Published online Nov 18, 2021. doi: 10.5312/wjo.v12.i11.899
Peer-review started: April 1, 2021
First decision: June 7, 2021
Revised: June 18, 2021
Accepted: September 27, 2021
Article in press: September 27, 2021
Published online: November 18, 2021
Following the successful Perioperative Surgical Home (PSH) practice for total knee arthroplasty (TKA) at our institution, the need for continuous improvement was realized, including the deimplementation of antiquated PSH elements and introduction of new practices.
To investigate the transition from femoral nerve blocks (FNB) to adductor canal nerve blocks (ACB) during TKA.
Our 13-month study from June 2016 to 2017 was divided into four periods: a three-month baseline (103 patients), a one-month pilot (47 patients), a three-month implementation and hardwiring period (100 patients), and a six-month evaluation period (185 patients). In total, 435 subjects were reviewed. Data within 30 postoperative days were extracted from electronic medical records, such as physical therapy results and administration of oral morphine equivalents (OME).
Our institution reduced FNB application (64% to 3%) and increased ACB utilization (36% to 97%) at 10 mo. Patients in the ACB group were found to have increased ambulation on the day of surgery (4.1 vs 2.0 m) and lower incidence of falls (0 vs 1%) and buckling (5% vs 27%) compared with FNB patients (P < 0.05). While ACB patients (13.9) reported lower OME than FNB patients (15.9), the difference (P = 0.087) did not fall below our designated statistical threshold of P value < 0.05.
By demonstrating closure of the “knowledge to action gap” within 6 mo, our institution’s findings demonstrate evidence in the value of implementation science. Physician education, technical support, and performance monitoring were deemed key facilitators of our program’s success. Expanded patient populations and additional orthopedic procedures are recommended for future study.
Core Tip: This study showed improved immediate postoperative outcomes of total knee arthroplasty patients through effective anesthetic management, specifically in regard to increased mobility (4.1 vs 2.0 m) and decreased oral morphine equivalents (13.9 vs 15.9) by employing adductor canal block instead of femoral nerve block. Our data supports the value of implementation science to generate institutional change though the application of guidelines from the modified Consolidated Framework for Implementation Research. It is proposed that the key enablers of implementation success, and in our case achieved a “knowledge to action” gap closure in 6 mo, are physician education, technical support, and performance monitoring.
- Citation: Crain N, Qiu CY, Moy S, Thomas S, Nguyen VT, Lee-Brown M, Laplace D, Naughton J, Morkos J, Desai V. Implementation science for the adductor canal block: A new and adaptable methodology process. World J Orthop 2021; 12(11): 899-908
- URL: https://www.wjgnet.com/2218-5836/full/v12/i11/899.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i11.899
The “knowledge to action gap” is notoriously large in clinical medicine and translation implementation can take up to 17 years[1]. The apparent disconnect, deemed the “second translational gap,” is one of the most daunting tasks facing the global healthcare system as declared by the World Health Organization (WHO)[2-3]. Although the Enhanced Recovery Program (ERP), Perioperative Surgical Home (PSH) and the WHO’s Surgical Safety Checklist (SSC) program have achieved remarkable success in the perioperative setting, these programs have varied significantly in their clinical effectiveness at the institutional level, often due to the uneven implementation effectiveness.
Historically, there are delays in two factors which enable success: the foundation of strong clinical evidence and a sound implementation process. The latter is challenging to achieve with consistency at an institutional level. In 2016, we previously reported the success of PSH practice for ambulatory total knee arthroplasty (TKA) at our institution’s pilot program[4]. Within approximately 24 mo, we spread the practice through our 21 hospitals and surgical centers guided by the Consolidated Framework for Implementation Research (CFIR)[5]. By employing CFIR principles, we achieved both clinical and implementation effectiveness in all our facilities, which led to significant reductions in length of stay (LOS) for all TKA patients regardless of where they received the care in our system.
The need for continuous improvement was made aware at our institution, including the removal of antiquated PSH elements and the introduction of new practices. Specifically, the substitution of the routine femoral nerve block (FNB) for the adductor canal block (ACB) was deemed important due to demonstrated improvements in postoperative quadriceps strength, patient mobility, and knee recovery in TKA patients[6]. While ACB practice was not novel, its strategy for effective and rapid implementation was of utmost interest, particularly to investigate how change management could be translated to other interventions.
The primary three goals of our study were to investigate the role of implementation guidelines adapted from the Consolidated Framework for Implementation Research (CFIR) to phase-out the routine FNB and phase-in the alternative ACB[7]. to assess our institution’s implementation process measured through utilization rates by neuraxial anesthesia type; to compare perioperative outcomes between FNB and ACB patient. By using CFIR guidelines,[7]. we deimplemented the routine FNB and implemented the abductor ACB as the new standard at our institution. We report here the principle, process and effectiveness of such an implementation method.
