Published online Mar 18, 2022. doi: 10.5312/wjo.v13.i3.278
Peer-review started: April 28, 2021
First decision: September 29, 2021
Revised: October 11, 2021
Accepted: February 19, 2022
Article in press: February 19, 2022
Published online: March 18, 2022
The Ilizarov bone transport (IBT) and the Masquelet induced membrane technique (IMT) have specific merits and shortcomings, but numerous studies have shown their efficacy in the management of extensive long-bone defects of various etiologies, including congenital deficiencies. Combining their strong benefits seems a promising strategy to enhance bone regeneration and reduce the risk of refractures in the management of post-traumatic and congenital defects and nonunion that failed to respond to other treatments.
To combine IBT and IMT for the management of severe tibial defects and pseudarthrosis, and present preliminary results of this technological solution.
Seven adults with post-traumatic tibial defects (subgroup A) and nine children (subgroup B) with congenital pseudarthrosis of the tibia (CPT) were treated with the combination of IMT and IBT after the failure of previous treatments. The mean number of previous surgeries was 2.0 ± 0.2 in subgroup A and 3.3 ± 0.7 in subgroup B. Step 1 included Ilizarov frame placement and spacer introduction into the defect to generate the induced membrane which remained in the interfragmental gap after spacer removal. Step 2 was an osteotomy and bone transport of the fragment through the tunnel in the induced membrane, its compression and docking for consolidation without grafting. The outcomes were retrospectively studied after a mean follow-up of 20.8 ± 2.7 mo in subgroup A and 25.3 ± 2.3 mo in subgroup B.
The “true defect” after resection was 13.3 ± 1.7% in subgroup A and 31.0 ± 3.0% in subgroup B relative to the contralateral limb. Upon completion of treatment, defects were filled by 75.4 ± 10.6% and 34.6 ± 4.2%, respectively. Total duration of external fixation was 397 ± 9.2 and 270.1 ± 16.3 d, including spacer retention time of 42.4 ± 4.5 and 55.8 ± 6.6 d, in subgroups A and B, respectively. Bone infection was not observed. Postoperative complications were several cases of pin-tract infection and regenerate deformity in both subgroups. Ischemic regeneration was observed in two cases of subgroup B. Complications were corrected during the course of treatment. Bone union was achieved in all patients of subgroup A and in seven patients of subgroup B. One non-united CPT case was further treated with the Ilizarov compression method only and achieved union. After a follow-up period of two to three years, refractures occurred in four cases of united CPT.
The combination of IMT and IBT provides good outcomes in post-traumatic tibial defects after previous treatment failure but external fixation is longer due to spacer retention. Refractures may occur in severe CPT.
Core Tip: This study presents preliminary outcomes and the protocol of a developed technology that includes phase 1 of the Masquelet technique for induced membrane generation and Ilizarov bone transport. The technology did not comprise bone grafting or skin flaps. It was used in 16 patients with post-traumatic tibial defects and congenital pseudarthrosis of the tibia (CPT), after multiple failed treatments. The results were rated as good in patients with post-traumatic tibial defects. Congenital cases showed similar rates of pseudarthrosis union as other means currently used for CPT. Refractures may be expected in severe types of CPT after multiple previous treatments
- Citation: Borzunov DY, Kolchin SN, Mokhovikov DS, Malkova TA. Ilizarov bone transport combined with the Masquelet technique for bone defects of various etiologies (preliminary results). World J Orthop 2022; 13(3): 278-288
- URL: https://www.wjgnet.com/2218-5836/full/v13/i3/278.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i3.278
The challenges of long-bone defect management have increased in contemporary orthopedic practice due to the severity of high-energy trauma and its complications[1]. These defects can lead to a multi-stage, long and costly treatment. The Ilizarov method of bone transport (IBT) and the Masquelet induced membrane technique (IMT) have been used in a great variety of challenging clinical situations including post-traumatic bone loss, infected nonunion, tumor resection, and congenital deficiency, such as congenital pseudarthrosis of the tibia (CPT)[2-4]. Both techniques have specific merits and shortcomings, but numerous studies have shown their efficacy in the management of extensive long-bone defects of various etiologies, including congenital defects[2-5].
The IBT has been praised for high union rates and its biological aspect of growing authentic bone tissue to close bone defects[5-7]. Its followers believe that it is an ideal type of non-free bone grafting by which a vascularized autologous osteotomized bone fragment is transported gradually in the interfragmental gap within the soft tissue envelope to grow the missing bone part of a required length and shape[2,6-8]. The Ilizarov system has been criticized due to complications such as pin-tract infection, pain, possible joint contractures, risk of ischemic regeneration in compromised soft-tissues around a large defect and impaired quality of life due to the long time needed to provide treatment tasks and new bone remodeling[9]. The IMT is also based on the biological tissues of the induced membrane (IM) and autologous bone grafting, and utilizes internal or external fixation[10,11]. It is not devoid of characteristic complications either, being a staged treatment that takes months to complete bone remodeling. However, it provides a better quality of life, especially if pathology is located in the upper limb and femur[3,10].
