Published online Aug 6, 2015. doi: 10.4292/wjgpt.v6.i3.89
Peer-review started: April 24, 2015
First decision: June 3, 2015
Revised: June 21, 2015
Accepted: July 11, 2015
Article in press: July 14, 2015
Published online: August 6, 2015
Kaposi’s sarcoma (KS) of the gastrointestinal tract is not an uncommon disease among individuals with acquired immunodeficiency syndrome (AIDS). The majority is asymptomatic, and for this reason, gastrointestinal KS (GI-KS) remains undiagnosed. With continued tumor growth, considerable variation in clinical presentation occurs including abdominal pain, nausea, vomiting, iron deficiency anemia (either chronic or frank gastrointestinal bleeding), and rarely mechanical obstruction alone or combined with bowel perforation. Endoscopy with biopsy allows for histological and immunohistochemical testing to confirm the diagnosis of GI-KS among those with clinical symptoms. In previous studies, dual treatment with highly active antiretroviral therapy and systemic chemotherapy have been associated with improved morbidity and mortality in individuals with visceral KS. Therefore, investigators have suggested performing screening endoscopies in select patients for early detection and treatment to improve outcome. In this review, we describe a 44 years old man with AIDS and cutaneous KS who presented for evaluation of postprandial abdominal pain, vomiting, and weight loss. On upper endoscopy, an extensive, infiltrative, circumferential, reddish mass involving the entire body and antrum of the stomach was seen. Histologic examination later revealed spindle cell proliferation, and confirmatory immunohistochemical testing revealed human herpes virus 8 latent nuclear antigen expression consistent with a diagnosis of gastric KS. Following this, we present a comprehensive review of literature on KS with emphasis on gastrointestinal tract involvement and management.
Core tip: Gastrointestinal Kaposi’s sarcoma (GI-KS) is more common than originally thought and the majority of patients are asymptomatic and remain undiagnosed. Therefore, clinicians should maintain a high suspicion, especially among acquired immunodeficiency syndrome patients. Screening endoscopies are frequently performed and a recent retrospective study suggested using certain clinical factors (low CD4 cell count of < 100 cell/μL, men who have sex with men, and presence of cutaneous Kaposi’s) which may predict occurrence of GI-KS endoscopically. Endoscopy to detect GI-KS prior to clinical symptom development is desired as initiation of combined highly active antiretroviral therapy and liposomal doxorubicin therapy has shown to improve outcome.
- Citation: Lee AJ, Brenner L, Mourad B, Monteiro C, Vega KJ, Munoz JC. Gastrointestinal Kaposi’s sarcoma: Case report and review of the literature. World J Gastrointest Pharmacol Ther 2015; 6(3): 89-95
- URL: https://www.wjgnet.com/2150-5349/full/v6/i3/89.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v6.i3.89
Kaposi’s sarcoma (KS) is a low grade tumor of the vascular endothelium[1]. In the United States, the majority of affected individuals have human immunodeficiency virus (HIV) infection[2]. Although KS can develop at any HIV infection stage, it occurs more commonly in the setting of advanced immune suppression[2,3]. In the Western population, the prevalence of acquired immunodeficiency syndrome (AIDS) associated KS is 6%-30%, and affected individuals usually manifest with cutaneous disease and/or visceral involvement, of which the gastrointestinal (GI) tract is the most common extracutaneous site[4]. In untreated AIDS, the GI tract is involved in approximately 40%-51%, but only one in five of affected individuals have GI symptoms[5]. The majority are clinically silent (79%), and therefore, most visceral KS remain unidentified[4]. In recent studies, treatment using combined highly active antiretroviral therapy (HAART) and systemic chemotherapy was associated with a significant improvement in morbidity and mortality[6]. Therefore some experts have suggested screening endoscopy in select patients for early detection and initiation of treatment to improve survival outcome[7].