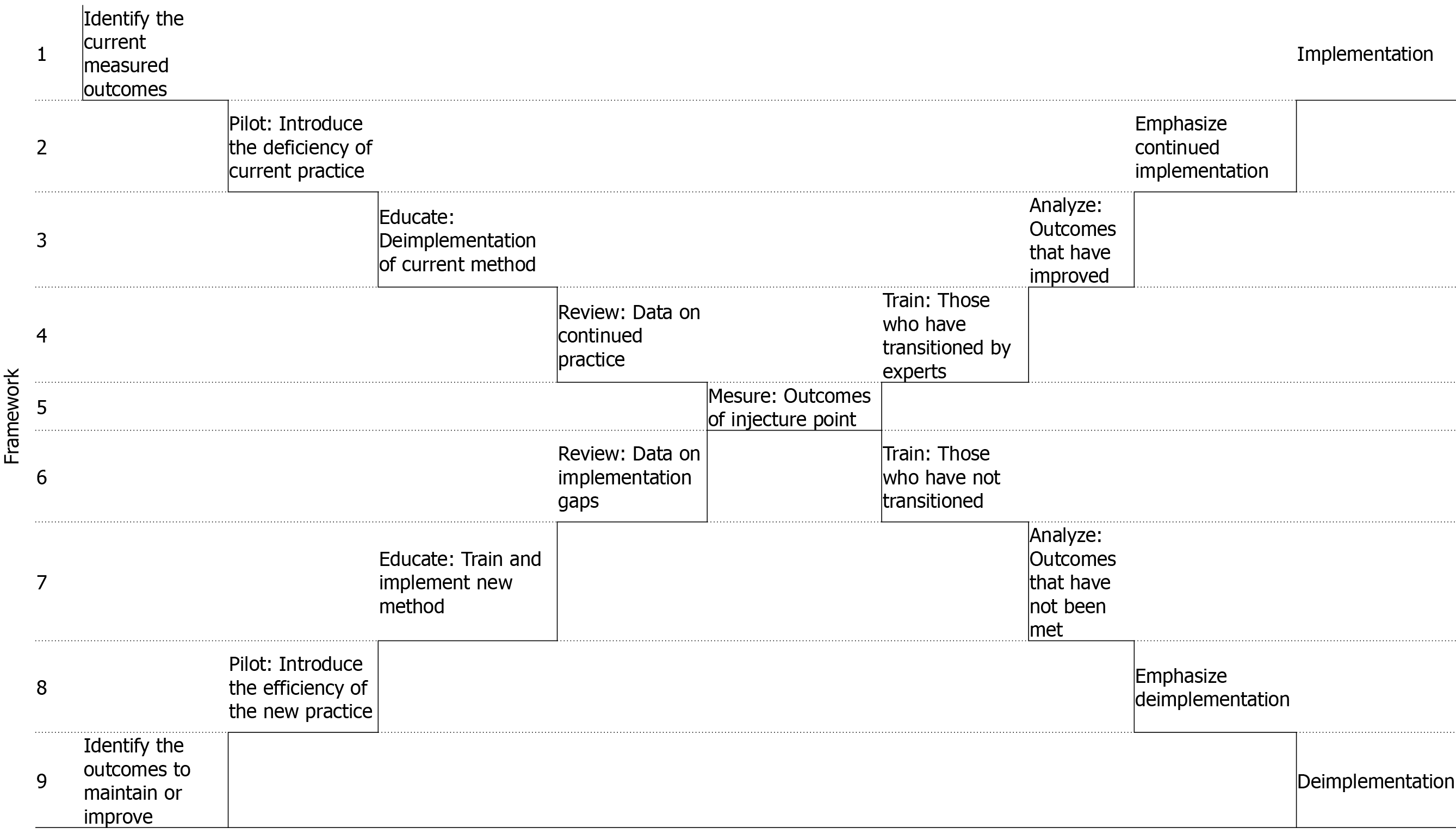
To evaluate the step-by-step implementation and deimplementation roadmap depicted in Figure 1. Specific implementation factors at our institution were part of an overall change management plan adopted from the Consolidated Framework for Implementation Research[7].
A baseline period (103 patients) was established from June to August 2016. Following a one-month pilot (47 patients) in September 2016 during which those trained in the ACB educated the providers in the team. Patients were informed of the change if they had received the FNB for their previous procedure. The dosage and technicality of the blocks were standardized and disseminated at the beginning of the pilot and reminders were given at each phase.