Recent available studies have reported mostly good final outcomes of both procedures[6,7,10,11]. A study that compared the IBT (37 sources) and the IMT (41 sources) did not find statistical differences and reliable advantages between them in regard to consolidation, infection risks and failures that ended with amputation[12]. However, the study found that IBT patients had a higher rate of refractures. This may be associated with the fact that bone regeneration in large defects requires a longer time for remodeling and needs supportive internal fixation[8]. Nevertheless, several reviews and clinical studies doubt the superiority of IMT over IBT for long-bone defects in the lower extremity and point out that bone consolidation time may be unpredictable while non-weight bearing is prolonged in IMT[13,14]. High rates of infection and even amputation were reported for tibial defects after open fractures treated with IMT[14]. On the contrary, IBT allows weight-bearing from the first days. It is primarily used in patients with an infected tibia and rarely results in amputation[2,5]. In pediatrics, IMT has been frequently used for cancer surgery reconstructions[4]. Congenital anomalies, including СPT, may be treated with both options[4,15,16].
The importance of improving bone regeneration in the management of large bone defects and CPT is a very relevant issue due to treatment failures that diminish bone potential for regeneration. Management of CPT may take years in a growing child due to frequent recurrences and has a negative impact on the child’s development. Therefore, a combination of the biological merits of IBT and IMT seemed to us a promising strategy in the management of cases with a history of failed attempts and impaired regeneration potential. Following use of the combined technique in an experimental canine model[17], we aimed to conduct clinical studies on the use of this new technological solution that integrates the IMT and IBT techniques for treating non-viable tibial defects of post-traumatic (PTD) etiology and CPT to improve bone regeneration at the docking site, bone consolidation and reduce the refracture rate.
We retrospectively studied the treatment course and outcomes in a case series that included seven PTD patients (subgroup A) and nine CPT cases (subgroup B) managed using the combination of IMT and IBT. The patients were treated at the same specialized department in our orthopedic center by one team of surgeons in 2014–2019.
Tibial defects in subgroup A were caused by falls from a height, injuries at production sites and traffic accidents (Table 1). Time since injury was from one to 12 years (mean, 3.7 ± 0.9 years) and all subjects were adults (six males and one female with a mean age of 38.5 ± 4.1 years). Six cases had a history of infection and one had delayed wound healing. Patients’ inclusion criteria in subgroup A were bone defects of post-traumatic origin after several failed previous treatments, with a disease history of one year or more, and non-viable types of nonunion (hypotrophic, torsion-wedge, defect-pseudarthrosis). Patients with active infection or hematogenous osteomyelitis were excluded. Subgroup B included nine children with a mean age of 6.1 ± 0.9 years and severe CPT types (Paley types 4 a-c)[15], mostly due to neurofibromatosis type I, who had had numerous failed interventions to unite pseudarthrosis and had no active infection (Table 2). Mean preoperative data of both subgroups are given in Table 3.
| Patient | Age (yr), Gender | Mechanism of injury/Туре the fracture | Disease duration (yr) | Type (number) of previous surgeries | Type of nonunion/Infection | Shortening/Bone defect (cm) | Joint Function before surgery | Regenerate/nonunion consolidation completeness | Nonunion consolidation (mo) | Postoperative complication (Paley classification) | Follow-up (mo) | Residual limb length discrepancy (cm) | Further surgery |
| PTD-1 | 51, F | MVA, OF | 1 | EF (1) | TW; Delayed wound healing | 3/3 | Knee and ankle stiffness | +/+ | 11 | Pin-tract infection Regenerate deformity; Deep vein thrombosis | 17 | 2 | - |
| PTD-2 | 50, M | MVA, OF | 4 | Plate (1); EF (1) | HN; History of infection | 3/5 | Ankle ankyloses | +/+ | 10 | Regenerate deformity | 24 | 5 | Rejected further surgery |
| PTD-3 | 48, M | IF, OF | 3 | Plate (1); EF (1) | HN; History of infection | 0/3 | Ankle stiffness | +/+ | 7 | Pin-tract infection | 12 | - | - |
| PTD-4 | 18, M | IF, OF | 3 | Plate (2); EF (2) | HN; History of infection | 6/3 | Ankle ankyloses | +/+ | 11 | Knee joint stiffness | 36 | 6 | 3-cm lengthening |
| PTD-5 | 21, M | IF; OF | 1 | EF (2) | HN | 0/4 | Full function | +/+ | 5 | Regenerate deformity | 24 | - | - |
| PTD-6 | 39, M | CT; CF | 12 | Plate (1); EF (1) | HN; History of infection | 1/3 | Ankle stiffness | +/+ | 7 | Pin-tract infection | 12 | - | - |
| PTD-7 | 43, M | CT | 2 | Plate (1) | HN; History of infection | 0/4 | Ankle stiffness | +/+ | 8 | - | n/a | - | N/A |
