Published online Nov 18, 2021. doi: 10.5312/wjo.v12.i11.931
Peer-review started: April 30, 2021
First decision: July 28, 2021
Revised: August 9, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: November 18, 2021
Allergic contact dermatitis (ACD) secondary to Dermabond Prineo™ is rare, but documented. To our knowledge, there are no described reports of this ACD reaction within the pediatric population following arthroscopic surgery.
We report two cases of pediatric ACD upon second exposure to Dermabond Prineo™ after knee arthroscopy. Both cases presented within two weeks of the inciting second exposure. The cases resolved with differing described combinations of sterile cleaning, diphenhydramine, and antibiotic administration. No long-term sequelae were found.
This case report elucidates the rare complication of allergic dermatitis secondary to Dermabond Prineo™ repeat exposure use in pediatric arthroscopy.
Core Tip: Dermabond Prineo™ has shown to be advantageous as a wound closure device with regards to operative efficiency, cosmetic results, and decreased postope
- Citation: Robinson J, Smidt KP, Houk G, McKie J, Barton RS, Massey P. Allergic dermatitis after knee arthroscopy with repeated exposure to Dermabond Prineo™ in pediatric patients: Two case reports. World J Orthop 2021; 12(11): 931-937
- URL: https://www.wjgnet.com/2218-5836/full/v12/i11/931.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i11.931
Efforts to decrease total operative time during a given surgical procedure are becoming more critical as both surgeons and administrators consider cost savings for hospital systems and surgical centers. It is estimated that one minute in the operating room can cost up to over $130 depending on the facility[1,2]. With the advent of rapid wound closure products such as Dermabond™ and Dermabond Prineo™ (Ethicon Endo-Surgery, Cincinnati, OH), operative times can be shortened, resources saved, and operative efficiency and post-operative patient comfort increased[3-5].
Prineo™ is a wound closure system that utilizes a self-adhering polyester-based mesh in combination with a monomeric 2-octyl cyanoacrylate formulation and the colorant D&C Violet No. 2. The wound closure system is intended to be used in conjunction with deep dermal stitches. Reported benefits of Prineo™ include a protective microbial barrier, greater skin holding strength when compared to skin staples or subcuticular sutures, more evenly distributed tension away from wound edges, easy removal, and reduction in overall wound closure time[3,6-8].
While there are reported cases of post-operative allergic contact dermatitis (ACD) with the use of Dermabond™, there are few reported cases of such dermatitis associated with the Prineo™ wound closure system, and even fewer associated with a pediatric age group[9-11]. This case report describes instances of ACD following exposure to Prineo™ in a pediatric age group.
Case 1: Six days after an arthroscopic left medial meniscus repair and bone marrow aspirate injection, a 15-year-old female reported increasing itching and a burning sensation around the incision sites that progressed to feeling like her left knee was “on fire.”
Case 2: The second patient is a 12-year-old female who presented one week after her left medial meniscal allograft transplantation and reconstruction of anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), and medial collateral ligament (MCL) with complaints of two days of itching around her operative sites.