A three-month implementation and hardwiring period (100 patients) from October to December 2016 was executed for the replacement of FNB for ACB. From January to June 2017, there was a six-month evaluation period (185 patients). During the evaluation period, the dataset was analyzed to determine providers for whom there remained obstacles to implementation; these barriers were addressed biweekly and resolved. In total, 435 TKA patients were reviewed over 13 mo from June 2016 to June 2017.
Data on patient demographics (e.g., sex, age, BMI, ASA status), anesthesia and analgesia (e.g., OME), intraoperative data (e.g., length of operation, estimated blood loss, site infection, transfusion), and perioperative outcomes (e.g., pain scores, distance traveled, buckling, LOS, 30 d readmission, MI or stroke, UTI, and fall) were collected and reviewed. Data was collected prospectively; however, it was retrospectively analyzed as a cohort over time. Reports were generated to evaluate progress initially biweekly and then monthly and during each phase until full implementation. Oral morphine equivalents (OME) were determined based on the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) equianalgesic calculator as an average over 24 h after surgery. Analysis was conducted in imperial units and then converted to International System of Units (SI) equivalents (e.g., feet to meters).
Statistical analysis was performed to compare between ACB and FNB groups using JMP® Pro, Version 13 (SAS Institute Inc., Cary, NC, 1989-2020) at a P value < 0.05. Continuous variables were summarized using descriptive statistics, such as mean, median, and range, and evaluated using two-tailed Student’s t-test. Proportions were calculated for ordinal variables and compared using Pearson chi-squared test. Outliers were removed as defined as three times outside 10% tail quantile.
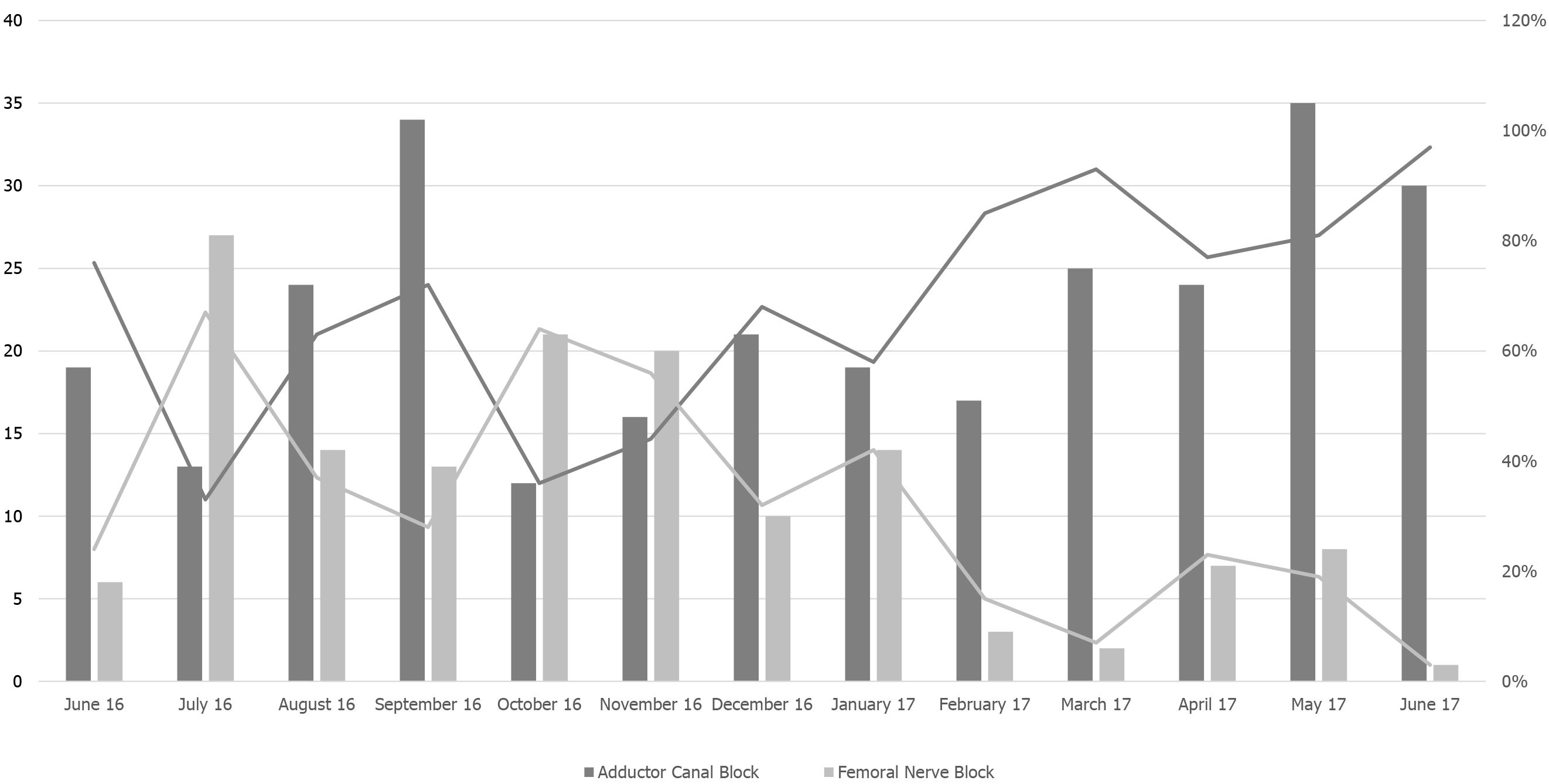
Overall, study population characteristics shown in Table 1 were similar to our reported baseline. Figure 2 illustrates the phase-out of the FNB and phase-in of the ACB over the 13-month study period. The preference for ACB vs FNB as peripheral nerve block improved after the pilot from October 2016 (36% vs 64%) to post-implementation in June 2017 (97% vs 3%).
