurements demonstrated over time fracture migrations of less than 2 mm in both cases. However, Patient 2, who followed the postoperative weight bearing instructions most closely, displaced less (0.3 mm vs 1.6 mm).
CONCLUSION: This study demonstrates the potential of using a combination of RSA, gait analysis and activity monitoring to obtain a comprehensive evidence base for postoperative weight bearing schedules during fracture healing.
Core tip: The extent to which patients follow rehabilitation instructions, likely affects not only the early recovery, but also the long-term outcomes that are dependent on maintenance of fracture reduction. We have demonstrated the feasibility of using radiostereometric analysis, gait analysis and activity monitoring to assess early fracture healing. Future larger clinical studies using this novel combination of assessment tools may provide an evidence base for particular rehabilitation schedules following different fracture types and fixation techniques.
- Citation: Callary SA, Thewlis D, Rowlands AV, Findlay DM, Solomon LB. Collecting a comprehensive evidence base to monitor fracture rehabilitation: A case study. World J Orthop 2013; 4(4): 259-266
- URL: https://www.wjgnet.com/2218-5836/full/v4/i4/259.htm
- DOI: https://dx.doi.org/10.5312/wjo.v4.i4.259
Outcomes of tibial plateau fractures (TPF) have been correlated with the degree of initial articular fragment reduction and the maintenance of reduction[1-3]. In the laboratory, articular steps of greater than 1.5 mm of the tibial condyles were shown to cause significantly increased pressure on the surrounding cartilage[4]. In clinical practice, reduction of the articular surface of the tibia with articular steps of less than 2 mm have been labeled as “anatomical”[5,6], while articular steps of more than 3 mm have been associated with worse outcomes and identified as a risk factor for post-traumatic knee osteoarthritis[2,3]. The problem with correlating outcomes of TPF, and for that matter of any articular fracture, with small articular steps measured on standard radiographs, is that the method is known to have a poor accuracy of ± 5 mm[7-10]. This has limited the previous reported results and outcome correlations considerably. In contrast to standard radiographs, radiostereometric analysis (RSA) has been shown to be a highly accurate and precise method to measure fracture displacement in clinical practice[11]. When applied to small TPF fragments, the method has been shown to have an accuracy of ± 0.037 mm and a precision of ± 0.016 mm[12]. The limitations of RSA are that it requires expensive equipment and software, is time consuming, requires experienced personnel and can only be used in prospective studies. However, imaging techniques with such a degree of accuracy are required to establish any objective correlations between the quality of reduction and maintenance of reduction and outcomes in these fractures.
To date, there is no consensus on how TPF patients should be rehabilitated after open reduction and internal fixation (ORIF), with current recommendations varying from non-weight bearing for up to 12 wk[13] to partial weight bearing in all cases[5,6]. It is reasonable to assume that most surgeons would recommend various postoperative weight bearing prescriptions for their patients, depending on the type of fracture and the “quality” of fixation achieved. Such prescriptions are largely empirical. In addition, it is well established that most patients cannot observe, or disregard, the postoperative weight bearing instructions set by their surgeon[14-16].
To our knowledge, we have performed the only study to investigate the stability of TPF in patients allowed to weight bear in the first six postoperative weeks[17]. We used RSA and differentially loaded RSA (DLRSA) performed under weight bearing load to assess fracture fragment stability[17]. Patients were recommended a postoperative partial weight-bearing regimen of 20 kg for the first six postoperative weeks, followed by progressive weight bearing as tolerated. However, during the DLRSA examinations performed under weight-bearing as tolerated at two and six postoperative weeks, two out of seven patients exceeded 20 kg at 2 wk and three were full weight bearing at 6 wk. Despite all fracture movements under load being reported to be elastic (the final position of the fracture returned to the preload position), the fractures were found to have migrated over time up to 2 mm in the cranio-caudal and medio-lateral directions[17]. These results suggest that during the first six postoperative weeks, the patients either loaded more than their body weight through their injured knee and/or the direction of the joint reaction forces during ambulation differed from that during standing for the DLRSA examinations. Monitoring patients’ activity and investigating their joint reaction force through gait analysis combined with musculoskeletal modeling, may provide a better understanding of fracture migration during different rehabilitation protocols. In addition, combining the results provided by monitoring fracture stability during healing with an accurate technique, namely RSA, with objective snapshots of patient activity level and objective biomechanical data from gait analysis has the potential to provide an evidence base for particular rehabilitation schedules to be recommended following different fractures types and fixation techniques.