| Patient | Age (yr), Gender | Neurofibromatosis | Type (number) of previous surgeries | Paley CPT Type | Shortening/Bone defect (cm) | Joint Function | Regenerate/nonunion consolidation completeness | Consolidation time (mo) | Complications (Paley classification) | Follow-up (mo) | Residual limb length discrepancy (cm) | Recurrence/Further surgery |
| СPT-1 | 4, M | I type | - | 4C | 5/3 | Full | +/+ | 7 | Regenerate deformity | 24 | 3 | Refracture |
| СPT -2 | 3, M | - | EF (1); Nail (1) | 4C | 5/2 | Ankle stiffness | +/+ | 9 | Pin-tract infection | 36 | 3 | Refracture |
| СPT -3 | 15, F | I type | More than 10 including EF, Nail | 4A | 15/3 | Ankle stiffness | +/+ | 10 | Pin-tract infection | 12 | 12 | |
| СPT -4 | 5, M | I type | - | 4B | 3/1.5 | Full | +/- | 7.5 | Pin-tract infection | 24 | 3 | Ilizarov monofocal compression |
| СPT -5 | 8, F | I type | Plate (1); Nail (2); EF (4) | 4B | 10/3 | Ankle ankylosis | +/+ | 9 | - | 36 | 12 | Twice Ilizarov lengthening by 6 cm |
| СPT -6 | 4, M | I type | EF (1); Autograft (1) | 4C | 5/1.5 | Full | -/- | 8 | Ischemic regenerate | 24 | 6 | Bone defect, rejected further treatment |
| СPT -7 | 6, F | - | EF and allograft (2) | 4A | 5/5 | Full | +/+ | 13.5 | Ischemic regenerate | 12 | 5 | - |
| СPT -8 | 6, F | - | Plate (1); Nail (2); EF (4) | 4B | 4/3 | Full | +/+ | 7 | Pin-tract infection | 24 | 2 | Refracture |
| СPT -9 | 4, F | - | Plate (1); Nail (2); EF (4) | 4A | 2/2 | Ankle stiffness | +/+ | 8 | - | 36 | 2 | Refracture |
| Parameter | Subgroup A | Subgroup B |
| Number of previous surgeries per patient | 2.0 ± 0.2 | 3.3 ± 0.7 |
| LLD at admission (cm) | 3.5 ± 0.5 | 6.0 ± 1.0 |
| Defect size (сm) | 3.6 ± 0.3 | 2.7 ± 0.3 |
| True defect (LLD + bone gap) after debridement relative to the contralateral limb (%) | 13.3 ± 1.7 | 31.0 ± 3.0 |
| Time of spacer retention (d) | 42.4 ± 4.5 | 55.8 ± 6.6 |
| Duration of distraction (d) | 43.0 ± 4.2 | 31.9 ± 4.2 |
| Distraction regenerate size (cm) | 3.1 ± 0.2 | 2.6 ± 0.2 |
| Completeness of defect filling (%) | 75.4 ± 10.6 | 34.6 ± 4.2 |
| External fixation index per cm | 143.5 ± 13.2 | 117.8 ± 8.5 |
| Duration of total external fixation, including spacer retention time (d) | 397.0 ± 15.3 | 270.1 ± 16.3 |
| Mean follow-up time (mo) | 20.8 ± 2.7 | 25.3 ± 2.3 |
Step 1: Ilizarov frame mounting + spacer implantation. The Ilizarov frame was constructed of three ring supports with three wires in the proximal and distal rings and two wires in the middle ring at the level of the tibial diaphysis. Fibular osteotomy was performed in order to eliminate segment deformities. For pseudarthrosis resection, an anterior approach to the tibia was used. In subgroup A, the resection started from the level of the endplate and extended to the margin with the bleeding bone. The "blood dew" sign indicated an adequate level of resection. In subgroup B, the pseudarthrosis zone along with the surrounding pathologically altered periosteum was resected. After resection, the limb was fixed with the Ilizarov frame in a neutral position according to the tension of soft tissues with the correct anatomical axis of the segment. Next, the defect size was measured. A pre-shaped spacer was prepared from methyl methacrylate cement by molding in a syringe. Its diameter corresponded to the bone diameter of the specific patient, coinciding with the level of the cortical plates, or going beyond the cortices by 2-3 mm. The spacer was placed into the defect gap after being hardened and was fixed in the gap by applying compression with the Ilizarov frame. One dose of vancomycin was added to the spacer material for infection prevention in subgroup A. Wounds were closed in the regular manner. We used only the first phase of the IMT procedure.
Step 2: Osteotomy for bone fragment transport. The spacer was accessed through the previous incision. Careful handling was required to maximize preservation of the induced membrane. Upon removal of the spacer, the induced membrane that enveloped it remained in the interfragmental gap and the wound was sutured. In the frame being unchanged, a mainly proximal osteotomy for bone transport was performed. The distal fragment was osteotomized in PTD-case 5 (Table 1); osteotomy was performed at two levels in CPT-case 7 (Table 2). Distraction was initiated from day 5 to 7 at a rate of 1 mm/d produced with 4 increments. Condition of the regeneration was checked radiographically every ten days. In low optical density of the regenerate or its deformity, the rate of distraction was adjusted or reduced to 2 or 3 increments, a quarter of a mm each. The transported bone fragment ran in the membrane without technical problems. Distraction was carried out until close docking of the fragments. Upon docking, supportive compression of 1 mm was provided once every two weeks in the consolidation phase. Autologous grafting was not added.