| Demographics | Results |
| Total, n | 435 |
| Anesthesia, n (%) | |
| Spinal | 368 (85) |
| General | 67 (15) |
| Spinal converted to General | 8 (2) |
| Sex, n (%) | |
| Male | 148 (34) |
| Female | 287 (66) |
| Age, Mean, Median [Range] | 72.3, 71 [65.0-91.0] |
| Age Group, n (%) | |
| 65 to 75 | 329 (76) |
| 76 to 85 | 93 (21) |
| 86 to 91 | 13 (3) |
| ASA status, n (%) | |
| I or II | 283 (65) |
| III, IV, or V | 152 (35) |
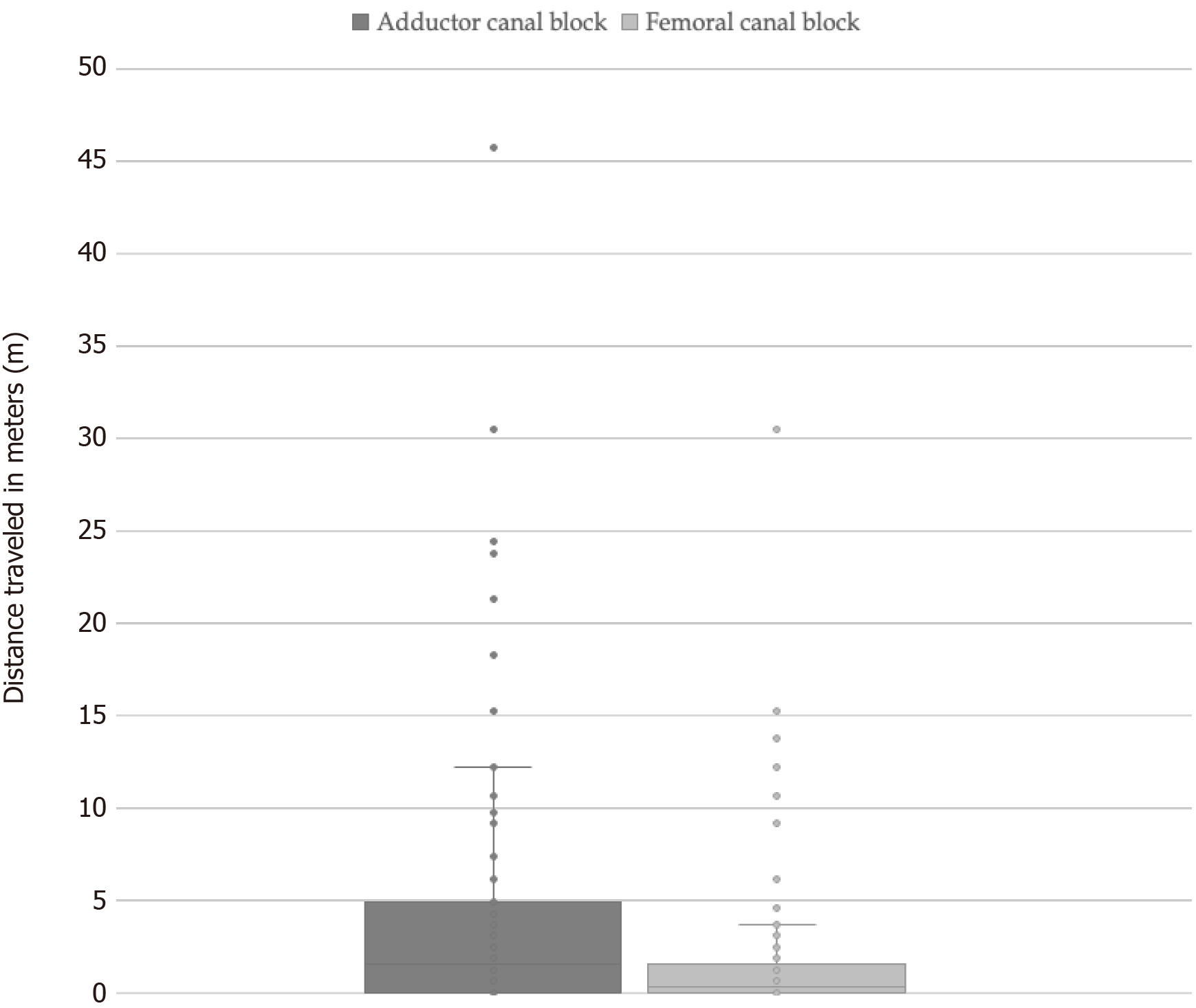
Table 2 demonstrates a summary of patient outcomes between the two groups. While FNB cases utilized lower amounts of local anesthetic (mg), ACB cases were shown to have lower estimated blood loss (mL), fall rates, and incidence of buckling during physical therapy. While the ACB group (13.9) reported lower OME vs FNB group (15.9), the difference did not meet our statistical threshold of P < 0.05 (P = 0.087). On the day of surgery, ACB patients were observed to have an increased mean distance traveled during mobilization compared with FNB patients (4.1 vs 2.0 m) as demonstrated in Figure 3.