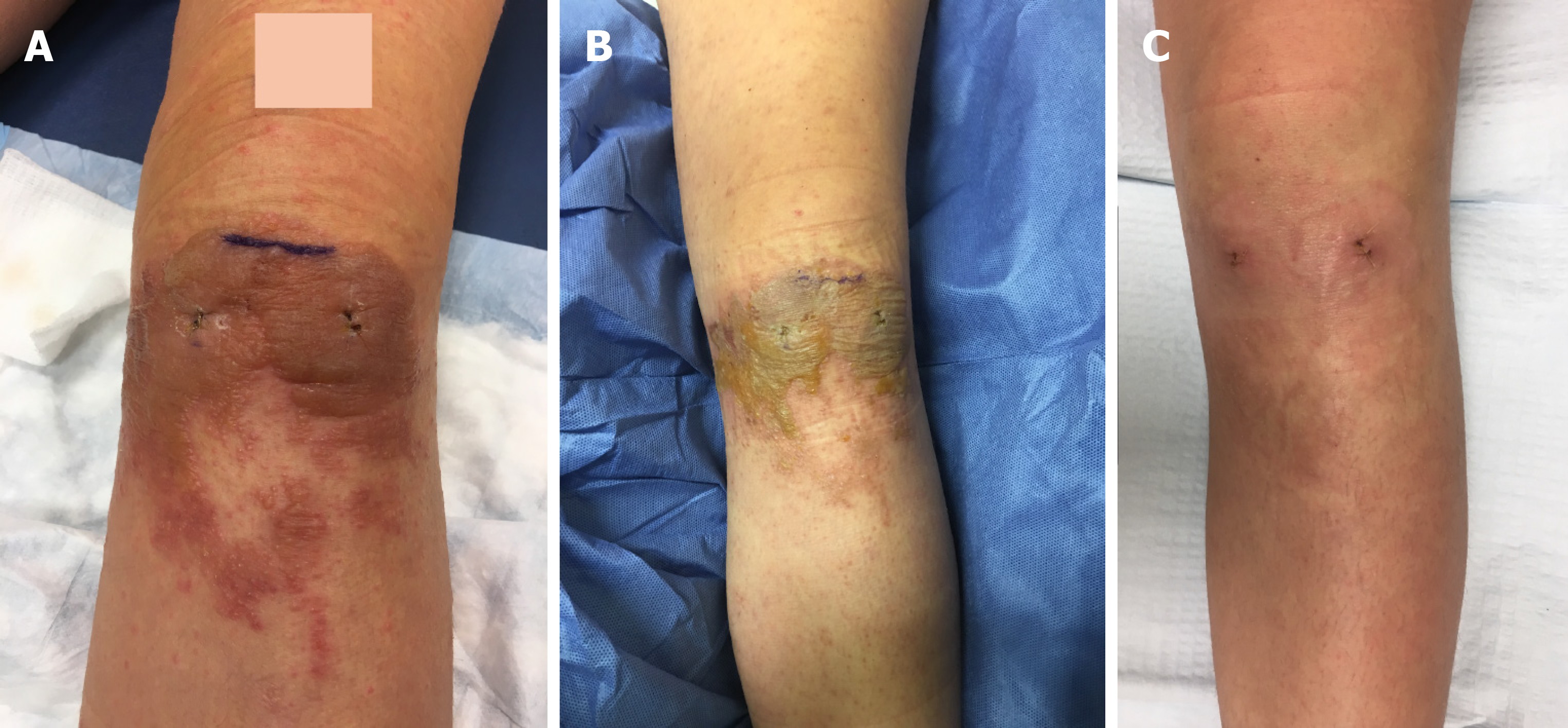
Case 1: The patient underwent an arthroscopic left medial meniscus repair and bone marrow aspirate injection in which the portal incision sites were closed with Prineo™. Thrombo-Embolus-Deterrent (TED) hose were applied after the surgical drapes were taken down. The procedure was uncomplicated. Upon the patient’s return for her one-week postoperative follow up appointment, she was noted to have large blisters covering the anterior portal sites (Figure 1A). The Prineo™ mesh dressing was removed and it was noted that there were large blisters to the anterior left knee.
Case 2: The patient underwent a left meniscal allograft transplantation with reconstruction of the ACL, PCL, and MCL for congenital absence of these structures. All incisions and portal sites were closed with Prineo™. Surgical drapes were taken down and TED hose were applied bilaterally. The procedure was uncomplicated. The patient then returned for her one-week postoperative follow-up appointment with a red papular rash surrounding the anterior knee and surgical sites. She complained of itching around these sites.
Case 1: This patient had a right knee ACL reconstruction two years prior in which the incisions were closed with Prineo™. There was no allergic reaction to the closure device at that time. She then sustained a left knee injury while playing softball. She was found to have a medial meniscus tear that was subsequently treated surgically as presented in this case.
Case 2: She had previously undergone a right medial meniscal allograft transplan
Case 1: This patient had an unremarkable personal and family medical history.