Postoperative care and radiographic checks followed the standards of the Ilizarov method. Radiographic evidence of bony union, external fixation time, defect filling rate and complications were assessed. The primary outcome measure was radiographic bone union. Secondary outcomes were correction of limb length discrepancy and deformities.
Thin fragments of the biomembrane formed around the cement spacer were harvested prior to bone transport for histological examination in all patients. The material was collected intraoperatively at step 2.
All adult patients and the children’s parents gave informed consent for surgical treatment and inclusion in the study. The study was approved by the ethics board of our institution.
The subgroups had different etiologies of the defects and belonged to different age groups. Thus, we did not aim to compare them. The statistical method included calculation of mean values and their deviations using Microsoft Excel 2019. Moreover, the sample size of subgroups was small; therefore, only descriptive statistics were used.
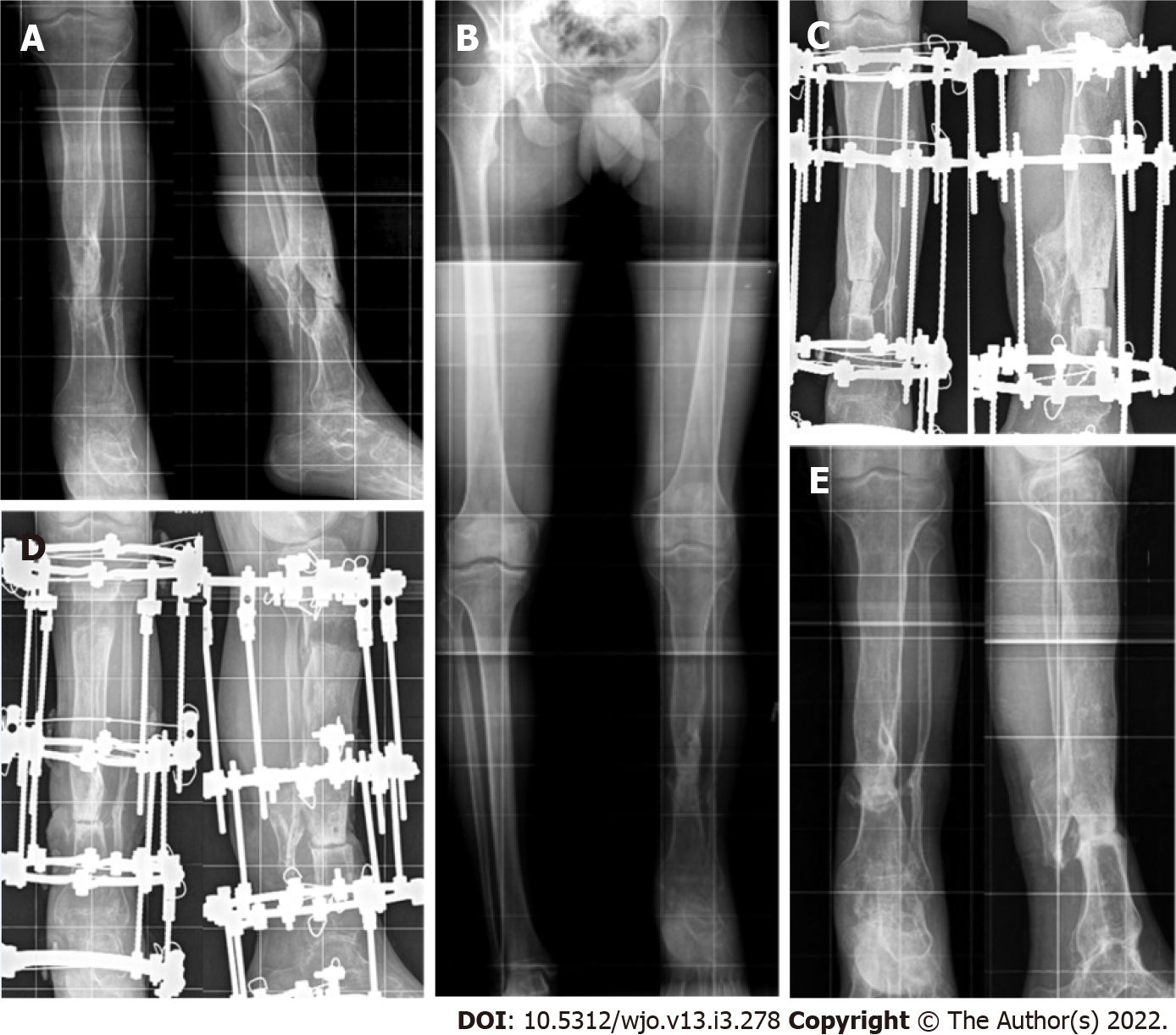
Table 1 and Table 2 present the main preoperative and treatment parameters along with outcomes of the combined technique of IMT + IBT in all patients. The mean values of the main measures are shown in Table 3. Bone union was achieved in all patients of subgroup A (Figure 1) and in seven patients of subgroup B (Figure 2). Total duration of external fixation was 397.0 ± 15.3 and 270.1 ± 16.3 d, including spacer retention time of 42.4 ± 4.5 and 55.8 ± 6.6 d, in subgroups A and B, respectively. One non-united CPT case was further treated with the Ilizarov compression method only and achieved union. Another failed CPT case was lost. After a follow-up period of one to three years, there were no refractures in subgroup A. Refractures occurred in four cases of CPT due to severe disease (mostly Paley CPT type 4 C) and multiple previous treatments. Cases CPT-8 and CPT-9 had seven previous surgeries each.
Bone transport in the membrane ran smoothly. Postoperative complications included several cases of pin-tract infection and regenerate deformity in both subgroups. Insufficient ischemic regeneration was observed in two cases of subgroup B. Bone regenerate deformity and pin-tract infection were resolved during the course of treatment. The regenerate zone was perforated with wires and supportive compression was performed with the same frame in ischemic hourglass-shaped regeneration for its stimulation. We prefer delayed lengthening to finally correct the length in non-viable nonunion, after bone consolidation has been secured. Thus, we subsequently performed this in two patients of subgroup A who applied for length compensation. Further treatment reduced limb length discrepancy from 12 to 6 cm in CPT-case 5 with two procedures.
Subgroup A patients could walk with crutches after frame removal gradually increasing weight-bearing. Subgroup B patients were recommended to use crutches for one month and then leg braces for one year.
Several surgeries are often required to manage extensive segmental bone loss after multiple failures or severe congenital deficiency. They may result in prolonged recovery times, poor outcomes, and even amputation as a complex of mechanical issues and biological factors should be utilized for reconstruction[1,2]. IBT has established itself as an efficient tool for long bone defect management, including patients with infections, especially in the tibia[5-9]. It is able to resolve the problematic triad of bone loss, soft-tissue compromise and bone infection. The IMT has recently been used for extensive defects in any long-bone segment[3,4,18]. According to several authors, the advantage of IMT over IBT lies in the fact that the consolidation time does not depend on the bone defect size as it is filled with autologous graft material[3,11]. Nevertheless, extensive defects need a lot of bone graft substance, especially in the lower extremities[18]. Alternately, the distraction procedure, being a part of IBT, is able to supply new regenerated bone substance[2].
We assumed that defect filling would provide a particularly favorable environment for bone regeneration and the reparative process with the combined use of IMT and IBT. After extraction of a spacer there is a tunnel in the interfragmental gap the walls of which are formed by the induced membrane which was found to be a type of neoperiosteum[16,19]. Apart from a favorable mechanical effect, the combined conditions could provide a biological effect of the induced membrane on osteogenesis. It was shown that multiple microvessels of the biomembrane penetrate into the regenerate zone and promote the inflow of low differentiated pluripotent cells[16]. The cells of the membrane basal layer and perivascular osteopontin-positive cells that possess osteogenic differentiation ability contribute to the formation of a low mineralized bone matrix on the surface of the spacer. This could cause an osteoinductive effect on the pluripotent cells in the region of the compression regenerate formed at the docking site. According to the reported findings, the osteoinductive membrane is adequately vascularized and produces growth factors (vascular endothelial growth factor, transforming growth factor-beta 1) and bone morphogenetic protein-2 that play a role in regeneration and may prevent lysis[19]. It is also assumed that the biomembrane features antimicrobial activity related to the synthesis of antioxidants which are secreted locally along with growth factors[20]. Another mechanism of the supposed bacteriostatic effect is the presence of local peptides in the membrane which are able to inhibit secretion of the bacterial biofilm[16]. There were no foci of infection in the biomembrane fragments harvested at step 2 of our procedure in all cases. In addition, none of the patients developed infection.
The results of the subgroups in our series could not be compared due to different etiologies and the pathogenesis of nonunion. For this reason, the outcomes were presented separately. Despite the absence of active infection, we chose the primary task to achieve radical debridement in order to prevent possible infection. In subgroup A, the spacer’s role was also to sanitize the site of previous infection. The absence of infection recurrence is attributed equally to the impact of radical debridement and that of the vascularized membrane. The interval between the first operation and the osteotomy was a period of infection control that was based on the results of bacteriological tests for selection of antibiotic therapy. The spacer maintained the shape of the defect gap to exclude soft tissues invagination into the defect.
We also promoted osteogenesis at bone fragments docking. As the role of the periosteum in CPT pathogenesis has already been proven, we expected that the neoperiosteum-like nature of the induced membrane would have an effect on bone union and regeneration in the CPT subgroup. The induced membrane was supposed to supply blood to the area with a new vascular network, thereby excluding osteolysis. However, the results in subgroup B were similar to other current techniques used for this pathology[15].