| Variable | Adductor | Femoral | P value |
| Demographics | |||
| Number of patients | 289 (66%) | 146 (34%) | - |
| Sex | 0.57 | ||
| Male | 101 (35%) | 47 (32%) | - |
| Female | 188 (65%) | 99 (68%) | - |
| Age | 72.3, 71 [65-91] | 72.3, 71 [65-91] | 0.90 |
| BMI in kg/m2 | 31.2, 31.0 [19.3-50.0] | 30.8, 29.8 [21.0-51.1] | 0.55 |
| ASA status | 0.52 | ||
| I or II | 185 (64%) | 98 (67%) | - |
| III, IV, or V | 104 (36%) | 48 (33%) | - |
| Intraoperative data | |||
| Anesthesia | 0.067 | ||
| Spinal | 246 (85%) | 114 (78%) | - |
| General | 38 (13%) | 29 (20%) | - |
| Spinal converted to General | 5 (2%) | 3 (2%) | - |
| Length of operation in min | 122.7, 118 [83-235] | 121.7, 107 [83-199] | 0.64 |
| Estimated blood loss in mL | 57.6, 45 [20-200] | 68.2, 75 [20-200] | 0.0031b |
| Local anesthetic in mg | 94.2, 100 [11.3-225] | 89.4, 93.8 [11.3-150] | 0.036a |
| Site infection or redness | 2 (1%) | 1 (1%) | 0.99 |
| Transfusion | 1 (0%) | 1 (1%) | 0.62 |
| Day of surgery outcomes | |||
| Pain score from 0 to 10 | 1.9, 1.7 [0-6.6] | 2.0, 1.7 [0-6.1] | 0.59 |
| OME | 13.9, 12.8 [0-66] | 15.9, 15 [0-50] | 0.087 |
| Distance traveled in meters | 4.1, 1.5 [0-45.7] | 2.0, 0.3 [0-30.5] | 0.0004b |
| Buckling | 14 (5%) | 40 (27%) | < 0.0001b |
| Physical therapy complication | 7 (2%) | 2 (1%) | 0.47 |
| Postoperative outcomes | |||
| Length of stay in days | 1.9, 1.4 [1.1-8.4] | 2.1, 2.1 [1.1-6.2] | 0.091 |
| 30 d readmission | 8 (3%) | 7 (5%) | 0.27 |
| MI and stroke | 0 (0%) | 0 (0%) | - |
| UTI | 1 (0%) | 1 (1%) | 0.69 |
| Fall | 0 (0%) | 2 (1%) | 0.046a |
While the average duration to translate new practice into routine adoption is 17 years, only half of evidence-based changes end up reaching broad medical usage[8-9]. In 2012, the replacement of low-value care, defined as inefficient or unwarranted health care practices, received widespread recognition by the American Board of Internal Medicine Foundation through its Choosing Wisely (CW) initiative[10-13]. Although CW campaigns gained initial enthusiasm, promising recommendations often stood in isolation, which resulted in poor adoption rates and lacked the capacity for sustained change[14-17]. The reasons for delayed or missed uptake of evidence-based practices include inadequate resources for mobilizing change, competing demands of providers, and dissonance between operational and research priorities[18]. In addition, the context of current practices, including both the barriers and facilitators of change, is an overlooked, yet imperative, consideration for successful deimplementation[19]. Thus, there is the need to develop targeted strategies to increase the proliferation of evidence-based practices, chiefly by learning through case studies in hospital systems[20].
As of today, implementation science remains an overlooked opportunity for accelerating patient care and improving clinical outcomes. Annually, there are nearly seven million complications and one million deaths shortly after surgery, despite the fact that the perioperative patient care accounts for more than 60% of hospital expenditure[2]. Moreover, it has been shown that roughly half of adverse outcomes are potentially avoidable[21]. In spite of many established clinical pathways and strategies that have been tailored to minimize negative impacts, the clinical outcomes have been staggering not due to lack of evidence and knowledge, but because of lack of implementation framework and strategies to sustain the effect of positive changes.
By applying the principle of implementation science, we replaced the femoral nerve block for TKA with the abductor canal block within 13 mo in our established PSH pathway. The learning for the new technique was rapid, the group adoption and transition of the practice was immediate, and consolidation of learning and practice was persistent. We found that ACB patients had increased ambulation and decreased falls and buckling compared with FNB patients, thereby validating an institutional practice change to enhance short-term patient outcomes after surgery. Our findings on improvement mobility are consistent with explanations that ACB may help assist in speedier knee recovery and maintenance of quadriceps strength[6]. We demonstrate that significant healthcare performance improvement can be achieved through the synergistic effect of evidence-based practice and evidence-based implementation science. Furthermore, successful implementation can be achieved through the simultaneous deimplementation of old practices within established PSH pathways.
Past research suggests that ERP initiatives are facilitated by successful pilot programs that generate preliminary evidence and demonstrate local effectiveness for further implementation[22-23]. As defined by Proctor et. al, our institution achieved high penetration, or diffusion rate of intervention, and sustainability, or continued use of intended practice, in the replacement of ACB for FNB during our 13-month study period[24]. Furthermore, it has been suggested that increasing ERP visibility, such as advertising pilot start dates, are beneficial to the implementation process[25,26]. In our case, much attention was focused around our program’s launch, as evidenced by the spike in ACB uptake (72%) during the September 2016 pilot. Although there was a subsequent dip in the following two months (36% and 44%), the steady adoption and study’s inverse relationship between ACB implementation and FNB deimplementation indicate strong adherence to our program’s intended outcome.
Comprehensive transition packages are recommended for dissemination across other regions[13,27]. The Institute for Healthcare Improvement (IHI) advocates that “care bundles” provide solid evidence for change in practice, limited debate over efficacy, and robust acceptance[28]. Gilhooly et al[29] categorized compliance to practice changes into three levels: high (70%-100%), medium (40%-69%), and low (0%-39%). Specifically, while high and medium compliance groups leveraged interdisciplinary teams, champion networks, and structured audits and feedback loops, low compliance groups employed less interactive strategies, such as posters and screen saver reminders[29]. Our institution serves as another case study of how high engagement strategies, including one-on-one coaching and timely program evaluations, helped a large provider team realize 97% ACB utilization rate by our study’s endpoint.
It is often discussed that the primary end goal of implementation science is to achieve “sustainability”, in which new knowledge and reformed practices are embedded in routine care[30]. As Rapport et al[30] propose in their “diffusion-dissemination-implementation” continuum, the concept of sustainability, along with adoption, is only one of five critical stages in the feedback loop that ensure sound implementation. With the goal for implementation science to seek long-term impact, purposeful language (i.e., terminology that can be refined and fit future needs) and shared agendas with the greater hospital organization help support sustainable change[30]. In our case, we ensured that our program’s messaging mirrored our group’s strategic initiatives, as well as the broader transformational goals of our hospital management organization. In building the case for practice change, it has been recommended that pre-implementation data includes, at a minimum, one year of prior data to support the endorsement of senior leaders and assignment of resources and capital[31]. While post-implementation cost savings analysis can facilitate future programs, there may be incalculable benefits, such as expanded experiential learning opportunities for residents and encouragement in critical thinking and evaluation of therapeutic interventions[31].
There are limitations to our study. By prescribing exclusion criteria to patients under 65 years old, our findings on ACB mobility benefits and lower incidence of falls and buckling may be narrowed to the older patient and more representative of the demographics of our specific medical center. In a previous analysis of data on 9580 total hip and knee patients across 11 of our region’s medical centers, it was found that 40% of patients were under 65 years old[32]. Future studies should explore younger patient populations and various demographics. Furthermore, there was variability in how the estimated distance traveled during postoperative physical therapy was recorded. For example, while some providers noted mobility progress in imperial units (e.g., “80 feet”), others included more qualitative measurements (e.g., “2 sidesteps”) which needed to be normalized in our database by adopting consistent assumptions (i.e., 1 sidestep = 1 foot). There is an opportunity for standardization in approach for tracking key physical therapy metrics as we continue to build our dataset across our regional network. In the future, there is value for implementation strategies to include cost to benefit analyses on the allocated change management resources (e.g., training, dedicated staff, campaign awareness) and perioperative patient outcomes to quantify the financial impact of such programs.