Case 2: This patient had an unremarkable family medical history with a personal medical history of congenital absence of bilateral ACL, MCL and medial meniscus.
Case 1: The blisters were intact and raised. She also had pruritic scattered papules on the thigh and lower leg. She had a negative Homan sign and the remainder of her physical exam was unremarkable for her postoperative course.
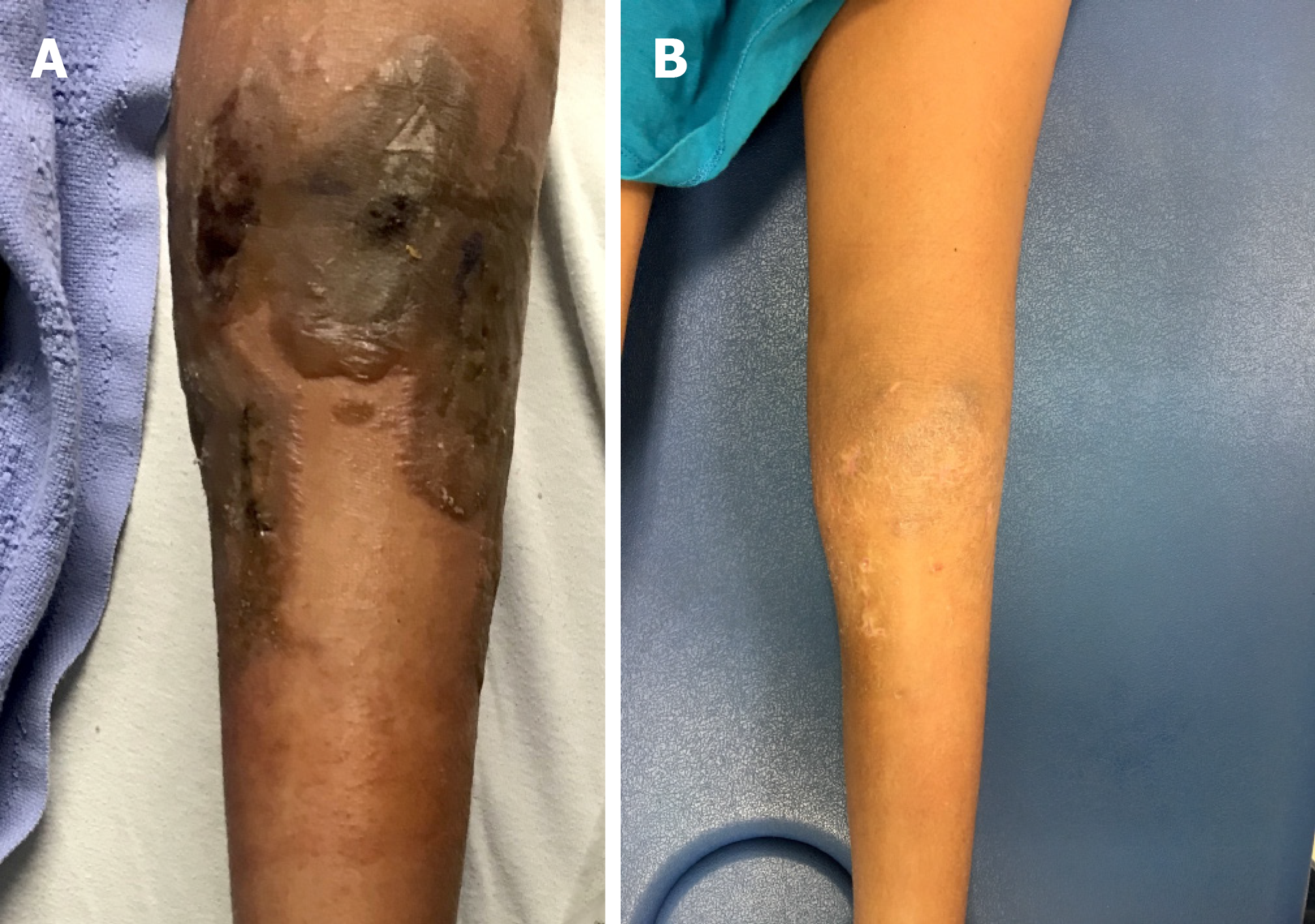
Case 2: One week post-operatively, the dressings covering the operative knee were removed and she was noted to have significant skin inflammation with blisters and welts along the entirety of her surgical incisions (Figure 2A). She also had scattered papules from her groin to her left ankle that were erythematous but not draining nor pustular. The surgical incisions and portal sites were noted to be well approximated with no evidence of drainage.