The removal of the spacer presupposes repeated trauma to the skin and soft tissues in the pseudarthrosis zone. However, if we draw a parallel, classical bone transport involves an open co-aptation for fragments docking. According to the protocol for our combined technique, docking was performed in a closed way by compression at the junction of the fragments without grafting. The known approach to create the maximum "bone mass" in the area of pseudoarthrosis was implemented by the technique[15]. Therefore, to add autologous bone grafting or internal fixation to the described combination seemed to us extremely invasive. However, open docking and a graft were used in an earlier study of infected tibial defects treated with a similar technology[21]. Thus, there could be options to synergistically widen the integrated approach.
Consolidation of nonunion was achieved in all the defects of post-traumatic etiology but it should be noted that the IM effect was not strong enough for CPT consolidation and did not help to eliminate refractures in the long term. The refracture rate was comparable with the literature data on the use of other methods, including the Ilizarov method used separately[15].
The management of СPT has been much discussed recently and there is plenty of clinical research with variable results[15,22-30]. The superiority of one of the techniques for reconstruction in CPT has not yet been confirmed. The latest clinical studies predominantly describe patients where the Ilizarov method is the main component of CPT management in conjunction with intramedullary nailing and bone grafting[22-25]. The combined technique of the Ilizarov external fixation, stabilization with an intramedullary rod and corticocancellous bone autograft yields a statistically significant reduction in the number of refractures compared with standalone fixation methods. It was stated that the four methods of CPT treatment might achieve primary union of about 50% without refracture and this was attributed to the biological nature of CPT[15]. Improved union rates in IMT assisted by the Ilizarov external fixator and grafting for previously failed CPT treatment were reported[26].
However, regardless of the primary bone fusion rates, most of the authors state that the probability of long-term bone union retention remains unpredictable due to biological factors of the disease characterized by low osteogenic potential. Therefore, methods to enhance this potential have been identified such as wrapping, grafting, crossunion of the tibia and fibula, and application of several biological agents to promote osteogenesis[27-30]. Our technology might also be used.
The combination of technologies to treat orthopedic pathology is largely associated with the need to obtain a faster and a more efficient result in the most severe cases. Apart from our previous study[16], we found only three case reports that used the combined principles of IMT and IBT with satisfactory outcomes, although not quite the same as our technology[21,31,32]. The limitation of our series is the small sample of patients with two different etiologies of defects and various clinical situations, but all severe cases. Our preliminary results suggest that the etiological factor plays a significant role in the use of this combined technique. Both subgroups had impaired bone regeneration potential due to multiple previous failures and a worsened condition of the tibia, but undoubtedly this was greater in subgroup B.
We did not complete limb length compensation in our patients due to the severity of their tibial defects and pseudarthrosis. The primary goal was bone union. Of course, residual limb length discrepancy is the factor affecting the final result in post-traumatic cases. We recommend IBT for defects less than 12 cm, and free vascularized fibula or transverse Ilizarov transport of the fibular fragment for bigger defects[2]. Due to the fact that IBT is able to realize the potential of human bone regeneration for anatomical and functional restoration in large long-bone defects with minimal trauma, it is extensively used after the failure of other established methods of treatment or infection. The arguments against it as a primary treatment option are the complexity of the Ilizarov apparatus mounting and its size, the number of adjustments, pin-tract infection, multi-stage and long treatment course that needs a lot of compliance both from the patient and the surgeon. Although IMT seems simple, it is not so easy to complete successfully in severe cases[33]. Finally, it is worth noting the significant disadvantage of the combined approach which is an increase in the duration of total external fixation[21]. Due to these facts, the integration is a more complex procedure. Its effects, modification or failures should be studied further.
The combination of IMT and IBT may provide good outcomes in post-traumatic tibial defects after previous treatment failures, although the external fixation is longer due to spacer retention time. This combination might also be used for severe types of CPT despite possible refractures.
The challenges of long-bone defect management have increased in contemporary orthopedic practice due to the severity of high-energy trauma and its complications. They lead to a multi-stage, long and costly treatment. The Ilizarov method of bone transport (IBT) and the Masquelet induced membrane technique (IMT) have been used in a great variety of challenging clinical situations including post-traumatic bone loss, infected nonunion, tumor resection, and congenital pseudarthrosis of the tibia (CPT).
The importance of improving bone regeneration in the management of large bone defects and CPT is a very relevant issue due to treatment failures that diminish bone potential for regeneration. Therefore, a combination of the biological merits of IBT and IMT seemed a promising strategy for the management of cases with a history of failed attempts and impaired regeneration potential.
We aimed to conduct clinical studies on the use of a new technological solution that integrates the IMT and IBT techniques for treating non-viable tibial defects of post-traumatic (PTD) etiology and CPT to improve bone regeneration at the docking site, bone consolidation and reduce refracture rate.