In this study, we closed the “knowledge to action gap” within 6 mo, proving the implementation effectiveness of the Consolidated Framework for Implementation Research and implementation science in our setting. The inverse relationship between the adoption of ACB utilization and phasing out of FCB suggests the benefits of implementation science guided by a roadmap of physician education, technical support, and performance monitoring. Moreover, our study demonstrates evidence that transition to ACB as the choice regional anesthesia technique during TKA may improve patient mobility and physical therapy outcomes following surgery. There is an opportunity to bridge our growing knowledge in improving perioperative techniques with an effective implementation framework. Next steps including expanded patient populations, additional medical centers, and other orthopedic procedures are warranted.
In 2016, we employed Perioperative Surgical Home (PSH) practice change for ambulatory total knee arthroplasty (TKA) resulting in reduced length of stay in our system. Nevertheless, we acknowledged the need for continuous improvement and implementation of new practices to optimize short-term outcomes in our TKA patient population.
We employed a new look at implementation science to remove outdated PSH elements and adopt modified consolidated framework for implementation research (mCFIR) practices. Our motivation was to investigate the transition from femoral nerve blocks (FNB) to adductor canal nerve blocks (ACB) and how learnings on change management could be applied to other surgical areas.
To execute our institution’s implementation process during the phase-out of FNB and phase-in of ACB during TKA. While the rationale for ACB practice was not novel, we focused on identifying the enablers of success practice change.
We tracked our institution’s implementation progress through utilization rates by neuraxial anesthesia type. Goals of enhancing patient care were validated through the comparison of perioperative outcomes between FNB and ACB patients.
Application of the mCFIR was shown to be successful in implementing institutional practice change for ACB during TKA within 6 mo. Increased patient mobility and improved physical therapy outcomes were demonstrated in ACB vs FNB patients.
Our institution’s successful phase-out of FNB and phase-in of ACB within 6 mo demonstrates the valuable role of implementation science. Effective physician education with technical support and metrics evaluation are critical methods to achieve swift practice change.
Future research should be focused on younger patient populations and different orthopedic procedures.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Anesthesiology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: DeSousa K, Kaçmaz M S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1802] [Cited by in F6Publishing: 1577] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 2. | Hull L, Athanasiou T, Russ S. Implementation Science: A Neglected Opportunity to Accelerate Improvements in the Safety and Quality of Surgical Care. Ann Surg. 2017;265:1104-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Peters DH, Tran NT, Adam T. Implementation Research in Health: A Practical Guide. Alliance for Health Policy and Systems Research, World Health Organization, 2013. [cited 15 April 2021]. Available from: http://who.int/alliance-hpsr/alliancehpsr_irpguide.pdf. [Cited in This Article: ] |
| 4. | Qiu C, Cannesson M, Morkos A, Nguyen VT, LaPlace D, Trivedi NS, Khachatourians A, Rinehart J, Kain ZN. Practice and Outcomes of the Perioperative Surgical Home in a California Integrated Delivery System. Anesth Analg. 2016;123:597-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Klein KJ, Sorra JS. The challenge of innovation implementation. Acad Manage Rev. 1996;21:1055-1080. [DOI] [Cited in This Article: ] |
| 6. | Wang D, Yang Y, Li Q, Tang SL, Zeng WN, Xu J, Xie TH, Pei FX, Yang L, Li LL, Zhou ZK. Adductor canal block vs femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Rojas Smith L, Ashok M, Morss Dy S, Wines RC, Teixeira-Poit S. Contextual Frameworks for Research on the Implementation of Complex System Interventions [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. [PubMed] [Cited in This Article: ] |
| 8. | Balas EA, Boren SA. Managing Clinical Knowledge for Health Care Improvement. Yearb Med Inform. 2000;65-70. [PubMed] [Cited in This Article: ] |
| 9. | Grant J, Green L, Mason B. Basic research and health: a reassessment of the scientific basis for the support of biomedical science. Res Eval. 2003;12: 217-224. [Cited in This Article: ] |
| 10. | Brownlee S, Chalkidou K, Doust J, Elshaug AG, Glasziou P, Heath I, Nagpal S, Saini V, Srivastava D, Chalmers K, Korenstein D. Evidence for overuse of medical services around the world. Lancet. 2017;390:156-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 518] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 11. | Canadian Institute for Health Information. Unnecessary Care in Canada: Technical Report. Ottawa, ON: CIHI; 2017. [cited 20 February 2021]. Available from: https://www.cihi.ca/en/unnecessary-care-in-canada. [Cited in This Article: ] |
