Copyright
©The Author(s) 2025.
World J Orthop. May 18, 2025; 16(5): 106377
Published online May 18, 2025. doi: 10.5312/wjo.v16.i5.106377
Published online May 18, 2025. doi: 10.5312/wjo.v16.i5.106377
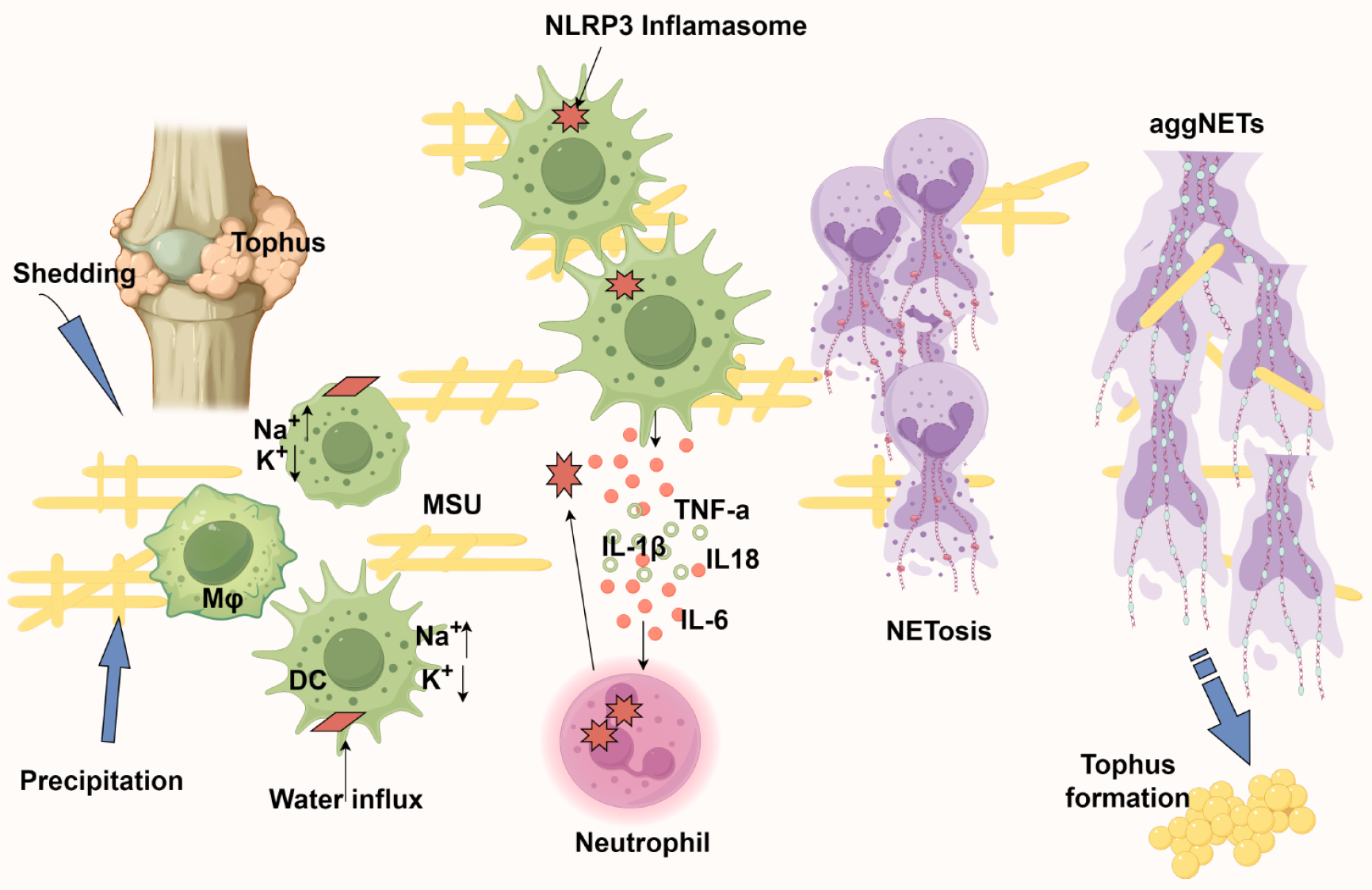
Figure 6 The neutrophil extracellular traps alleviate inflammation caused by monosodium urate crystals.
1: Monosodium urate crystals nucleate in joints either through de novo precipitation or release from existing tophi, subsequently being phagocytosed by antigen-presenting cells; 2: Intracellular crystal dissolution creates osmotic stress - sodium influx drives water entry, critically reducing potassium concentration below the threshold required for nucleotide-binding domain, leucine-rich repeat and pyrin domain-containing protein 3 inflammasome assembly; 3: Inflammasome activation triggers interleukin-18 (IL-18) and IL-1β release, establishing a chemokine gradient for neutrophil recruitment; 4: Infiltrating neutrophils amplify inflammation through proteinase 3-mediated pro-IL-1β processing while simultaneously engaging in crystal clearance via NETosis; 5: The resulting neutrophil extracellular trap (NET) structures serve dual roles: Physically encapsulating monosodium urate crystals to limit their bioavailability and forming aggregated NETs that proteolytically degrade inflammatory mediators; 6: Paradoxically, persistent aggregated NET deposition may nucleate new tophi, completing the pathogenic cycle. Created by Figdraw, ID: UAWAYbf41e. AggNETs: Aggregated neutrophil extracellular traps; NLRP3: Nucleotide-binding domain, leucine-rich repeat and pyrin domain-containing protein 3; DC: Dendritic cells; MSU: Monosodium urate; IL: Interleukin; TNF: Tumor necrosis factor.
- Citation: Sun GJ, Xu F, Jiao XY, Yin Y. Advances in research of neutrophil extracellular trap formation in osteoarticular diseases. World J Orthop 2025; 16(5): 106377
- URL: https://www.wjgnet.com/2218-5836/full/v16/i5/106377.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i5.106377