The aim of this study was therefore to investigate the feasibility of monitoring the healing of TPF patients with RSA, gait analysis and physical activity monitoring. Using these combined assessment tools in larger clinical studies may provide more comprehensive and objective data to confirm the appropriateness of current postoperative weight bearing regimes in lower limb articular fractures.
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by the Royal Adelaide Hospital Research Ethics Committee (Protocol Numbers 060621 and 080107). All patients provided informed written consent.
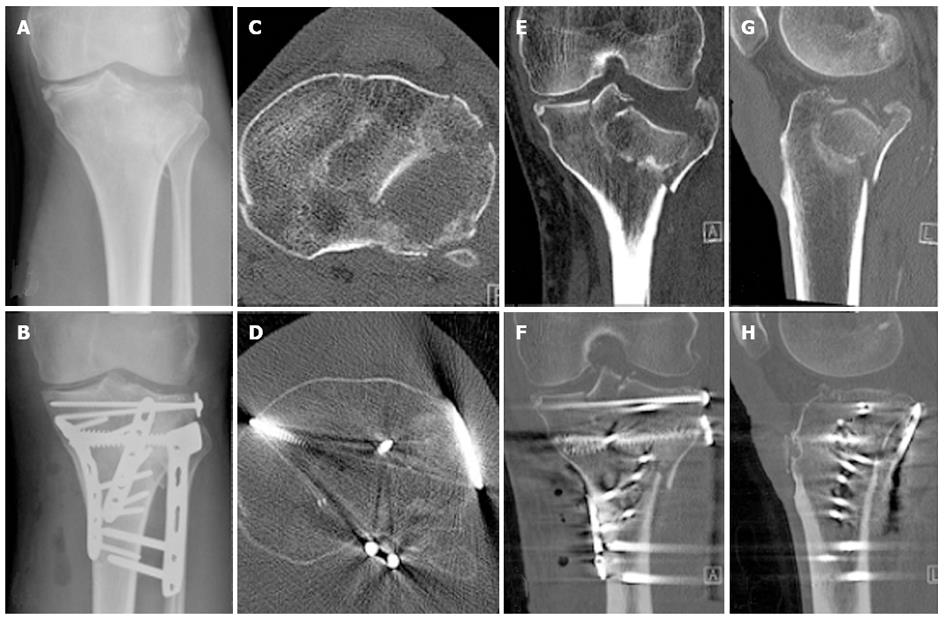
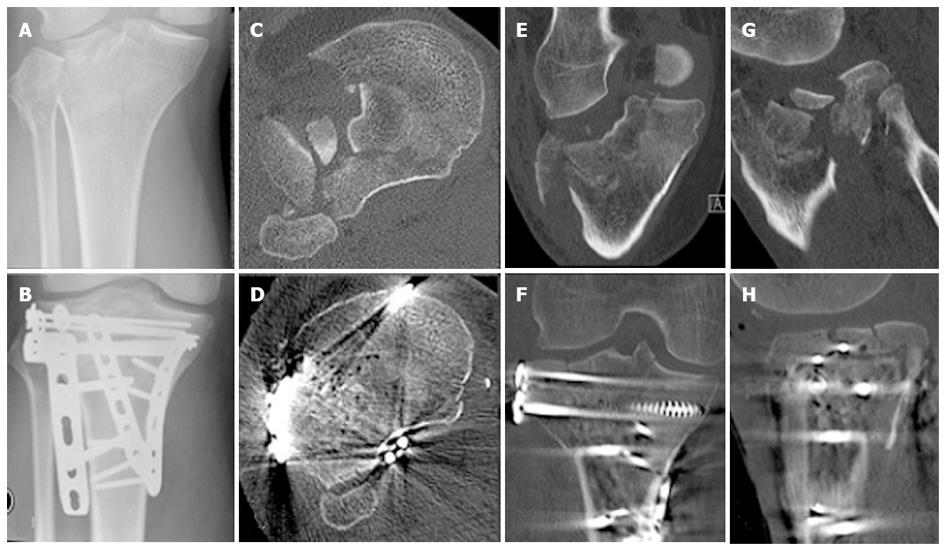
Two consecutive patients treated in our institution for a similar pattern 41B3 fracture, involving a split component of the posterior medial tibial condyle and a split depression of the lateral tibial condyle, who agreed to have their fracture healing monitored with RSA, activity monitoring and gait analysis were included in this study (Figures 1 and 2). Both patients were male of a similar age (56 and 60 years respectively) and both sustained their TPF as an isolated injury in a low speed motorbike accident. Both patients lived with supportive families. At the time of the accident, Patient 1 was on a long-standing disability support pension for a traumatic amputation of two fingers, while Patient 2 was self-employed as an electrician. Each fracture had multiple split and depressed fragments with the maximum depression on preoperative CT scans measured 19 and 22 mm respectively. Both patients had ORIF by the same surgeon within 24 h of their injury through a combined anterolateral and posteromedial approach to allow buttressing of the posteromedial, posterolateral and anterolateral cortical components of the fracture and to contain the lateral tibial condyle cancellous bone defect to allow impaction bone grafting. The internal fixation was completed by impaction bone grafting and a raft of subchondral screws (Figures 1 and 2). At the time of surgery, tantalum beads (1.0 mm, RSA Biomedical, Umea, Sweden) were inserted in the largest depressed fracture fragment as well as in the unfractured tibial metaphysis, to allow for RSA and DLRSA. Postoperatively, the patients were instructed and educated to partial weight bear to 10 kg on the injured limb for the first six postoperative weeks and to progress to full weight bearing as tolerated afterwards. Unrestricted knee range of motion was encouraged immediately after surgery.
In the first postoperative days, the patients had standard radiographs and a fine cut computer tomographic scan to assess the fracture reduction. An RSA radiographic examination was taken as a baseline for measurement of fracture displacement. Following radiographic imaging, each patient had gait analysis.
The patients were reviewed at 2, 6 and 12 postoperative weeks. Clinical examination, plain radiographs, RSA radiographs and gait analysis were repeated at each of these times. The patients were given a physical activity monitor to wear between their 2 and 6 wk outpatient appointments. Lysholm patient reported outcomes were completed at the 6 and 12 wk outpatient appointments. Knee range of motion and stability were recorded during the 6 and 12 wk outpatient appointments.
Baseline RSA radiographs were taken in the supine position, centering the patient’s knee over the RSA calibration cage (Cage No. 43, RSA Biomedical, Umea, Sweden). Radiographs were analyzed using specialized software (UmRSA v6.0, RSA Biomedical, Umea, Sweden). The mean error of rigid body fitting accepted was less than 0.35 mm.
DLRSA examinations were performed at 6 and 12 wk with each patient standing on a custom built platform that allows centering of the patient’s knee in front of the RSA calibration cage, as described previously[17]. During the RSA examination, a digital foot scale was used to measure the load applied whilst weight bearing. RSA radiographs were taken with the foot placed on the scale but applying 2-5 kg (pre-load), followed by radiographs with the patient applying a maximum weight bearing load on the scales, as tolerated without pain (loaded). A final pair of radiographs was taken while applying 2-5 kg load (post-load). Parallel bars were used by patients as support during examination to allow a “controlled static” weight bearing. Inducible displacement of fracture fragments was calculated as the difference in position of the fracture fragment relative to the proximal tibia using the pre-load radiographs as the reference examination. Two dimensional (2D) migration was calculated as the vectorial sum of proximal-distal and medio-lateral migrations. The subsequent apparent stiffness was calculated as the force (applied maximum tolerated load) divided by the displacement (2D migration).
The gait analysis was undertaken with the patients walking at their self-selected pace along a 10-m walkway. A minimum of five successful trials were recorded at all assessments. Patients were allowed rest periods between trials, if required. Small reflective markers were attached to the lower limbs and pelvis of the patient[18]. The trajectories of the markers were recorded using a 12-camera Vicon MX-F20 motion capture system (Vicon Inc., Oxford, United Kingdom) at 100 Hz. Ground reaction forces were recorded simultaneously through Vicon Nexus 1.8 (Vicon Inc., Oxford, United Kingdom) with the motion data, using two AMTI (AMTI, Watertown, United States) and two Kistler (Kistler Inc., Switzerland) force platforms at 400 Hz. Data were exported to Visual3D (C-Motion, Inc., United States) where the motion was reconstructed[19] and the knee joint angles and loads (forces and moments) calculated. Joint angles were calculated using the joint coordinate system[20]. Joint loads were computed using inverse dynamics. From Visual 3D, further processing was performed in Matlab (Mathworks, United States). Using a musculoskeletal model[21], the joint reaction forces were calculated. From the gait analysis, the peak vertical ground reaction force, peak vertical knee joint reaction force, the peak knee flexion and extension moments, the peak knee adduction moment and the knee joint range of motion were extracted.
Each patient was asked to wear a GENEActiv accelerometer on their non-dominant wrist after their discharge from the hospital. These activity monitors are small, lightweight, wireless devices that provide a continuous record of activity patterns over extended periods of time. Data obtained were used to quantify the amount of time the patients spent in activity of light and moderate-to-vigorous intensity following their discharge.
The patients were instructed to wear the wrist monitor 24 h a day, including during water-based activities (showering/bathing) and sleeping. The GENEActiv is a triaxial accelerometry-based activity monitor with a dynamic range of +/- 8 g (http://www.geneactiv.co.uk, Gravity Estimator of Normal Everyday Activity, ActivInsights Ltd, Cambridgeshire, United Kingdom). GENEActiv PC software was used to configure the GENEActivs to collect data at 10 Hz, upload the data and convert the .bin files to 15 s epoch .csv files for data analysis. The sampling frequency of 10 Hz was selected to enable data collection for over one month.
Periods of non-wear were identified using an algorithm that identified minute-by-minute changes of position. No changes of position in a rolling 60-min window were classified as non-wear. Sixty-minute windows of no movement have been used previously to classify non-wear[22]. A minimum of 10-hour wear time during daytime was required for a valid day[22]. The sum and mean of the vector magnitude (VM) of the 15 s epochs were calculated for each valid day. Outcome variables for each valid day were: time in light and moderate-to-vigorous intensity physical activity (classified using published cut-points[23]). Light activity includes activity greater than one and a half times resting metabolic rate and moderate-to-vigorous activity includes activities with an intensity at and above that of a brisk walk. Data analysis was performed using Microsoft Excel 2010.
There were no complications. Both patients had an uneventful recovery with a continuous improvement in their knee symptoms and function. The postoperative CT scan demonstrated that both fractures were reduced anatomically (Figures 1 and 2). No measurable fracture displacement over time was identifiable on plain radiographs in any of the patients. At six weeks Patient 1 reported a Lysholm score of 51, his knee was stable and had a range of motion of 5°-100°. At 12 wk Patient 1 reported a Lysholm score of 48, his knee was stable and had a range of motion of 0°-110°. At 6 wk Patient 2 reported a Lysholm score of 49, his knee was stable and had a range of motion of 10°-90°. At 12 wk Patient 2 reported a Lysholm score of 47, his knee was stable and had a range of motion of 5°-100°.
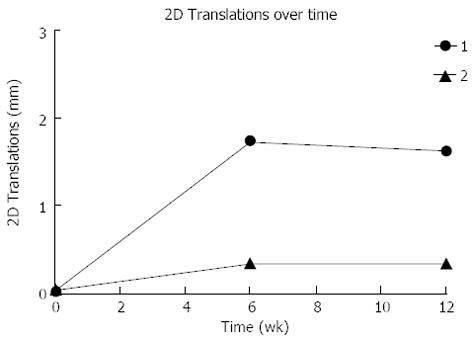
RSA measurements demonstrated that the two-dimensional (2D) fracture migration over time was less than 2mm in both cases (Figure 3). There was a difference in size and pattern of fracture migration, with the fracture fragment of Patient 1 migrating almost 1.6 mm compared to 0.3 mm for Patient 2. The majority of the migration occurred within the first six postoperative weeks (Figure 3).
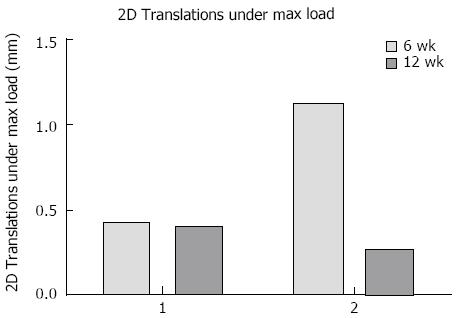
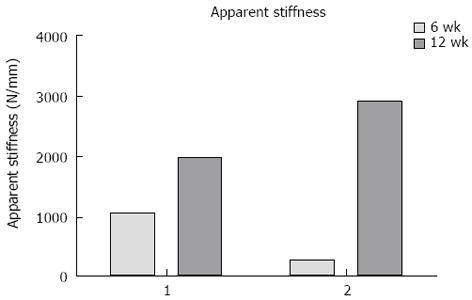
Fracture displacement during DLRSA examinations was elastic, with the post-load examinations demonstrating that that the fracture fragments returned to their pre-load position. During DLRSA examinations, Patients 1 and 2 applied 47 kg and 30 kg respectively, at 6 wk and both applied 80 kg at 12 wk. The 2D translations under the 80 kg loads at 12 wk were both below 0.4 mm (Figure 4). The calculated apparent stiffness value for the fracture construct of Patient 2 at 6 wk (261 N/mm) was lower compared to that of Patient 1 (1064 N/mm) (Figure 5). Conversely, Patient 2 had a higher apparent stiffness at 12 wk than Patient 1 (Figure 5). Both fractures showed increased stiffness over time, consistent with other measures of fracture healing.
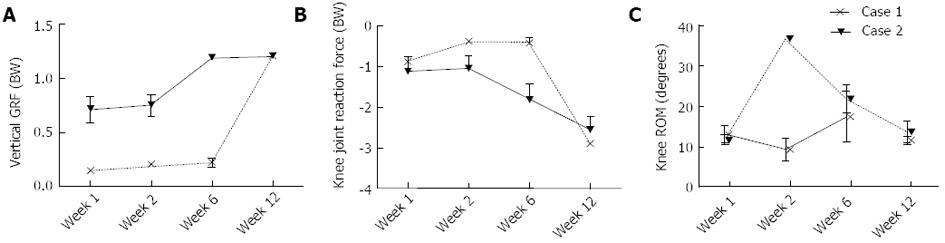
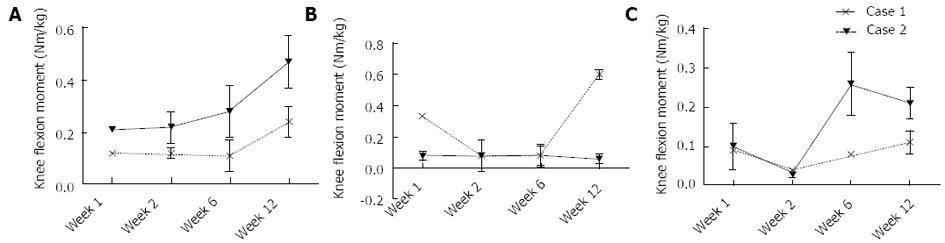
At one week postoperative, Patient 1 had a peak Fz of 0.7 times their total body weight (bw). In contrast, Patient 2 loaded to only 0.15 bw. Between 1 wk and 12 wk, increases of 0.49 and 1.05 bw were observed in the peak Fz for Patients 1 and 2, respectively (Figure 6A). At 1 wk postoperative, Patient 1 had a peak knee JRFz of 1.11 bw, which was comparable to that calculated for Patient 2 of 0.86 bw. Between 1 and 12 wk, increases of 1.45 and 2.04 bw were observed in the peak JRFz for Patient 1 and 2, respectively (Figure 6B). Besides a peak in the knee ROM at week 2 for Patient 2, no substantial changes were observed in the knee ROM (Figure 6C). Patient 1 showed an increase of both the knee flexion and adduction moment between 1 and 12 wk postoperative, with a magnitude of 0.25 and 0.11 Nm/kg, respectively. No change was observed in the knee extension moment. Patient 2 showed a similar percentage increase in the knee flexion moment to Patient 1, with the peak moment increasing by 0.12 Nm/kg between 1 and 12 wk (Figure 7A). The knee extension moment increased by 0.27 Nm/kg (Figure 7B); however, no change was noted in the knee adduction moment (Figure 7C).
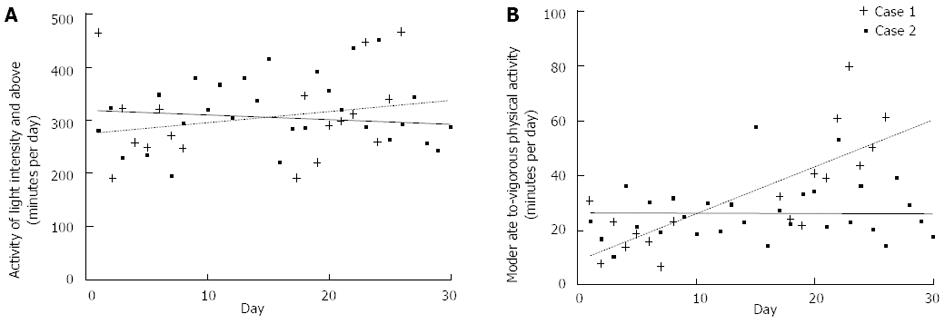
Patient 1 did not wear the monitor for a period of 10 days in the middle of the investigated period. Between 2 and 6 wk post-surgery, light activity increased by approximately one hour per day in Patient 1 (from 270 min/d to 330 min/d) and stayed fairly constant in Patient 2 (approximately 300 min/d) (Figure 8A). Patient 1 also increased from approximately 20 min/d of moderate-vigorous intensity physical activity at 2 wk post-surgery to approximately 60 min/d of moderate-vigorous intensity physical activity 6 wk post-surgery (Figure 8B). In contrast, Patient 2 maintained fairly stable levels of 20-30 min/d moderate-vigorous intensity physical activity between 2 and 6 wk post-surgery.
The influence of rehabilitation instructions and the extent to which patients follow them likely affects not only the early recovery, but also the long-term outcomes that are dependent on maintenance of fracture reduction. This study investigated the feasibility of using a number of postoperative outcome tools to provide a better understanding of the stability of TPF during rehabilitation and healing. The highly accurate technique of RSA was used in conjunction with physical activity monitors, patient assessment forms and specific gait analyses.
This case study of two fracture patients was, to the best of the authors knowledge, the first time that RSA, activity monitors and gait analysis had all been used to monitor fracture healing and functional improvement during the early postoperative period. While the results suggest the feasibility of applying these particular analyses, clearly no conclusion can be drawn from this case study regarding the way patients follow rehabilitation instructions and their long-term outcomes. Larger scale studies using RSA, gait analysis and activity monitoring are required to obtain an evidence base for particular schedules following different fracture types and fixation techniques.
The postoperative rehabilitation of fractures is arguably as important for uncomplicated fracture healing as their surgical management. It is well known that lengthy periods of immobilization can have severe detrimental effects on wellbeing of patients, while over aggressive mobilization can equally lead to severe complications. Such complications have implications not only for the wellbeing of the patient but also on the direct and indirect costs of treatment to the health care providers. Conversely, significant improvements have been made in fracture care over time due partly to more aggressive rehabilitation of patients. Unfortunately, changes in fracture rehabilitation remain empirical and speculative. Such changes are compounded by most patients’ inability to follow partial weight bearing instructions[14-16] and, until recently, by the absence of objective tools to assess fracture stability during healing.
Objective biomechanical data and activity monitoring reported in this study are examples of the different compliance of patients when following rehabilitation instructions. The amount of activity patients perform in the first few postoperative weeks can be extremely variable along with the amount of weight bearing. Interestingly in the cases investigated, the non-compliant weight bearing applied by Patient 1 did not lead to excessive displacement of the fracture according to current definitions of anatomical reduction (less than 2 mm of articular step)[5,6]. The results of Patient 1 also suggested that his fracture stabilization was adequate and in these cases weight bearing as tolerated after a TPF may lead to a quicker rehabilitation. Recovery of a gait pattern similar to the uninjured limb was observed as early as 6 wk after surgery for Patient 1 compared to 12 wk for Patient 2. Finally, Patient 2, who did adhere to postoperative weight bearing instructions, displayed larger elastic displacement under load at 6 wk, but did not displace as much over time. Larger scale clinical studies that apply the combination of technologies used in the current investigation may provide more information to suggest which postoperative weight bearing regimes are appropriate for different fracture types and internal fixation techniques. In addition, the accurate measurement of fracture stability, combined with objective activity monitoring and patient outcomes, could assist to better define terms such as “anatomical reduction” and objective correlations between articular fracture displacements and outcomes.
In conclusion, this case study demonstrates the potential of using a combination of RSA, gait analysis and activity monitoring to provide, for the first time, an evidence base for the application of particular rehabilitation schedules following fracture. Such large scale studies are required before rehabilitation protocols can be optimized by objective data.
There is no consensus on the recommended rehabilitation protocol after lower limb trauma, including tibial plateau fractures. The results of previous studies investigating different weight bearing regimes have been limited due to the lack of relevant assessment tools available to identify clinically significant differences in patient cohorts.
Recent improvements in monitoring fracture stability with radiostereometric analysis, and monitoring patient movement with specific gait analysis and patient activity monitors may allow novel research to be undertaken.
Authors have demonstrated the feasibility of using radiostereometric analysis, gait analysis and activity monitoring to assess rehabilitation protocols after lower limb trauma.
Using this combination of assessment methods in larger clinical studies has the potential to provide, for the first time, an evidence base for the application of particular rehabilitation schedules following fracture.
RSA: A very accurate measurement method that uses two simultaneous X-rays to monitor the movement of a fracture fragment over time and under load.
This case report presents a good concept of combining three assessment tools to monitor two patients after tibial plateau fracture. Using these methods in larger studies may allow novel improvements to clinical practice.
P- Reviewers Shafi M, van den Bekerom MPJ S- Editor Gou SX L- Editor A E- Editor Wu HL
| 1. | Honkonen SE, Järvinen MJ. Classification of fractures of the tibial condyles. J Bone Joint Surg Br. 1992;74:840-847. [PubMed] [Cited in This Article: ] |
| 2. | Honkonen SE. Indications for surgical treatment of tibial condyle fractures. Clin Orthop Relat Res. 1994;199-205. [PubMed] [Cited in This Article: ] |
| 3. | Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9:273-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 231] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Brown TD, Anderson DD, Nepola JV, Singerman RJ, Pedersen DR, Brand RA. Contact stress aberrations following imprecise reduction of simple tibial plateau fractures. J Orthop Res. 1988;6:851-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 134] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Eggli S, Hartel MJ, Kohl S, Haupt U, Exadaktylos AK, Röder C. Unstable bicondylar tibial plateau fractures: a clinical investigation. J Orthop Trauma. 2008;22:673-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Russell TA, Leighton RK. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am. 2008;90:2057-2061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Borrelli J, Goldfarb C, Catalano L, Evanoff BA. Assessment of articular fragment displacement in acetabular fractures: a comparison of computerized tomography and plain radiographs. J Orthop Trauma. 2002;16:449-456; discussion 456-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Cole RJ, Bindra RR, Evanoff BA, Gilula LA, Yamaguchi K, Gelberman RH. Radiographic evaluation of osseous displacement following intra-articular fractures of the distal radius: reliability of plain radiography versus computed tomography. J Hand Surg Am. 1997;22:792-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Liow RY, Birdsall PD, Mucci B, Greiss ME. Spiral computed tomography with two- and three-dimensional reconstruction in the management of tibial plateau fractures. Orthopedics. 1999;22:929-932. [PubMed] [Cited in This Article: ] |
| 10. | Martin J, Marsh JL, Nepola JV, Dirschl DR, Hurwitz S, DeCoster TA. Radiographic fracture assessments: which ones can we reliably make? J Orthop Trauma. 2000;14:379-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Madanat R, Mäkinen TJ, Moritz N, Mattila KT, Aro HT. Accuracy and precision of radiostereometric analysis in the measurement of three-dimensional micromotion in a fracture model of the distal radius. J Orthop Res. 2005;23:481-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Solomon LB, Stevenson AW, Callary SA, Sullivan TR, Howie DW, Chehade MJ. The accuracy and precision of radiostereometric analysis in monitoring tibial plateau fractures. Acta Orthop. 2010;81:487-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Whittle AP. Fractures of the lower extremity. In: eds. Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. Mosby Elsevier, Philadelphia 2007; 3085-3236. [Cited in This Article: ] |
| 14. | Hurkmans HL, Bussmann JB, Selles RW, Benda E, Stam HJ, Verhaar JA. The difference between actual and prescribed weight bearing of total hip patients with a trochanteric osteotomy: long-term vertical force measurements inside and outside the hospital. Arch Phys Med Rehabil. 2007;88:200-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Tveit M, Kärrholm J. Low effectiveness of prescribed partial weight bearing. Continuous recording of vertical loads using a new pressure-sensitive insole. J Rehabil Med. 2001;33:42-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Vasarhelyi A, Baumert T, Fritsch C, Hopfenmüller W, Gradl G, Mittlmeier T. Partial weight bearing after surgery for fractures of the lower extremity--is it achievable? Gait Posture. 2006;23:99-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Solomon LB, Callary SA, Stevenson AW, McGee MA, Chehade MJ, Howie DW. Weight-bearing-induced displacement and migration over time of fracture fragments following split depression fractures of the lateral tibial plateau: a case series with radiostereometric analysis. J Bone Joint Surg Br. 2011;93:817-823. [PubMed] [Cited in This Article: ] |
| 18. | Cappozzo A, Catani F, Croce UD, Leardini A. Position and orientation in space of bones during movement: anatomical frame definition and determination. Clin Biomech (Bristol, Avon). 1995;10:171-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1147] [Cited by in F6Publishing: 1019] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 19. | Lu TW, O’Connor JJ. Bone position estimation from skin marker co-ordinates using global optimisation with joint constraints. J Biomech. 1999;32:129-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 667] [Cited by in F6Publishing: 563] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 20. | Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2694] [Cited by in F6Publishing: 2485] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 21. | Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng. 1990;37:757-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1386] [Cited by in F6Publishing: 1106] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 22. | Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181-188. [PubMed] [Cited in This Article: ] |
| 23. | Esliger DW, Rowlands AV, Hurst TL, Catt M, Murray P, Eston RG. Validation of the GENEA Accelerometer. Med Sci Sports Exerc. 2011;43:1085-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 389] [Article Influence: 29.9] [Reference Citation Analysis (0)] |