| 12. | Kirkham KR, Wijeysundera DN, Pendrith C, Ng R, Tu JV, Laupacis A, Schull MJ, Levinson W, Bhatia RS. Preoperative testing before low-risk surgical procedures. CMAJ. 2015;187:E349-E358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Levinson W, Kallewaard M, Bhatia RS, Wolfson D, Shortt S, Kerr EA; Choosing Wisely International Working Group. 'Choosing Wisely': a growing international campaign. BMJ Qual Saf. 2015;24:167-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7:50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1424] [Cited by in F6Publishing: 1318] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 15. | Mafi JN, Parchman M. Low-value care: an intractable global problem with no quick fix. BMJ Qual Saf. 2018;27:333-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Nieuwlaat R, Schwalm JD, Khatib R, Yusuf S. Why are we failing to implement effective therapies in cardiovascular disease? Eur Heart J. 2013;34:1262-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Rosenberg A, Agiro A, Gottlieb M, Barron J, Brady P, Liu Y, Li C, DeVries A. Early Trends Among Seven Recommendations From the Choosing Wisely Campaign. JAMA Intern Med. 2015;175:1913-1920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 18. | Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM. An introduction to implementation science for the non-specialist. BMC Psychol. 2015;3:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1016] [Cited by in F6Publishing: 877] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 19. | Rasooly IR, Beidas RS, Wolk CB, Barg F, Landrigan CP, Schondelmeyer A, Brady PW, McLeod LM, Bonafide CP; Pediatric Research in Inpatient Settings (PRIS) Network. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5:68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299:211-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 961] [Cited by in F6Publishing: 862] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 21. | Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1608] [Cited by in F6Publishing: 1592] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 22. | Bona S, Molteni M, Rosati R, Elmore U, Bagnoli P, Monzani R, Caravaca M, Montorsi M. Introducing an enhanced recovery after surgery program in colorectal surgery: a single center experience. World J Gastroenterol. 2014;20:17578-17587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 41] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | McEvoy MD, Wanderer JP, King AB, Geiger TM, Tiwari V, Terekhov M, Ehrenfeld JM, Furman WR, Lee LA, Sandberg WS. A perioperative consult service results in reduction in cost and length of stay for colorectal surgical patients: evidence from a healthcare redesign project. Perioper Med (Lond). 2016;5:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, Griffey R, Hensley M. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2680] [Cited by in F6Publishing: 3420] [Article Influence: 263.1] [Reference Citation Analysis (0)] |
| 25. | Gotlib Conn L, McKenzie M, Pearsall EA, McLeod RS. Successful implementation of an enhanced recovery after surgery programme for elective colorectal surgery: a process evaluation of champions' experiences. Implement Sci. 2015;10:99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Wick EC, Galante DJ, Hobson DB, Benson AR, Lee KH, Berenholtz SM, Efron JE, Pronovost PJ, Wu CL. Organizational Culture Changes Result in Improvement in Patient-Centered Outcomes: Implementation of an Integrated Recovery Pathway for Surgical Patients. J Am Coll Surg. 2015;221:669-77; quiz 785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Levinson W, Born K, Wolfson D. Choosing Wisely Campaigns: A Work in Progress. JAMA. 2018;319:1975-1976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 28. | Resar R, Griffin FA, Haraden C, Nolan TW. Using Care Bundles to Improve Health Care Quality. IHI Innovation Series white paper. Cambridge: Institute for Healthcare Improvement; 2012. [cited 20 February 2021]. Available from: http://www.ihi.org/resources/Pages/IHIWhitePapers/UsingCareBundles.aspx. [Cited in This Article: ] |
| 29. | Gilhooly D, Green SA, McCann C, Black N, Moonesinghe SR. Barriers and facilitators to the successful development, implementation and evaluation of care bundles in acute care in hospital: a scoping review. Implement Sci. 2019;14:47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Rapport F, Clay-Williams R, Churruca K, Shih P, Hogden A, Braithwaite J. The struggle of translating science into action: Foundational concepts of implementation science. J Eval Clin Pract. 2018;24:117-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 31. | Trumbo SP, Iams WT, Limper HM, Goggins K, Gibson J, Oliver L, Leverenz DL, Samuels LR, Brady DW, Kripalani S. Deimplementation of Routine Chest X-rays in Adult Intensive Care Units. J Hosp Med. 2019;14:83-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Crain N, Goharderakhshan R, Reddy N, Apfel A, Navarro R. The role of intraoperative urinary catheters on postoperative urinary retention after total joint arthroplasty: a multi-hospital retrospective study on 9580 patients. Arch Bone Jt Surg. 2020;. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |