INTRODUCTION
Osteoclasts are the only multinucleated cells in the body that resorb bone, a process essential for postnatal bone remodeling. Humans and mice with deficient osteoclast generation or function form skeleton during embryonic bone development, but develop severe osteopetrosis in later stages of life. In osteopetrosis, the bone marrow cavity is filled with un-resorbed bone matrix[1,2]. Osteopetrosis is often accompanied by early death due to bone marrow failure-associated with immune deficiency and a high risk of infection[3,4]. Thus, osteoclasts are not required for embryonic bone development and modeling, but they are essential for postnatal bone remodeling. Osteoclasts are hematopoietic cells originating from embryonic mesoderm along with bone and cartilage. They are formed from bone marrow myeloid progenitor cells[5]. Under the influence of macrophage colony-stimulating factor (M-CSF), these progenitor cells survive and proliferate to expand themselves[6]. Receptor activator for NF-κB ligand (RANKL, also named TNSF11 or osteoprotegerin ligand) triggers the differentiation of M-CSF-dependent progenitor cells to osteoclast precursors and then to mature osteoclasts, which are stained positive for Tartrate-resistant acid phosphatase (TRAP, also named acid phosphatase 5)[7,8]. Osteoclast precursors are TRAP+ mono-nucleated pre-osteoclasts (pre-OCs), which fuse to form multinucleated mature TRAP+ osteoclasts. TRAP+ pre-OCs do not resorb bone in in vitro cultures and mice with defective pre-OC fusion develop osteopetrosis[9,10]. Thus, pre-OC fusion is a critical cellular event for osteoclast function, and understanding its regulation will have an important impact on the development of a new therapy to control bone loss via targeting osteoclast cell fusion.
OSTEOCLAST FUSION
Under physiological conditions, both TRAP+ mono-nucleated pre-OCs and TRAP+ multinucleated osteoclasts are found only on the bone surface, indicating that pre-OC formation from TRAP- myeloid progenitor cells and subsequent fusion must occur on the bone surface. Since TRAP- myeloid progenitor cells are present in multiple organs and tissues while TRAP+ pre-OCs and osteoclasts only are present on the bone surface within the bone marrow, to initiate cell fusion, progenitor cells must be recruited, migrated and attached to the bone surface and differentiate to pre-OCs. Thus in a broad sense, factors that affect recruitment, migration and attachment of progenitor cells should also be considered factors that regulate cell fusion. However, although the events that are involved in pre-OC fusion are continuous events in vivo, they can be divided into a series of steps experimentally in cell cultures. Myeloid progenitor cells isolated from multiple tissues, such as peripheral blood[11], spleen[12], liver[13] and even lymph nodes (our unpublished observation), can all give rise to TRAP+ mono-nucleated pre-OCs in the presence of M-CSF and RANKL, which fuse spontaneously within 1-3 d to form multinucleated osteoclasts on plastic culturing plates. Thus, the fusion of pre-OCs, at least in vitro, is an independent process, apart from recruitment, migration and adhesion. In this review we will specifically discuss factors that control the fusion process of pre-OCs. We will not include factors that regulate progenitor recruitment, migration and adhesion, although these are prerequisite to fusion in vivo[14,15]. Because mouse genetic studies indicate that depletion of Dendritic cell-specific transmembrane protein (DC-STAMP, TM7SF4)[9,10] in mice affects only the cell-cell fusion process, we will discuss the recent findings of DC-STAMP and its regulation in detail.
FACTORS THAT REGULATE OSTEOCLAST FUSION
Information on factors that regulate osteoclast fusion comes from two lines of study. One is from in vitro experiments in which samples from different stages (d) of osteoclast differentiation are used for the expression levels of factor that are known to regulate macrophage fusion; and the loss or gain of function experiments to determine the effect of manipulating these factors on pre-OC fusion. Another is from in vivo experiments in which bone phenotype and TRAP+ osteoclast morphology (mono-nucleated vs multi-nucleated) are examined in genetically modified mice to determine if a specific gene is responsible for or only responsible for pre-OC fusion.