The above cases demonstrate the occurrence of pediatric ACD upon second exposure to Prineo™. Both cases presented with this within one week of the surgical procedure.
Case 1: She was prescribed diphenhydramine 25 mg twice daily and placed on doxycycline 100 mg daily for seven days for treatment of concurrent folliculitis. Her wounds were cleaned with sterile water and patted dry. They were then redressed with a nonadherent dressing over the blistering area followed by soft dressings over top. Her TED hose were discontinued until the blisters dried up.
Case 2: The patient’s TED hose were discontinued on the operative side (left) and the skin was cleaned above and below the incisions. The incision sites were then redressed with a non-adhesive dressing followed by soft dressings. She was placed on diphenhydramine 25 mg twice daily which was increased to four times per day as needed for persistent itching. She underwent daily dressing changes through postoperative day nine when the blisters became flaccid.
Case 1: At her 2-wk post-operative visit, the raised plaques had flattened, the erythema had decreased, and her pruritis had resolved (Figure 1B). She was followed weekly and noted to have significant improvement of the contact dermatitis at her three-week postoperative visit. Her blisters had resolved and no active drainage was appreciated on exam (Figure 1C). The patient was followed two years postoperatively and had no recurrence of any skin reaction surrounding the surgical incisions or elsewhere on her body.
Case 2: On postoperative day fifteen, all blisters had drained and epithelialization of the underlying skin was appreciated. At the patient’s three-week postoperative appointment, she was instructed to shower and refrain from using any lotions or creams as scabbing of the blisters was noted. At her six-week postoperative appointment, the allergic dermatitis was completely resolved (Figure 2B). The patient was followed regularly for two years postoperatively and had no recurrence of any skin reaction surrounding the surgical incisions or elsewhere on her body.
The occurrence of allergic reaction to Dermabond™ and Dermabond Prineo™ is rare and infrequently reported in the literature. Durando et al[12] reported an incidence rate of 1.7% (15 of 912 patients) over a two-year span involving 912 total knee arthroplasty (TKA) cases using Dermabond™. Of these 15 patients who developed a suspected ACD, three agreed to participate in patch testing to determine if they were allergic to Dermabond™ or 28 other possible allergens. Prineo™ was not used in these patient’s cases and as such was not studied. Of the three who agreed to participate, two of the three developed a positive reaction to Dermabond™[12].
Chan et al[13] reported 3 cases of allergic reaction to Prineo™ out of 366 patients (1.8%) that were managed by a single surgeon following TKA. Each of the cases presented within 4-9 d postoperatively and the reaction resolved between 4 wk to 12 wk postoperatively. Each patient was referred to a dermatologist and 2 of the 3 patients received a course of topical corticosteroids. Similar to our cases, no long-term sequelae, including recurrence or superficial or deep joint infection, occurred when followed for at least one year[13].
In a study examining wound complications after 2-octylcyanoacrylate skin closure following total joint arthroplasty, Michalowitz et al[14] found a 19.2% superficial wound complication rate in hip and knee arthroplasty cases when Dermabond Prineo™ was used. As a retrospective cohort study, the specifics of what defined a superficial wound complication were not described[14].
Davis and Stuart[15] reported a single case of a 72-year-old woman who was found to have severe ACD following a left TKA that subsequently was found to have an extreme patch test reaction to Prineo™ upon patch testing. This patient reported a similar but milder rash a year prior when she underwent right TKA. This case study provides further evidence that occurrence and severity of ACD to Dermabond Prineo™ may be related to second exposure. Similar to other reported cases, it should be noted that their patient’s symptoms resolved over a 3-4 wk treatment of topical corticosteroids[15].
Regarding current treatment standards, once surgical site infection is ruled out, the treatment of ACD requires an accurate severity assessment. In the post-operative setting, orthopaedic surgeons need to have a high index of suspicion for any dermatitis following the use of skin adhesives and treat immediately based on the severity of the dermatitis. In accordance with the International Contact Dermatitis Research Group classification, a mild reaction (1+ grade) has light erythema and is nonvesicular[16]. Mild reactions can be monitored for progression and consideration can be given to remove the Prineo™ dressing[17]. Conservative treatment entails dressing removal and oral antihistamines for pruritus. A moderate reaction (2+ grade) has edema, erythema, and discrete vesicles[16]. The removal of the Prineo™ dressing is necessary and the ACD is treated with topical steroids and oral antihistamines[17]. A severe reaction has coalescing vesiculobullous papules and is treated the same as a moderate reaction with the additional consideration of oral steroids[16,17]. The current guidance from the American Academy of Allergy, Asthma, and Immunology is to use 0.5 to 1 mg/kg daily oral steroids for 7 d when more than 20% of the body surface area is affected[16]. The selection of topical steroid potency is based on the location of the dermatitis, the lesion size, and the severity of the reaction[16]. In orthopaedic cases that do not involve flexural surfaces, mid to high potency topical steroids, such as triamcinolone 0.1% or clobetasol 0.05%, are appropriate[18]. Topical steroids application should be after hydration of the skin for optimal effectiveness[16]. In our cases, we did not use corticosteroids due to concerns with wound complications that have been previously reported with steroid use and postoperative incisions[19,20].
In a case report by Dunst et al[21], a 44-year-old woman who underwent reduction mammoplasty with Prineo™ wound closure presented 10 d postoperatively complaining of severe itching with an extensive skin reaction in the vicinity of the Prineo™ skin closure device. She was referred to dermatology and underwent allergy testing where a moderate positive allergic reaction to both components of the Prineo™ wound closure device was noted. The authors described a noticeable reduction in operating time in their use of Prineo™ in over 50 cases of excisional body contouring procedures with this case being the only instance of any dermatitis complication[21].
While there are some reports of Prineo™ reactions, there are several studies demonstrating the benefits of shorter operative times. Shippert[1] performed a randomized controlled trial showing decreased operative time, leading to decreased costs[1]. Another randomized study concluded that Prineo™ has significantly faster closure and increased post-operative patient comfort[8]. The low risk of adverse reaction to Prineo™ combined with the benefits of increased patient comfort and operative efficiency provide rationale for its continued use. In the current era with a focus on cost savings, Prineo™ can significantly decrease operative times leading to overall cost savings for hospital systems and surgical facilities. Any previous occurrence of allergic dermatitis following use of Dermabond™ or Prineo™, however, should prompt a thorough history and further use of Prineo™ should be carefully considered, if not completely avoided.
This case report elucidates the rare complication of allergic dermatitis following second exposure use of Prineo™. This case report also brings forth the first, to our knowledge, reported cases of such allergic dermatitis in response to Prineo™ within the pediatric population. With the increased use of Prineo™ as a wound closure alternative, surgeons should be aware of potential risks, especially in cases with previous exposure to Dermabond™ or Prineo™.
