Copyright
©The Author(s) 2017.
World J Gastrointest Pharmacol Ther. May 6, 2017; 8(2): 137-146
Published online May 6, 2017. doi: 10.4292/wjgpt.v8.i2.137
Published online May 6, 2017. doi: 10.4292/wjgpt.v8.i2.137
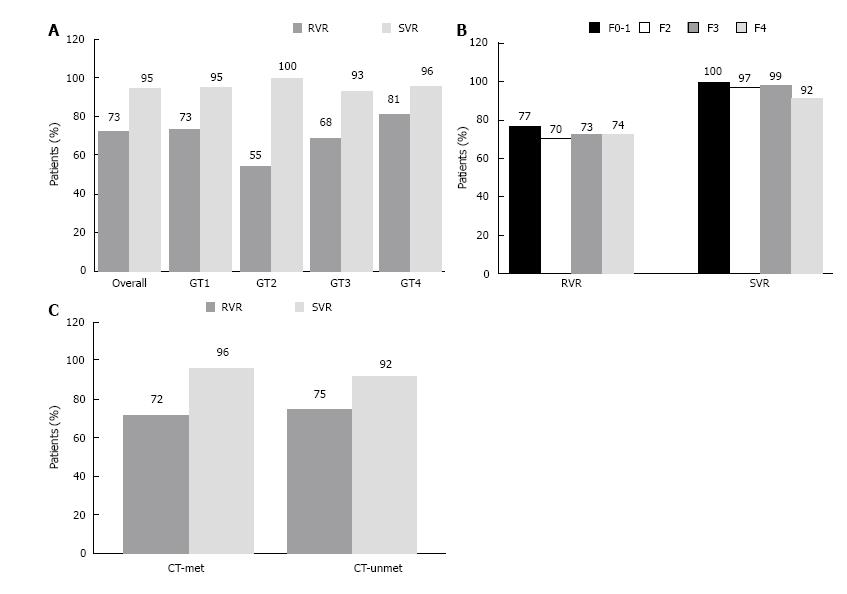
Figure 1 Rates of virological response.
Patients with undetectable viral loads during and post treatment. A: At treatment week 4 and post-treatment week 12 (sustained virological response) by genotype; B: At treatment week 4 and post-treatment week 12 (sustained virological response) by fibrosis stage; C: At treatment week 4 and post-treatment week 12 (sustained virological response) by CT-met and CT-unmet. Data for 5 patients were lost: genotype 1, data from three patients were lost; genotype 3 and 4, a patient data in each genotype were lost. Data for 4 patients were lost. Data for 1 patient were lost. GT: Genotype; RVR: Undetectable HCV RNA at week 4; SVR: Sustained virological response; CT: Clinical trial.
- Citation: Ramos H, Linares P, Badia E, Martín I, Gómez J, Almohalla C, Jorquera F, Calvo S, García I, Conde P, Álvarez B, Karpman G, Lorenzo S, Gozalo V, Vásquez M, Joao D, de Benito M, Ruiz L, Jiménez F, Sáez-Royuela F, Asociación Castellano y Leonesa de Hepatología (ACyLHE). Interferon-free treatments in patients with hepatitis C genotype 1-4 infections in a real-world setting. World J Gastrointest Pharmacol Ther 2017; 8(2): 137-146
- URL: https://www.wjgnet.com/2150-5349/full/v8/i2/137.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v8.i2.137