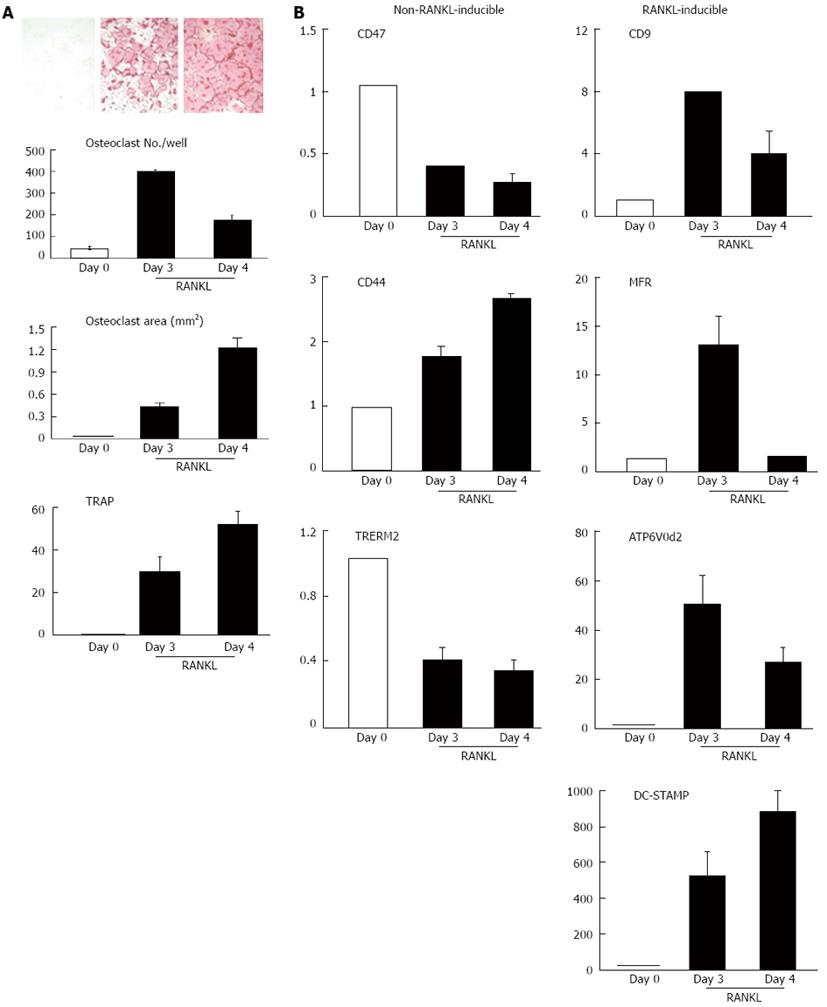
Several factors are important for macrophage fusion including CD44, CD47, macrophage fusion receptor, CD9, DC-STAMP[16,17]. To investigate the expression pattern of these factors during osteoclast differentiation, we treated primary bone marrow mono-nuclear cells, of which 30% of them expressed pan myeloid marker CD11b, with M-CSF for 2 d to enrich myeloid progenitor cells. At this stage more than 90% of the cells expressed CD11b and all of them were TRAP negative[18] (Figure 1). We then treated these myeloid progenitor cells with RANKL for 3 or 4 d and examined the expression levels of known fusion genes, and compared their status of osteoclast fusion and differentiation (Figure 1). M-CSF-induced myeloid progenitor cells expressed high levels of CD44, CD47, and triggering receptor expressed on myeloid cells-2 (TREM2) at day 0 before RANKL treatment and gave rise to multinucleated osteoclasts at day 3 after they were cultured with RANKL. Osteoclast formation was accompanied by significant up-regulation of a set of fusion genes including CD9, macrophage fusion receptor (MFR), ATP6V0d2 and DA-STAMP. Compared to the day 0 samples, the expression of ATP6V0d2 and DA-STAMP was induced by 50- and 500-fold, respectively. These data (Figure 1) are consistent with other reports regarding the changes of expression of these genes during pre-OC fusion in Raw264.7 cells, a mouse macrophage/osteoclast precursor cell line[19], and divide fusion factors into 2 groups: factors (CD44, CD47, TREM2) that are not regulated by RANKL or those (CD9, ATP6V0d2 and DC-STAMP) that are regulated by RANKL. The factors whose regulation is induced by RANKL express at a higher level in M-CSF-dependent myeloid progenitor cells and the changes of their expression levels are less than 3-fold during the period from the TRAP-cells at day 0 to the TRAP+ multinucleated osteoclasts at day 3. In contrast, the factors that highly respond to RANKL are induced more than 8-folds by RANKL in the same samples (Figure 1).
Figure 1 Receptor activator of NF-κB ligand increases the expression of a set of fusion genes in osteoclasts derived from WT bone marrow cells.
Bone marrow mono-nuclear cells were cultured with macrophage colony-stimulating factor for 2 d and were then treated with Receptor activator of NF-κB ligand (RANKL) for additional 3 or 4 d. Cells were harvested at day 0 (before RANKL), day 3 and day 4 after RANKL treatment. A: Tartrate-resistant acid phosphatase (TRAP) staining, osteoclast number and area, and TRAP mRNA expression; B: The expression levels of fusion genes by quantitative polymerase chain reaction. The values are the fold increase vs the value on Day 0 as 1. MFR: Macrophage fusion receptor; DC-STAMP: Dendritic cell-specific transmembrane protein
FACTORS THAT ARE NOT REGULATED BY RANKL
CD47
CD47 is an integrin-associated protein that binds to its receptor, MFR (also called signal regulatory protein alpha)[20]. MFR is the first molecule identified to be critical for macrophage fusion[21,22]. In macrophages, CD47 and MFR interaction mediates cell-cell recognition at the stage before cell-cell fusion rather than during the fusing process itself. This is also the case in osteoclast fusion (Figure 1). The involvement of CD47 in bone remodeling and bone cell regulation was first studied in vitro using neutralizing antibodies to CD47 or MFR to block the CD47/MFR pathway. These neutralizing antibodies strongly reduced the formation of TRAP+ multinucleated osteoclasts in cultures of murine bone marrow cells in the presence of RANKL and M-CSF[23]. The role of CD47 in bone was further studied in mice that had global depletion of CD47. The numbers of osteoclasts were reduced when Cd47-/- spleen or bone marrow cells were cultured in vitro. The mechanism of reduced osteoclast numbers was due to a lack of SHPS-1 phosphorylation, SHP-1 phosphatase recruitment, and subsequent dephosphorylation of non-muscle cell myosin IIA. However, despite reduced osteoclast formation in vitro, Cd47-/- mice developed low bone mass phenotypes with a significant decrease in osteoblastogenesis in bone marrow stromal cells, which overrides the defect in osteoclast formation and fusion. Because CD47 is an integrin-associated protein, it has functions other than just regulating fusion[24]. The role of CD47 on osteoclast fusion in bone remodeling in vivo needs to be studied in mice with specific depletion of CD47 deletion in osteoclast precursors and in mice with pathological bone disorders.
CD44
CD44, a receptor for hyaluronic acid, is a type I transmembrane glycoprotein that connects a variety of extracellular matrix proteins to the cell surface. It binds to chondroitin sulfates and osteoponin to inhibit macrophage fusion. CD44 is highly expressed in macrophages prior to fusion[25]. As cell fuse, the intracellular domain of CD44 is cleaved and translocated to the nucleus, where it activates the NF-κB pathway and stimulates fusion[26]. CD44 is involved in actin cytoskeletal organization in osteoclasts by linking to podosome cores[27]. The role of CD44 in osteoclast generation and fusion in vivo was examined in Cd44-/- mice. Cd44-/- mice had normal basal bone volume. Osteoclast size and numbers on the bone surfaces of Cd44-/- mice were similar to that of WT mice, indicating that CD44 expression is not essential for pre-OC fusion under physiological conditions[28]. To investigate if CD44 plays a role in pathological bone disorders, Cd44-/- mice were crossed with TNF-Tg, a mouse model of inflammatory arthritis due to over-expression of the human TNF transgene[29]. Compared to TNF-Tg mice, TNF-Tg/Cd44-/- mice developed much more severe joint inflammation and bone erosion and systemic bone loss. Increased osteoclast number, size and resorptive capacity were observed in TNF-Tg/Cd44-/- mice, but bone formation and osteoblast differentiation in these mice were normal. Cd44-/- osteoclasts had elevated activation of the p38 mitogen-activated protein kinase in response to TNF, indicating that CD44 is a critical inhibitor of TNF-driven joint destruction and inflammatory bone loss[30].
TREM2
TREM2 is the main DNAX activating protein 12 (DAP12)-associated receptor in osteoclasts. In the bone, TREM-2 induces fusion of pre-OCs into multinucleated cells; thus, osteoclast development is blocked in the absence of TREM-2, resulting in inefficient bone resorption[31,32]. In vitro loss of function experiments demonstrated that knock-down of DAP12 or TREM2 impairs osteoclast development and fusion[33]. DAP12/TREM-2 is also involved in the formation of foreign body giant cells and is induced by IL-4. IL-4 is a cytokine produced by T helper cells and plays a critical role in macrophage fusion[34]. Macrophages express an uncharacterized TREM-2 ligand[35]. It has been hypothesized that DAP12- and TREM-mediated macrophage fusion is mediated by the interaction between TREM-2 ligand-expressing macrophages and TREM-expressing macrophages. DAP12/TREM2 signaling can therefore be considered as an endogenous macrophage trigger leading to increased gene transcription and expression of factors required for efficient fusion. It can be separated from the exogenous IL-4 stimulus because both IL-4-induced and basal macrophage fusion levels are reduced in the absence of DAP12 signaling[31,32].
FACTORS THAT ARE REGULATED BY RANKL
CD9
CD9 is a member of the tetraspanin superfamily proteins, which are implicated in a variety of cell processes including fusion. Members of tetraspanin superfamily proteins that mediate cell fusion include CD9, CD63, CD81. Blockage of CD9 and CD81 with neutralizing antibodies enhances macrophage fusion to multinucleated giant cells[36]. CD9 is expressed in a specific membrane lipid raft of Raw267.4 cells, which is enhanced by RANKL treatment. Blockage of CD9 by a neutralizing antibody or RNA interference reduces osteoclast formation while over-expression of CD9 promotes cell fusion in the absence of RANKL[19]. CD9 protein expression is increased in activated osteoclasts on bone surfaces in mice with ovariectomy- or arthritis-induced bone loss, suggesting that CD9 plays important roles in bone destruction[37].
ATP6v0d2
Among numerous fusion regulators, gene knockout studies revealed that the expression of DC-STAMP (Dendritic cell-specific transmembrane protein, TM7SF4)[9,10] and ATP6V0d2 (ATPase, H+ transporting, lysosomal 38 kDa, V0 subunit d2) are required for osteoclast fusion under basal conditions. ATP6V0d2 is a component of the ATPase pump. However, osteoclasts from ATP6V0d2-/- mice do not have defective v-ATPase activity and differentiation, rather the fusion of pre-OCs to mature osteoclasts is blocked[38]. Apart from defective fusion, bone formation is significantly increased in ATP6V0d2-/- mice. Since osteoblasts do not express ATP6V0d2 and bone marrow stromal cells from ATP6V0d2-/- mice differentiate to osteoblasts in vitro normally, it is speculated that increased bone formation in ATP6V0d2-/- mice works through an indirect mechanism. The expression levels of DC-STAMP and MFR is un-changed in ATP6V0d2-/- cells. ADAM8 and ADAM12 expression levels are markedly reduced. Over-expression of ADAM8 or ADAM12 rescues pre-OC fusion defect of ATP6V0d2-/- cells[38].
A disintegrin and metalloprotease 8 (ADAM8) expresses in osteoclast precursors and stimulates osteoclast formation[39]. Recently, Dr. David Roodman’s lab generated TRAP-ADAM8 transgenic mice which specifically over-expressed ADAM8 in TRAP-expressing pre-OCs and osteoclasts, and global ADAM8-/- mice. TRAP-ADAM8 transgenic mice developed a low bone mass phenotype with increased osteoclast numbers. In vitro studies demonstrated that cells from TRAP-ADAM8 transgenic mice had increased RANKL-mediated signaling pathways including NF-κB, Erk and Akt. Apart from increased osteoclast differentiation and bone resorption, pre-OCs had increased fusion capacity. Interestingly, basal levels of DC-STAMP expression were significantly increased in TRAP-ADAM8 cells, which was further enhanced by RANKL. In contrast, the expression of other fusion proteins such as CD44, CD47 and ATP6v0d2 were only slightly increased in the later time of cultures. Increased DC-STAMP was considered due to increased RANKL-mediated signaling such as c-Fos, NFATc1 and NF-κB, all of them up-regulate DC-STAMP expression[40]. However, it is not clear why ATP6v0d2 expression was not changed since it is also regulated by RANKL-NFATc1 pathway and over-expression of ADAM8 in ATP6v0d2-/- cells rescues the fusion defect.
DC-STAMP
DC-STAMP is a seven-pass transmembrane protein which is encoded by the gene named Tm7sf4[41]. In 2006, Dr. Toshio Suda’s lab used a DNA subtraction screen between osteoclasts and mononuclear macrophages and identified that DC-STAMP was highly expressed in osteoclasts but not in macrophages. They generated DC-STAMP-/- mice and demonstrated that mice developed osteopetrosis due to a defect in osteoclast fusion. Cells from DC-STAMP-/- mice were still able to respond to RANKL-induced expression of osteoclast functional genes such as TRAP and cathepsin K. In contrast to ATP6V0d2-/- mice, osteoblast-mediated bone formation was normal in DC-STAMP-/- mice. Currently, DC-STAMP-/- mice is the only mouse model where the increased bone mass is solely due to a defect of pre-OC fusion. The foreign body giant cell reaction in DC-STAMP-/- mice was also defected, confirming the known role of DC-STAMP in macrophage cell fusion[9]. Cells from DC-STAMP-/- mice can still resorb the bone tissues with significantly reduced capacity, indicating that cell fusion is not essential for bone resorption but significantly affects its efficiency. These data suggest that cell fusion is an important step in osteoclast differentiation, and osteoclast size determines resorptive capacity and functions of osteoclasts.