The authors would like to thank Samantha Vance for editing and manuscript preparation and Tammy Bradford for manuscript preparation.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Orthopedics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kim WJ, Li HY, Saracco M S-Editor: Fan JR L-Editor: A P-Editor: Xing YX
| 1. | Shippert RD. A Study of Time-Dependent Operating Room Fees and How to save $100 000 by Using Time-Saving Products. Am J Cosmet Surg. 2005;22:25-34. [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Childers CP, Maggard-Gibbons M. Understanding Costs of Care in the Operating Room. JAMA Surg. 2018;153:e176233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 437] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 3. | Blondeel PN, Richter D, Stoff A, Exner K, Jernbeck J, Ramakrishnan V. Evaluation of a new skin closure device in surgical incisions associated with breast procedures. Ann Plast Surg. 2014;73:631-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Huemer GM, Schmidt M, Helml GH, Shafighi M, Dunst-Huemer KM. Effective wound closure with a new two-component wound closure device (Prineo™) in excisional body-contouring surgery: experience in over 200 procedures. Aesthetic Plast Surg. 2012;36:382-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Richter D, Stoff A, Ramakrishnan V, Exner K, Jernbeck J, Blondeel PN. A comparison of a new skin closure device and intradermal sutures in the closure of full-thickness surgical incisions. Plast Reconstr Surg. 2012;130:843-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Anderson FL, Herndon CL, Lakra A, Geller JA, Cooper HJ, Shah RP. Polyester Mesh Dressings Reduce Delayed Wound Healing and Reoperations Compared with Silver-Impregnated Occlusive Dressings after Knee Arthroplasty. Arthroplast Today. 2020;6:350-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | DERMABOND® PRINEO® Skin Closure System. J&J Medical Devices. [cited 23 June 2020]. Available from: https://www.jnjmedicaldevices.com/en-US/product/dermabond-prineo-skin-closure-system. [Cited in This Article: ] |
| 8. | Lee JC, Ishtihar S, Means JJ, Wu J, Rohde CH. In Search of an Ideal Closure Method: A Randomized, Controlled Trial of Octyl-2-Cyanoacrylate and Adhesive Mesh vs Subcuticular Suture in Reduction Mammaplasty. Plast Reconstr Surg. 2018;142:850-856. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | El-Dars LD, Chaudhury W, Hughes TM, Stone NM. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Lindsey RW, Harper A. Systemic and Cutaneous Immune Reactions Following Orthopaedic Procedures. JBJS Case Connect. 2017;7:e37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Durando D, Porubsky C, Winter S, Kalymon J, O'Keefe T, LaFond AA. Allergic contact dermatitis to dermabond (2-octyl cyanoacrylate) after total knee arthroplasty. Dermatitis. 2014;25:99-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Chan FJ, Richardson K, Kim SJ. Allergic Dermatitis After Total Knee Arthroplasty Using the Prineo Wound-Closure Device: A Report of Three Cases. JBJS Case Connect. 2017;7:e39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Michalowitz A, Comrie R, Nicholas C, Wagner M, Kehoe J. Wound Complications after 2-Octyl Skin Closure Systems for Total Joint Arthroplasty. J Bone Jt Infect. 2020;5:101-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Davis MD, Stuart MJ. Severe Allergic Contact Dermatitis to Dermabond Prineo, a Topical Skin Adhesive of 2-Octyl Cyanoacrylate Increasingly Used in Surgeries to Close Wounds. Dermatitis. 2016;27:75-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | American Academy of Allergy, Asthma and Immunology, American College of Allergy. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97:S1-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Chalmers BP, Melugin HP, Sculco PK, Schoch JJ, Sierra RJ, Pagnano MW, Stuart MJ, Taunton MJ. Characterizing the Diagnosis and Treatment of Allergic Contact Dermatitis to 2-Octyl Cyanoacrylate Used for Skin Closure in Elective Orthopedic Surgery. J Arthroplasty. 2017;32:3742-3747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255. [PubMed] [Cited in This Article: ] |
| 19. | Ismael H, Horst M, Farooq M, Jordon J, Patton JH, Rubinfeld IS. Adverse effects of preoperative steroid use on surgical outcomes. Am J Surg. 2011;201:305-8; discussion 308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Merkler AE, Saini V, Kamel H, Stieg PE. Preoperative steroid use and the risk of infectious complications after neurosurgery. Neurohospitalist. 2014;4:80-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Dunst KM, Auboeck J, Zahel B, Raffier B, Huemer GM. Extensive allergic reaction to a new wound closure device (Prineo). Allergy. 2010;65:798-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |