Copyright
©2012 Baishideng Publishing Group Co.
World J Orthop. Sep 18, 2012; 3(9): 142-150
Published online Sep 18, 2012. doi: 10.5312/wjo.v3.i9.142
Published online Sep 18, 2012. doi: 10.5312/wjo.v3.i9.142
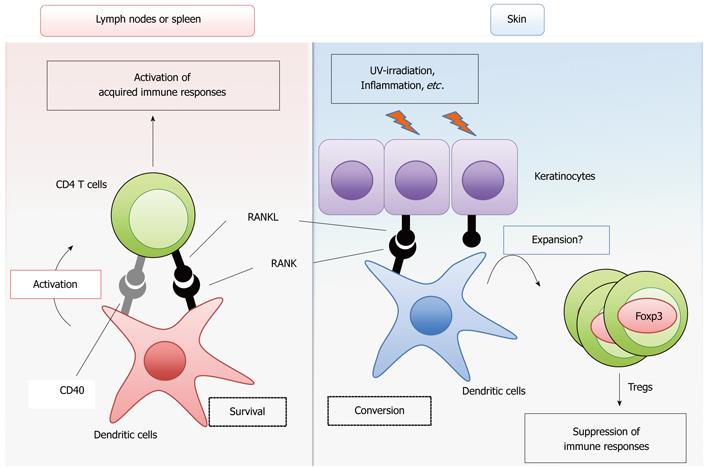
Figure 1 Modulation of dendritic cell functions by receptor activator of NF-κB ligand signaling.
Receptor activator of NF-κB ligand (RANKL) signaling promotes the survival of conventional dendritic cells (cDCs) and ensures T cell priming and activation, thereby enhancing the acquired immune response (left). CD40 signaling may have a redundant role in this function of RANK signaling. In skin and, most likely, the intestines, RANKL-treated DCs maintain the number of Foxp3-positive regulatory T cells to suppress the immune response against self-antigens, food and commensal flora (right). In the skin, inflammation and ultraviolet (UV)-irradiation upregulate the expression of RANKL in keratinocytes.
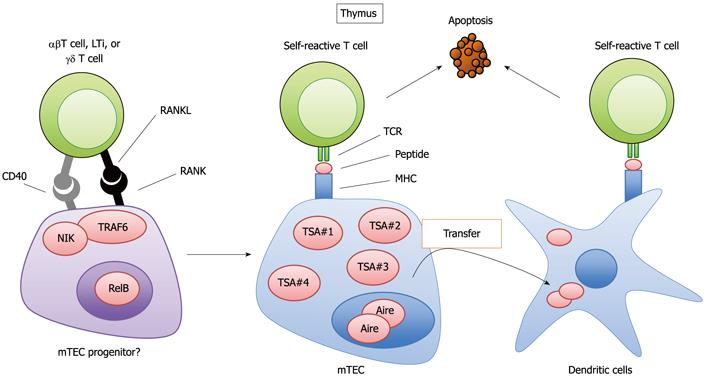
Figure 2 Regulation of the development and function of medullary thymic epithelial cells by receptor activator of NF-κB ligand signaling.
Receptor activator of NF-κB ligand (RANKL) signaling mediates the development of mature medullary thymic epithelial cells (mTECs) expressing Autoimmune regulator (Aire) and a wide variety of tissue-specific antigens (TSAs) from the potential progenitors. RANKL is expressed in several types of cells, including αβ T cells, lymphoid tissue inducer cells (LTi) and a subset of γδ T cells. The signal transducers NF-κB inducing kinase (NIK) and TNF receptor-associated factor 6 (TRAF6) mediate RANK signaling, culminating in activation of the NF-κB transcription factor member RelB. mTECs present peptides are derived from TSAs to eliminate self-antigen reactive T cells by apoptosis. The TSAs can be transferred to dendritic cells by mechanisms that are not yet fully identified. The dendritic cells receiving TSAs also induce the apoptosis of self-reactive T cells in the thymus.
- Citation: Akiyama T, Shinzawa M, Akiyama N. RANKL-RANK interaction in immune regulatory systems. World J Orthop 2012; 3(9): 142-150
- URL: https://www.wjgnet.com/2218-5836/full/v3/i9/142.htm
- DOI: https://dx.doi.org/10.5312/wjo.v3.i9.142