REGULATION OF DC-STAMP BY FACTORS IN THE RANKL PATHWAY
RANK and RANKL are key regulatory molecules for osteoclast formation and activation[13,42-44]. RANKL is expressed on the surface of osteoblastic cells in the marrow cavity and by osteocytes embedded within bone[45,46] and it interacts with its receptor RANK located on the surface of osteoclasts and osteoclast precursors. RANKL/RANK interaction leads to recruitment of TNF receptor-associated factors (TRAFs), adaptor proteins to which enzymes, including MAP kinases and Src tyrosine kinase, bind and are activated to mediate downstream signaling. TRAF-mediated signaling leads to activation of NF-κB, c-Fos, and NFATc1 (nuclear factor of activated T cells cytoplasmic 1)[13,42-44], transcription factors that are essential for osteoclast differentiation. These transcription factors also regulate osteoclast fusion, mainly via DC-STAMP.
NFATc1
NFATc1 is a RANKL downstream gene[47]. Dr. Miyamoto’s lab (2007) demonstrated that the DC-STAMP promoter contains AP-1 and NFATc1 binding sites, which are required for RANKL-induced DC-STAMP expression. c-Fos and NFATc1 are indispensable for DC-STAMP expression and cell-cell fusion in osteoclasts, but both factors are dispensable for giant cell formation by macrophages, suggesting that DC-STAMP transcription is regulated in a cell type-specific manner[48]. Over-expression of NFATc1 in WT bone marrow-derived macrophages does not affect the expression levels of CD9, CD44, and MFR, but significantly increases Atp6v0d2 and DC-STAMP expression, indicating that among fusion regulators, Atp6v0d2 and DC-STAMP are RANKL target genes. Both the Atp6v0d2 and DC-STAMP promoters contain multiple NFATc1 binding sites and are bound by NFATc1 protein in chromatin immunoprecipitation assay[49].
NF-κB
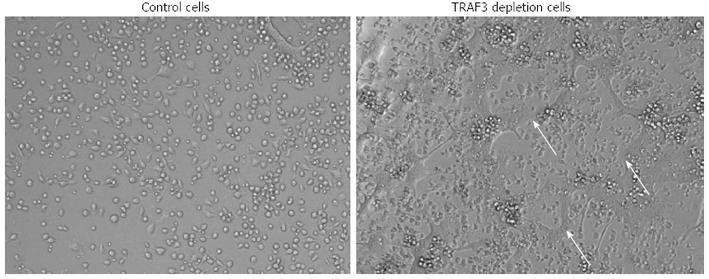
The NF-κB family includes NF-κB1 (p50 and its precursor p105), NF-κB2 (p52 and its precursor p100), RelA, RelB and c-Rel. Homo- and hetero-dimers of the 5 NF-κB proteins activate the transcription of target genes, through the canonical (RelA:p50) and the non-canonical (RelB:p52) pathways. NF-κB (typically refers to canonical RelA-mediated transcription) regulates many aspects of cellular activity[47]. Expression of both NF-κB1 and NF-κB2 are essential for RANKL-induced osteoclast formation[13,42,50]. Basal osteoclast formation is normal in NF-κB1-/- or NF-κB2-/- mice[51], but TNF and RANKL-induced osteoclast formation is increased in NF-κB2-/- mice, indicating that NF-κB2-mediated cellular events negatively regulate osteoclast formation[52]. IL-4 inhibits the RANKL-induced osteoclast differentiation and promotes macrophage fusion. IL-4 inhibition of osteoclastogenesis is mediated by suppressing the RANKL-induced activation of NF-κB. A recent study reported that IL-4 did not block proximal, canonical NF-κB signaling. Instead, IL-4 inhibited NF-κB signaling by inducing NF-κB1 expression. Formation of both RANKL-induced osteoclasts and IL-4-induced foreign body giant cells are impaired in bone marrow-derived macrophages from NF-κB1-/- mice, and in WT cells that were treated with the NF-κB inhibitors, IκB kinase 2 inhibitor or NF-κB essential modulator inhibitory peptide. Over-expression of p50, p65, p52, and RelB individually in NF-κB1-/- or WT bone marrow-derived macrophages increased the formation of multinucleated osteoclasts and foreign body giant cells. Interestingly, knockdown of NF-κB2 in WT BMM dramatically enhanced both osteoclast and foreign body giant cell formation[53]. NF-κB2 is composed of p52 and its precursor p100. RANKL promotes the processing of p100 to p52 via NIK (NF-κB inducing kinase). Under basal conditions, cells have very low levels of NIK protein because it is degraded in a protein complex inducing TRAF3[54,55]. In mice whose osteoclasts carrying a mutated form of NIK that lacks its TRAF3 binding domain, osteoclast formation and fusion is increased and mice develop mild osteoporosis. Osteoclasts from these mice express high levels of NFATc1 and DC-STAMP[56]. Consistent with this finding, we observed increased RANKL-induced osteoclast fusion in M-CSF-dependent myeloid precursors that are isolated from osteoclast specific TRAF3 knockout mice (Figure 2). Together, these findings suggest that NF-κB1 stimulates while NF-κB2 inhibits osteoclast fusion. The detailed mechanisms by which NF-κB2 negatively regulates osteoclast fusion need to be further studied.
Figure 2 Increased osteoclast fusion in cells from TRAF3f/f; CSK-Cre mice.
Bone marrow mono-nuclear cells from control mice (TRAF3f/f) and TRAF3f/f; CSK-Cre mice were cultured with M-CSF for 2 d and then treated with RANKL for additional 2 d. Photos show multi-nuclear mature osteoclasts (arrows) in cells from TRAF3f/f; CSK-Cre mice. TNF: Tumor necrosis factor; TRAF: TNF receptor-associated factors; M-CSF: Macrophage colony-stimulating factor.
Calcium
RANKL induces calcium (Ca2+) oscillations, leading to up-regulation of NFATc1. Cellular Ca2+ comes from both intracellular and extracellular sources. Inositol 1,4,5-trisphosphate affects osteoclast formation by stimulating Ca2+-release from the endoplasmic reticulum[57-59]. However, it is less clear the extent to which extracellular Ca2+ influx is involved in osteoclast biology. Ca2+ entering cells is mainly mediated by the Ca2+-release-activated Ca2+ channel on plasma membrane[60,61]. Orai1 is a subunit of the Ca2+-release-activated Ca2+ channel, which provides a major Ca2+ influx pathway in hematopoietic cells and plays a critical role in the maintenance of Ca2+ oscillations[62]. Orai1 is required for the activation of NFATc1 in T cells[63]. Knockdown of Orai1 by RNA interference in human monocytes[64] or RAW264.7 cells[65] reduces Ca2+ channel and inhibits RANKL-induced osteoclastogenesis by suppressing the induction of NFATc1. Orai1-/- cells are defective in cell fusion and bone resorption, which is associated with down-regulation of ATP6v0d2, suggesting that Orai1 might be a potential therapeutic target for the treatment of bone loss caused by osteoclasts.
REGULATION OF DC-STAMP BY FACTORS OTHER THAN RANKL
Recent studies reveal that DC-STAMP can be regulated by factors other than RANKL, indicating the complexity of cell fusion regulation.
Interleukin-32
Interleukin-32 gamma is a cytokine that is produced mainly by T cells and NK cells[66]. The expression of interleukin-32 gamma is increased in synovial tissues from patients with rheumatoid arthritis[67]. Interleukin-32 gamma increases the expression levels of NFATc1, DC-STAMP and ATP6V0d2, and promotes RANKL-induced osteoclast formation and fusion. Interleukin-32 gamma-induced DC-STAMP and ATP6V0d2 up-expression is abolished in cells treated with NFATc1 inhibitor. These data indicate that T cell cytokine interleukin-32 gamma promotes osteoclast fusion via NFATc1-mediated up-regulation of DC-STAMP and ATP6V0d2, and T cells may affect osteoclast fusion via Interleukin-32 gamma in patients with rheumatoid arthritis or other similar types of diseases[68].
Tal1
T-cell acute lymphocytic leukemia 1/stem cell leukemia 1 (Tal1/Scl1) is a basic helix-loop-helix transcription factor essential for hematopoiesis. Tal1-/- mice died in early embryo due to failure of hematopoietic stem cell and blood development[69]. Tal1 functions as a transcription repressor or activator, depending on the cofactors with which it is associated[70]. Embryonic stem cells derived from Tal1-/- mice fail to give rise to osteoclasts[71]. A recent study indicates that Tal1 is involved in pre-OC fusion. Over-expression of Tal1 in RAW264.7 cells or primary bone marrow-derived macrophages inhibits osteoclast formation, but has no effect on TRAP+ pre-OC formation. Knockdown of Tal1 with RNA interference increases pre-OC fusion. Tal1 binds to the DC-STAMP promoter and inhibits DC-STAMP transcription. During osteoclast differentiation, the Tal1 occupancy of DC-STAMP promoter decreases while PU.1 occupancy of DC-STAMP promoter increases, cells fuse[72]. However, the exact role of Tal1 in osteoclast fusion in bone volume needs to be studied in mice with osteoclast specific depletion of Tal1.
CCN2/CTGF
CCN family 2/connective tissue growth factor (CCN2/CTGF) promotes endochondral ossification[73]. Ccn2-/- mice have an expanded hypertrophic zone[74], indicating that the resorption of the cartilage extracellular matrix by osteoclasts or chondroblasts is impaired. Expression of the Ccn2 gene is increased along RANKL-induced osteoclast differentiation. Recombinant CCN2 plus RANKL significantly enhances TRAP+ multinucleated cell formation compared with RANKL alone. CCN2 induces DC-STAMP expression and binds to DC-STAMP protein. Furthermore, RANKL-induced osteoclastogenesis is impaired in fetal liver cells from Ccn2-/- mice, which can be rescued by the addition of exogenous recombinant CCN2 or the forced expression of DC-STAMP by a retroviral vector. These results suggest that CCN2 expressed during osteoclastogenesis promotes osteoclast formation via induction of and interaction with DC-STAMP[75].
LDLR
Osteoporosis is often associated with atherosclerosis and vascular calcification due to hyperlipidemia. A recent study demonstrates that RANKL-induced osteoclast formation is decreased in bone marrow-derived macrophages from mice with depletion of low-density lipoprotein receptor (LDLR). Osteoclast precursors constitutively express LDLR. PreOCs from LDLR-/- mice form smaller osteoclasts than WT cells. RANKL-activated downstream Erk and Akt signals and expression of NFATc1, cathepsin K, and TRAP are normal in LDLR-/- pre-OCs, but the protein expression of DC-STAMP and ATP6V0d2 is reduced in the cell surface of LDLR-/- pre-OCs. LDLR-/- mice have high bone mass, which is accompanied by a decrease in bone resorption parameters, with no changes in bone formation parameters. These findings provide a novel mechanism for osteoclast differentiation and improve the understanding of the correlation between osteoclast fusion and lipids[76].
Vitamin E
Vitamin E is an antioxidant that inhibits lipid peroxidation by scavenging reactive oxygen species and is believed to be protective against arteriosclerotic changes and the aging process[77]. Vitamin E is a mixture of tocopherols and tocotrienols. In liver α-tocopherol transfer protein (α-TTP) mediates the transfer of α-tocopherol into lipoproteins[78] and α-tocopherol is the most predominant isoform of vitamin E in the body. Dr. Takeda’s lab recently examined bone phenotypes of mice deficient in α-TTP (Ttpa-/- mice) and found that these mice develop high bone mass phenotypes. Ttpa-/- mice have normal osteoblasts and decreased osteoclasts in vivo. Interestingly, in vitro RANKL-induced osteoclast formation is normal when bone marrow-derived macrophages from Ttpa-/- mice are used. Serum from Ttpa-/- mice inhibits RANKL-induced WT osteoclast formation, suggesting that circulating α-tocopherol negatively regulates osteoclast formation. Consistent with this, additional α-tocopherol increases RANKL-induced osteoclast formation and the size of osteoclasts. α-tocopherol promotes DC-STAMP expression via mitogen-activated protein kinase and microphthalmia-associated transcription factor. It is directly recruited to the DC-STAMP promoter. These studies reveal a new role for vitamin E in bone homeostasis through regulation of osteoclast fusion[79].
DC-STAMP HAS FUNCTIONS OTHER THAN FUSION
DC-STAMP is expressed on the cell surface. A recent study reveals two new functions of DC-STAMP apart from that of a master fusion factor using osteoclasts and precursors from human peripheral blood. One is that DC-STAMP can serve as an osteoclast precursor biomarker in patients with psoriatic arthritis. In this study, peripheral blood mono-nuclear cells are isolated from psoriatic arthritis patients and healthy controls, and stained with FITC-labeled anti-DC-STAMP antibody. DC-STAMP+ and DC-STAMP- cells are used in RANKL-induced osteoclast formation assays. The majority of osteoclasts are derived from DC-STAMP expressing monocytes. Interestingly, psoriatic arthritis patients have a higher percentage of DC-STAMP expressing monocyte cells than those of healthy controls. Another new discovery is that DC-STAMP functions as a signaling molecule within the osteoclasts. The cytoplamic tail of DC-STAMP contains an immunoreceptor tyrosine-based inhibitory motif, which is phosphorylated on its tyrosine residues and physically interacts with SHP-1 and CD16, an SH2-domain-containing tyrosine phosphatase and an ITAM-associated protein, respectively[11].
OC-STAMP
DC-STAMP was originally identified on the cell surface of dendritic cells[81] and in IL-4-treated macrophages and osteoclasts[9,41,81]. To isolate factors that are specifically expressed on multinucleated osteoclasts after they are formed from pre-OC fusion, the OC-STAMP (osteoclast stimulatory transmembrane protein) is cloned in RANKL-treated RAW264.7 cells[82]. OC-STAMP-/- mice have a fusion defect in both osteoclasts and foreign body giant cells, both of which are macrophage-lineage cells. Interestingly, DC-STAMP expression in OC-STAMP-/- osteoclasts and foreign body giant cells is normal. Similarly, OC-STAMP expression in DC-STAMP-/- osteoclasts and foreign body giant cells is normal, suggesting that OC-STAMP and DC-STAMP may regulate cell-cell fusion in osteoclasts and foreign body giant cells independently[83]. The regulation of OC-STAMP and its relationship with DC-STAMP is currently not known.
Pro-inflammatory cytokines
Osteoclast fusion is also promoted by pro-inflammatory cytokines. In RAW264.7 cells, RANKL, lipopolysaccharide (LPS), and TNFα all induce cell fusion, but M-CSF has no effect. The cell fusion induced by RANKL, TNFα, and LPS is specifically blocked by osteoprotegerin (OPG), anti-TNFα antibody and polymyxin B, respectively. LPS-induced cell fusion is partly inhibited by the anti-TNFα antibody, but not by OPG. Osteoclast fusion induced by these cytokines is accompanied by the activation of signaling pathways including PI3K, Src, ERK, JNK, and p38. Consistently, the specific chemical inhibitors LY294002 (PI3K), PP2 (Src), U0126 (ERK), and SP600125 (JNK) effectively reduce cell fusion. However, the expression levels of NFATc1 and DC-STAMP are unchanged. Thus, pre-inflammatory induced cell fusion may involve factors other than DC-STAMP[84].
CONCLUSION
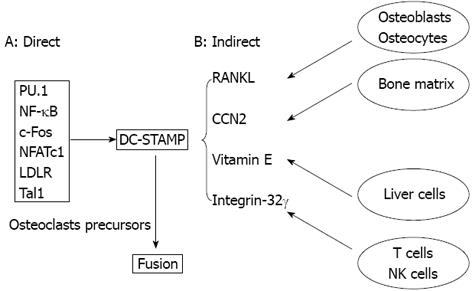
Many advances have been made in recent years for understanding of osteoclast fusion and its regulation, which is involved in numerous molecules and pathways. Among them, DC-STAMP is the major fusion regulator. DC-STAMP expression is regulated through direct and indirect mechanisms (Figure 3). In the direct mechanism, RANKL/RANK downstream transcription factors that are essential for RANKL-mediated osteoclast formation are also important for osteoclast fusion, which include PU.1, NF-κB, c-Fos, and NFATc1. Factors that do not work through the RANKL/RANK pathway control osteoclast fusion include LDLR and Tal1. These factors function in the osteoclast precursors to up-regulate DC-STAMP transcription. In the indirect mechanism, cells in the non-osteoclast lineage regulate DC-STAMP expression by producing soluble factors such as RANKL. Osteoblasts and osteocytes are major cell types that produce RANKL under physiological condition, which is a strong inducer of DC-STAMP production. CCN2, vitamin E, and integrin-32 are newly identified factors that regulate osteoclast fusion via DC-STAMP despite of precise mechanisms by which regulate DC-STAMP expression are not clear. ATP6V0d2 is another important fusion regulator and is regulated by the RANKL-NFATc1 pathway under certain conditions.
Figure 3 Osteoclast fusion is regulated by various factors mainly through dendritic cell-specific transmembrane protein.
A: In osteoclast precursors, factors including transcription factors essential for osteoclastogenesis control DC-STAMP expression, which regulates osteoclast fusion directly; B: Various cell types produce soluble factors that affect DC-STAMP expression in osteoclast precursors, which regulates osteoclast fusion indirectly. DC-STAMP: Dendritic cell-specific transmembrane protein.
However, there are still many open questions. For instance, all current fusion factors regulate the fusion process of both foreign body giant cell and osteoclast formation. It is not clear if osteoclast specific fusion factors exist. It has been proposed that in order to fuse, cells need to become fusion-component status. The techniques of identifying these fusion-component cells and their regulation are lacking. M-SCF is another factor that is essential for osteoclastogenesis, but M-SCF itself cannot induce TRAP+ pre-OC formation. It will be interesting to determine if M-CSF is the factor for fusion-competency while RANKL induces fusion mediators such as DC-STAMP. Because osteoclasts a play central role in physiologic bone remodeling and pathologic bone destruction, manipulation of their fusion is a promising therapeutic strategy to treat bone disorders due to the abnormality of osteoclasts.
Peer reviewers: Xiang-Hang Luo, Professor, Institute of Endocrinology and Metabolism, the Second Xiangya Hospital of Central South University, No. 139, Middle Renmin Road, Changsha 410011, Hunan Province, China; Chih-Shung Wong, MD, PhD, Professor and Director, Department of Anesthesiology, Cathay General Hospital, No. 280, Renai Road, Section 4, Taipei, Taiwan, China
S- Editor Huang XZ L- Editor A E- Editor Zhang DN