We retrospectively studied the treatment course and outcomes in a case series that included seven PTD patients (subgroup A) and nine CPT cases (subgroup B) managed by the combined technology of IMT and IBT. Adult patients in subgroup A had bone defects of post-traumatic origin after several previous treatments failed and non-viable types of nonunion (hypotrophic, torsion-wedge, defect-pseudarthrosis). Subgroup B included nine children with a mean age of 6.1 ± 0.9 years with severe CPT types who had numerous failed interventions to unite pseudarthrosis. Step 1 included Ilizarov frame placement and spacer introduction into the resected defect to generate the induced membrane which remained in the interfragmental gap after spacer removal. Step 2 was an osteotomy and bone transport of the fragment through the tunnel in the induced membrane, its compression and closed docking for consolidation without grafting. Upon docking, supportive compression of 1 mm was provided once every two weeks in the consolidation phase. Postoperative care and radiographic checks followed the standards of the Ilizarov method. Radiographic evidence of bony union, external fixation time, defect filling rate and complications were assessed. The primary outcome measure was radiographic bone union. Secondary outcomes were correction of limb length discrepancy and deformities. The outcomes were retrospectively studied after a mean follow-up period of 20.8 ± 2.7 mo in subgroup A and 25.3 ± 2.3 mo in subgroup B.
Upon completion of treatment, defects were filled by 75.4 ± 10.6% and 34.6 ± 4.2%, in subgroups A and B, respectively. Total duration of external fixation was 397 ± 9.2 and 270.1 ± 16.3 d, including spacer retention time of 42.4 ± 4.5 and 55.8 ± 6.6 d, respectively. Bone infection was not observed. Postoperative complications included several cases of pin-tract infection and regenerate deformity in both subgroups. Ischemic regeneration was observed in two cases of subgroup B. Complications were corrected during the course of treatment. Bone union was achieved in all patients of subgroup A and in seven patients of subgroup B. One non-united CPT case was further treated with the Ilizarov compression method only and achieved union. After a follow-up period of two to three years, refractures occurred in four cases of united CPT.
The combination of IMT and IBT may provide good outcomes in post-traumatic tibial defects after previous treatment failures, although the external fixation is longer due to spacer retention time. This combination might also be used for severe types of CPT, despite the fact that refractures may occur.
There are ways to further investigate the adjuncts to our protocol such as grafting at the docking site and intramedullary nailing, especially in severe CPT.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eamsobhana P, Wang P S-Editor: Wang JL L-Editor: Webster J P-Editor: Wang JL
| 1. | Hoogervorst LA, Hart MJ, Simpson PM, Kimmel LA, Oppy A, Edwards ER, Gabbe BJ. Outcomes of severe lower limb injury with Mangled Extremity Severity Score ≥ 7. Bone Joint J. 2021;103-B:769-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Borzunov DY, Kolchin SN, Malkova TA. Role of the Ilizarov non-free bone plasty in the management of long bone defects and nonunion: Problems solved and unsolved. World J Orthop. 2020;11:304-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 3. | Masquelet AC, Kishi T, Benko PE. Very long-term results of post-traumatic bone defect reconstruction by the induced membrane technique. Orthop Traumatol Surg Res. 2019;105:159-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 4. | Gouron R. Surgical technique and indications of the induced membrane procedure in children. Orthop Traumatol Surg Res. 2016;102:S133-S139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | El-Alfy BS. Unhappy triad in limb reconstruction: Management by Ilizarov method. World J Orthop. 2017;8:42-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Catagni MA, Azzam W, Guerreschi F, Lovisetti L, Poli P, Khan MS, Di Giacomo LM. Trifocal versus bifocal bone transport in treatment of long segmental tibial bone defects. Bone Joint J. 2019;101-B:162-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Li R, Zhu G, Chen C, Chen Y, Ren G. Bone Transport for Treatment of Traumatic Composite Tibial Bone and Soft Tissue Defects: Any Specific Needs besides the Ilizarov Technique? Biomed Res Int. 2020;2020:2716547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Bernstein M, Fragomen AT, Sabharwal S, Barclay J, Rozbruch SR. Does Integrated Fixation Provide Benefit in the Reconstruction of Posttraumatic Tibial Bone Defects? Clin Orthop Relat Res. 2015;473:3143-3153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Liu Y, Yushan M, Liu Z, Liu J, Ma C, Yusufu A. Complications of bone transport technique using the Ilizarov method in the lower extremity: a retrospective analysis of 282 consecutive cases over 10 years. BMC Musculoskelet Disord. 2020;21:354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Ayouba G, Lemonne F, Kombate NK, Bakriga B, Yaovi Edem J, André-Pierre Max U. Interest of nailing associated with the Masquelet technique in reconstruction of bone defect. J Orthop. 2020;20:228-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Gupta G, Ahmad S, Mohd Zahid, Khan AH, Sherwani MK, Khan AQ. Management of traumatic tibial diaphyseal bone defect by "induced-membrane technique". Indian J Orthop. 2016;50:290-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Mi M, Papakostidis C, Wu X, Giannoudis PV. Mixed results with the Masquelet technique: A fact or a myth? Injury. 2020;51:132-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Morelli I, Drago L, George DA, Gallazzi E, Scarponi S, Romanò CL. Masquelet technique: myth or reality? Injury. 2016;47 Suppl 6:S68-S76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 14. | Morris R, Hossain M, Evans A, Pallister I. Induced membrane technique for treating tibial defects gives mixed results. Bone Joint J. 2017;99-B:680-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Paley D. Congenital pseudarthrosis of the tibia: biological and biomechanical considerations to achieve union and prevent refracture. J Child Orthop. 2019;13:120-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Borzunov DY, Gorbach EN, Mokhovikov DS, Kolchin SN. Combined bone plasty interventions for rehabilitation of patients with congenital pseudarthrosis of the tibia. Genij Ortopedii. 2019;25:304-311. [DOI] [Cited in This Article: ] |
| 17. | Mokhovikov DS, Stupina TA, Varsegova TN, Diuriagina OV, Emanov AA, Borzunov DYu. Histomorphometric characteristics of the tibialis anterior muscle and the peroneal nerve in experimental repair of post-resection tibial defect using the Ilizarov external fixation and the Masquelet technique. Genij Ortopedii. 2020;26:216-221. [DOI] [Cited in This Article: ] |
| 18. | Piacentini F, Ceglia MJ, Bettini L, Bianco S, Buzzi R, Campanacci DA. Induced membrane technique using enriched bone grafts for treatment of posttraumatic segmental long bone defects. J Orthop Traumatol. 2019;20:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Wang X, Wei F, Luo F, Huang K, Xie Z. Induction of granulation tissue for the secretion of growth factors and the promotion of bone defect repair. J Orthop Surg Res. 2015;10:147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Roukoz S, El Khoury G, Saghbini E, Saliba I, Khazzaka A, Rizkallah M. Does the induced membrane have antibacterial properties? Int Orthop. 2020;44:391-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Marais LC, Ferreira N. Bone transport through an induced membrane in the management of tibial bone defects resulting from chronic osteomyelitis. Strategies Trauma Limb Reconstr. 2015;10:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | El-Rosasy MA. Congenital pseudarthrosis of the tibia: the outcome of a pathology-oriented classification system and treatment protocol. J Pediatr Orthop B. 2020;29:337-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Kocaoğlu M, Eralp L, Bilen FE, Civan M. Congenital pseudarthrosis of the tibia: Results of circular external fixation treatment with intramedullary rodding and periosteal grafting technique. Acta Orthop Traumatol Turc. 2020;54:245-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Kesireddy N, Kheireldin RK, Lu A, Cooper J, Liu J, Ebraheim NA. Current treatment of congenital pseudarthrosis of the tibia: a systematic review and meta-analysis. J Pediatr Orthop B. 2018;27:541-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Yan A, Mei HB, Liu K, Wu JY, Tang J, Zhu GH, Ye WH. Wrapping grafting for congenital pseudarthrosis of the tibia: A preliminary report. Medicine (Baltimore). 2017;96:e8835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Meselhy MA, Elhammady AS, Singer MS. Outcome of Induced Membrane Technique in Treatment of failed previously operated Congenital Pseudarthrosis of the Tibia. Orthop Traumatol Surg Res. 2020;106:813-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Vaidya SV, Aroojis A, Mehta R, Agashe MV, Dhawale A, Bansal AV, Sarathy K. Short Term Results of a New Comprehensive Protocol for the Management of Congenital Pseudarthrosis of the Tibia. Indian J Orthop. 2019;53:736-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Liu Y, Yang G, Liu K, Wu J, Zhu G, Tang J, Zheng Y, Mei H. Combined surgery with 3-in-1 osteosynthesis in congenital pseudarthrosis of the tibia with intact fibula. Orphanet J Rare Dis. 2020;15:62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Memeo A, Verdoni F, Minoli CF, Voto A, D'Amato RD, Formiconi F, Priano D, Montanari L, Panuccio E. Effectiveness of bone marrow aspirate concentrate (BMAC) as adjuvant therapy in the surgical treatment of congenital pseudoarthrosis of the tibia: a retrospective comparative study. J Biol Regul Homeost Agents. 2020;34:431-440. Congress of the Italian Orthopaedic Research Society. [PubMed] [Cited in This Article: ] |
| 30. | Richards BS, Anderson TD. rhBMP-2 and Intramedullary Fixation in Congenital Pseudarthrosis of the Tibia. J Pediatr Orthop. 2018;38:230-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Uzel AP, Lemonne F, Casoli V. Tibial segmental bone defect reconstruction by Ilizarov type bone transport in an induced membrane. Orthop Traumatol Surg Res. 2010;96:194-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Mitrofanov AI, Al Delamy OK, Al Harris MS. Repair of tibial bone defects with fibular fragment and the induced membrane technique. Genij Ortopedii. 2019;25:239-242. [DOI] [Cited in This Article: ] |
| 33. | Mathieu L, Potier L, Ndiaye R, Choufani C, Mbaye E, Niang CD. Challenges of the induced-membrane technique in the reconstruction of traumatic tibial defect with limited resources : a cohort study. Acta Orthop Belg. 2020;86:606-613. [PubMed] [Cited in This Article: ] |