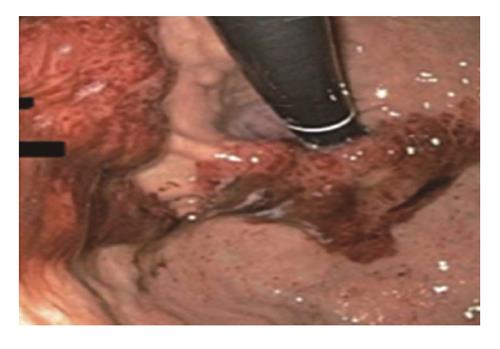
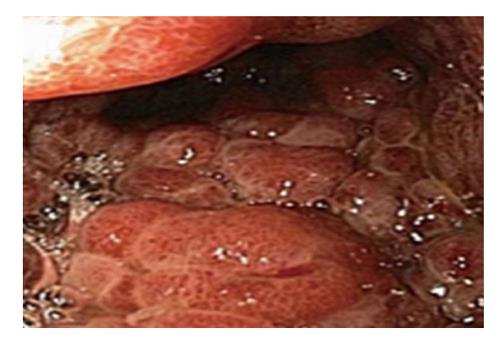
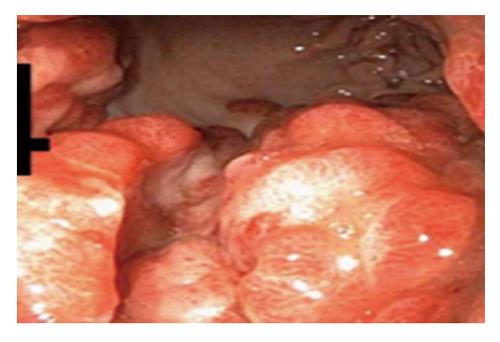
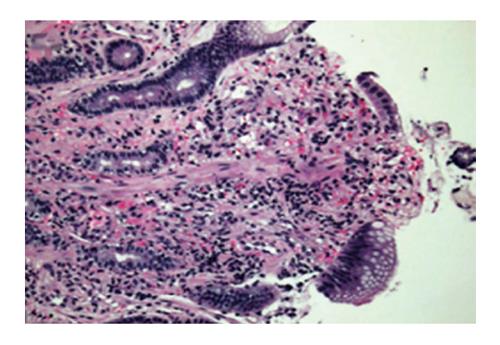
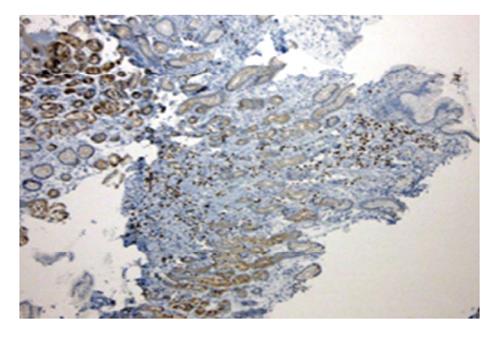
A 44 years old man presented to the emergency department for evaluation of worsening abdominal pain following oral intake. Abdominal pain was described as cramping and associated with severe nausea and vomiting of ingested food. Clinical symptoms were unrelated to food type (acidic, dairy, or gluten-containing groups), and occurred after either solid or liquid intake. At evaluation, he was unable to tolerate anything by mouth and reported an associated 10 pound weight loss over a 1 mo period. Past medical history was significant for AIDS and cutaneous Kaposi sarcoma diagnosed by skin biopsy 4 years previously. Home medications included topical Imiquimod therapy for cutaneous KS, and recent initiation of HAART (2 mo prior to presentation) for low CD4 cell count which was poorly tolerated due to GI discomfort. On physical examination, multiple, plaque-like, violaceous, skin lesions were found in both lower extremities with associated edema in the right leg and foot. In addition, similar lesions were also found in the neck and trunk; the patient reported these lesions to be increasing in size. Laboratory studies revealed an absolute CD4 cell count of 101 cells/μL, HIV-RNA level of 168 copies/mL, and a mild anemia with hemoglobin of 11 g/mL. Other biochemical markers were normal including amylase, lipase, and liver function panel. Further evaluation with computed tomography imaging of the abdomen/pelvis with and without contrast was remarkable for a prominent, concentric gastric wall thickening involving the majority of the stomach. An upper endoscopy revealed an extensive, circumferential, infiltrative, reddish mass throughout the entire gastric body which extended into the fundus and antrum (Figures 1-3). Endoscopic biopsy specimens were obtained from the gastric lesions, and hematoxylin and eosin examination revealed spindle cell proliferation with extensive lymphoplasmocytic cell infiltrates (Figure 4). A confirmatory immunohistochemical test showed human herpes virus 8 latent nuclear antigen (HHV8 LNA) expression suggestive of HHV8, supporting the diagnosis of gastric KS (Figure 5).
KS was described in 1872 by a Hungarian dermatologist, Moritz Kohn Kaposi, who was the first to identify five cases of “idiopathic multiple pigmented sarcomas of the skin”[8]. In one case, the patient expired from gastrointestinal bleeding with a postmortem report that revealed unspecified visceral lesions in the lung and GI tract. In 1981, with the onset of the HIV epidemic, the first case of AIDS-associated KS was described[9]. Since, an increasing number of KS among AIDS patients prompted speculations of a possible infectious agent involved in tumor pathogenesis[10]. By 1994, Chang et al[11] isolated a unique DNA sequence in KS tissue which was later classified as a new herpesvirus species now known as KS-associated herpesvirus or HHV8[11]. Since its discovery, HHV8 has been detected in over 95% of all AIDS and non-AIDS associated KS lesions, suggesting it as the causative agent in tumor development[12].
Numerous serological studies have demonstrated a direct correlation between HHV8 infection and KS incidence. This data also indicated significant geographic variation in HHV8 seroprevalence existed. Overall, low seroprevalence is reported in North America, Northern Europe and Asia, intermediate seroprevalence in Eastern Europe, Mediterranean, and Caribbean countries, and high seroprevalence in Central (Uganda, Zambia, and South Africa) and Southern Africa[12]. Although a cause and effect relationship between HHV8 and KS disease is clear, some exceptions to this pattern have been observed. For example in Africa, cases of endemic (or African) variant of KS were reported in Uganda and Zambia, but not in Gambia or the Ivory Coast although all four countries have similar seroprevalence rates[13]. Similar circumstances have been reported in Italy, Egypt, and Chinese subpopulations where despite high seroprevalence of HHV8, KS incidence is low[14-16]. These discrepancies suggest that other, at present, unidentified factors may be required for clinical expression.
KS is classified into four clinical variants which have coevolved in certain human populations: classic KS, endemic (or African) KS, Iatrogenic (or immunosuppression related) KS, and epidemic (or AIDS related) KS[12]. In the United States, AIDS and immunosuppression-related causes are the two most common forms of KS tumor, and also the more likely variants causing visceral organ involvement. Despite efforts to classify KS, all forms are associated with HHV8 infection, and differences are observed in primary tumor location and rate of disease progression. Therefore, these epidemiological variants of KS merely represent different clinical manifestations of the same pathologic process.
Classic KS: The classic variant occurs predominantly in elderly men of Mediterranean, Eastern Europe, and Ashkenazi Jewish descent, and is uncommon in North America. It is a slow growing tumor which typically presents as a solitary or multifocal cutaneous lesion in the lower extremities. Systemic progression is rare and visceral disease is observed in less than 10% of cases[17].
Endemic or African KS: The African or Endemic KS variant consists of two disease subtypes, African cutaneous and African lymphadenopathic. It is more aggressive, and generally affects children of endemic African countries. These entities are not associated with HIV infection[18]. Cutaneous and nodal manifestations are common, with systemic disease less frequent.
Iatrogenic or immunosuppression-related KS: Iatrogenic or immunosuppression-related KS affect individuals with suppressed immunity as a result of organ transplantation or other medical conditions requiring long term immunosuppressant therapy. Cutaneous lesions are typically the presenting feature with systemic progression occurring in up to 50% of cases. In two case series of renal transplant recipients, KS developed in approximately 0.5%-5% of organ recipients which occurred in a median time of 29-31 mo after surgery[19-21]. In these patients, treatment with either reduction or discontinuation of the offending agent often led to tumor regression, and in some circumstances, complete resolution of disease.
Epidemic or AIDS related KS: Epidemic or AIDS-related KS is the most common form encountered in the United States. It can present at any stage of HIV infection, but more frequently in the setting of severe immune suppression[22]. Clinical features are similar to those with immunosuppression-related KS, presenting with cutaneous lesions and visceral KS disease arising as disease progresses[2].
The majority of patient with GI-KS will have cutaneous lesions at diagnosis. In most cases, skin lesions are not physically debilitating, but can cause severe disfigurement. Cutaneous KS are generally well-demarcated, erythematous to violaceous, as well as flat, plaque-like or nodular in appearance[2]. A solitary skin lesion is common in the early stage, but it can evolve into multicentric, coalescing lesions with progression. Large lesions are often associated with edema, but isolated lymphedema have been also reported in the absence of cutaneous disease. In most cases, these lesions present initially in the distal segment of the lower limbs, with proximal region involvement that parallel disease progression[23]. However, in AIDS-related KS, skin lesions more frequently affects the head, neck and upper torso regions[2].
Forty to fifty-one percent of patients with cutaneous lesions develop visceral KS which can involve the gastrointestinal tract, lungs, and less commonly the liver, spleen, kidney, and heart[5]. The GI tract is the most common extracutaneous site, and the majority of affected individuals are without clinical symptoms[4,5]. However, with tumor growth, considerable variations in symptoms can present including abdominal pain, nausea, vomiting, and iron deficiency anemia from chronic or frank GI bleeding. In rare cases, the tumor burden has also led to mechanical obstruction, intussusception, and perforation of the bowel wall[1].
Given such high incidence of GI-KS, endoscopic evaluation is required in AIDS patients that present with GI symptoms. However, in patients that are asymptomatic, the benefit of endoscopy remain a topic of debate. In Japan, screening endoscopies are frequently performed in HIV infected patients with the primary aim of early detection and initiation of treatment. A recent retrospective study looked at predictive factors for GI-KS and reported that patients with low CD4 cell count of < 100 cells/μL, men who have sex with men (MSM), and presence of cutaneous KS were more likely to have GI-KS, suggesting that clinicians consider endoscopy in these select patients even in the absence of clinical symptoms[7].
Gastrointestinal KS can involve any part of the GI tract from the oropharynx to the rectum, but it occurs most frequently in the stomach and small intestine. In two case series of 83 AIDS patients, the incidence of GI-KS was 40%-51%, of which 12%-24% were in the upper tract, 8%-12% were in the lower tract, and 15%-20% had multifocal lesions involving both the upper and lower GI tract[5,24]. GI-KS is endoscopically evident by appearance which ranges from a red maculopapular lesion to a darker, nodular or polypoid lesion. In more severe disease, patients may present with a volcano-like mass with a central umbilication or ulceration which can bleed on contact[1].
Although the majority of GI-KS disease can be identified easily on endoscopy, in some situations it may resemble common benign lesions (peptic ulcer and/or granulation tissue) as well as malignant neoplasms (gastrointestinal stromal tumor, spindle cell melanoma, angiosarcoma)[25,26]. For these reasons, biopsy specimens should be obtained to allow confirmatory testing with histopathology and immunohistochemistry testing. However, an endoscopic biopsy diagnosis is possible in only 15%-23% of cases, mainly owing to the submucosal nature of tumor growth[24,27,28]. In the absence of mucosal invasion, an endoscopically obtained biopsy may be too superficial and key features may be difficult to appreciate on histopathology. In such cases, an endoscopic ultrasound guided biopsy increases the diagnostic yield and is recommended[7].
On histopathology, KS is classically characterized as spindle cell proliferation that forms irregular vascular channels or slits in the submucosal bowel layer. It is associated with extensive red blood cell extravasation and hemosiderin-laden macrophage deposits which gives it a characteristic red to dark, bruise-like appearance. Additionally, significant lymphoplasmocytic infiltration occur which can lead to tumor swelling and cause pain response[25].
The presence of spindle shaped cells can exclude many benign and malignant lesions. However, in some circumstances, subtle vascularity and certain histological features of KS may overlap with other GI spindle cell tumors (gastrointestinal stromal tumor-GIST, spindle cell melanoma, and angiosarcoma) causing diagnostic uncertainty. To make a diagnosis of KS, the presence of HHV8 is necessary and immunohistochemical testing is recommended for all specimens with spindle cell morphology. HHV8 LNA is an immunomarker for this viral agent, and expression in spindle cell nuclei is considered to be 99% sensitive and 100% specific for KS[29].
Other biomarkers that are expressed include CD34 and CD117 (or c-KIT), which overlap with other stromal tumors making it a less reliable marker for KS diagnosis. Similar to GI-KS, gastrointestinal stromal tumors also expresses CD34 and CD117, but it is distinguished by the absence of HHV8 LNA, and positive DOG 1 expression[29]. Furthermore, spindle cell melanomas also share similar endoscopic and histologic features with GI-KS, but these tumors typically lack the CD34 marker and express S100 immunopositivity[29].
The treatment approach varies depending on the epidemiological variant of KS, and therapy is individualized based on the disease profile. In this segment, we will be focusing on the current treatment options for AIDS-associated KS with gastrointestinal tract manifestations.
AIDS-related KS is currently not a curable malignancy and the rate of disease progression is variable. Treatment with HAART is recommended in affected individuals if not already on therapy at diagnosis. Aside from the obvious improvement in immune status, the use of HAART inhibits the production of HIV tat protein which is responsible for HIV viral replication and KS cell growth, invasion and angiogenesis[28]. Since the introduction of HAART in 1995, the incidence of KS has substantially declined (25.6 cases per 100 person-years in the early 1990’s to 7.5 per 100 person-years in 1996-1997), and patients stable on HAART with good virologic response had a slower rate of disease progression and prolonged survival[29,30].
Among patients with localized cutaneous KS, HAART with either topical gel therapy or local radiation may suffice. However, in patients with extensive mucocutaneous disease or visceral organ involvement, combined HAART and systemic chemotherapy is indicated. Liposomal doxorubicin is the chemotherapeutic agent of choice in which the pegylated liposome component allows for the drug to accumulate preferentially in KS tissue, reducing the dose requirement and drug toxicity. In one study, a combined HAART and liposomal doxorubicin therapy was superior to stand alone HAART therapy with an overall response rate of 72% compared to 20% in the HAART alone group[6]. Chemotherapy with vinca alkaloids, bleomycin, and doxorubicin has fallen out of favor due to variable response rates and substantial drug toxicity[31-34]. Moreover, in randomized control trials, liposomal doxorubicin was also superior to combined chemotherapy regimens, further supporting liposomal doxorubicin as the drug of choice[35,36].
Despite favorable outcomes with dual HAART and liposomal doxorubicin treatment, the incidence of disease relapse is reported to be 13% per year with the majority in the first year after completing chemotherapy[37]. Among patients that require retreatment with liposomal doxorubicin, one-third will have treatment failure. In clinical trials, paclitaxel has demonstrated a comparable response rate of 59% and is currently the second-line agent approved by the Food and Drug Administration[38]. A third option is interferon-alpha which was the initial medication approved for AIDS-related KS. It has a similar clinical response rate but requires several months for tumor response, limiting usefulness in clinical practice[2].
Other drugs have been studied including anti-angiogenic compounds thalidomide and IM-862, and immunomodulators such as retinoid compounds[2]. All have shown suboptimal response rates. Antiviral agents have also been investigated in small trials, and in a single in-vitro drug sensitivity evaluation, HHV8 was very sensitive to cidofovir, moderately sensitive to ganciclovir, and weakly sensitive to foscarnet and acyclovir suggesting it as a possible alternative therapy[39,40]. However, currently no treatment recommendations have been drawn based on this study data for these compounds.
KS of the GI tract is far more common than originally thought, but the majority of patients are asymptomatic and remain undiagnosed. Therefore, clinicians should always maintain a high index of suspicion for GI-KS especially in patients with AIDS. In Japan, screening endoscopies are frequently performed, and a recent retrospective study has suggested using certain clinical factors (low CD4 cell count of < 100 cells/μL, MSM, and presence of cutaneous KS) may predict the occurrence of GI-KS endoscopically. Performance of endoscopy to detect GI-KS prior to development of clinical symptoms is desired as initiation of combined HAART and liposomal doxorubicin therapy has shown to improve outcome.
A 44 years old man with a history of acquired immune deficiency syndrome presented with postprandial abdominal pain and nausea with vomiting.
Multiple plaque-like, violaceous skin lesions found in the lower extremities, trunk and neck suggestive of Kaposi’s lesions.
Peptic ulcer disease, gastric mass (adenocarcinoma, stromal tumor, Kaposi’s sarcoma), and infectious esophagitis secondary to candida, herpes simplex virus, cytomegalovirus.
Absolute CD4 cell count 101 cells/μL; human immunodeficiency virus-RNA 168 copies/mL; hemoglobin 11 g/mL; amylase, lipase, and liver function test were within normal limits.
Computed tomography scan showed a prominent concentric gastric wall thickening, and follow up upper endoscopy revealed a circumferential mass in the gastric body extending into antrum and fundus.
Hematoxylin and eosin examination revealed spindle cell proliferation with extensive lymphoplasmocytic cell infiltrates, and immunohistochemical testing confirmed human herpes virus 8 latent nuclear antigen (HHV8 LNA) expression supporting the diagnosis of gastrointestinal Kaposi’s sarcoma (GI-KS).
Patient was treated with highly active antiretroviral therapy and medical oncology referral was made to initiate systemic chemotherapy.
Early diagnosis and treatment of GI-KS has shown to improve patient outcome. Since the majority of patients are asymptomatic, screening endoscopies are being performed in Japan. One retrospective study suggested using specific clinical factors to predict the occurrence of GI-KS which should be confirmed in a larger trial.
The HHV8 LNA stain is a confirmatory immunohistochemical test which is used to distinguish GI-KS from mimickers such as gastrointestinal stromal tumor, spindle cell melanoma, and angiosarcoma.
This case suggests clinicians should maintain a high index of suspicion for GI-KS and a more judicious use of endoscopy may be helpful in improving patient outcome. Specific clinical factors have been shown to help predict the occurrence of GI-KS, which allows clinicians to identify at-risk patients for endoscopic evaluation. However, this should be confirmed in a larger trial.
It is a good case report of a gastrointestinal KS. Moreover, It is performed a comprehensive review and updating of literature about this.
P- Reviewer: Munoz M, Ohkoshi S S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Balachandra B, Tunitsky E, Dawood S, Hings I, Marcus VA. Classic Kaposi’s sarcoma presenting first with gastrointestinal tract involvement in a HIV-negative Inuit male--a case report and review of the literature. Pathol Res Pract. 2006;202:623-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Levine AM, Tulpule A. Clinical aspects and management of AIDS-related Kaposi’s sarcoma. Eur J Cancer. 2001;37:1288-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Berberi A, Noujeim Z. AIDS: An Epidemiologic and Correlation Between HIV- Related Oral Lesions and plasma levels of CD4, CD8 T Lymphocytes Counts and Ratio Among 50 Patients. Br J Med Med Res. 2015;6:859-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Stebbing J, Sanitt A, Nelson M, Powles T, Gazzard B, Bower M. A prognostic index for AIDS-associated Kaposi’s sarcoma in the era of highly active antiretroviral therapy. Lancet. 2006;367:1495-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Parente F, Cernuschi M, Orlando G, Rizzardini G, Lazzarin A, Bianchi Porro G. Kaposi’s sarcoma and AIDS: frequency of gastrointestinal involvement and its effect on survival. A prospective study in a heterogeneous population. Scand J Gastroenterol. 1991;26:1007-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Martin-Carbonero L, Barrios A, Saballs P, Sirera G, Santos J, Palacios R, Valencia ME, Alegre M, Podzamczer D, González-Lahoz J. Pegylated liposomal doxorubicin plus highly active antiretroviral therapy versus highly active antiretroviral therapy alone in HIV patients with Kaposi’s sarcoma. AIDS. 2004;18:1737-1740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Nagata N, Shimbo T, Yazaki H, Asayama N, Akiyama J, Teruya K, Igari T, Ohmagari N, Oka S, Uemura N. Predictive clinical factors in the diagnosis of gastrointestinal Kaposi’s sarcoma and its endoscopic severity. PLoS One. 2012;7:e46967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Stănescu L, Foarfă C, Georgescu AC, Georgescu I. Kaposi’s sarcoma associated with AIDS. Rom J Morphol Embryol. 2007;48:181-187. [PubMed] [Cited in This Article: ] |
| 9. | Centers for Disease Control (CDC). Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men--New York City and California. MMWR Morb Mortal Wkly Rep. 1981;30:305-308. [PubMed] [Cited in This Article: ] |
| 10. | Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi‘s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 800] [Cited by in F6Publishing: 812] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 11. | Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865-1869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4341] [Cited by in F6Publishing: 4016] [Article Influence: 133.9] [Reference Citation Analysis (0)] |
| 12. | Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342:1027-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 584] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Gompels UA, Kasolo FC. HHV-8 serology and Kaposi’s sarcoma. Lancet. 1996;348:1587-1588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Calabrò ML, Sheldon J, Favero A, Simpson GR, Fiore JR, Gomes E, Angarano G, Chieco-Bianchi L, Schulz TF. Seroprevalence of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 in several regions of Italy. J Hum Virol. 1998;1:207-213. [PubMed] [Cited in This Article: ] |
| 15. | Andreoni M, El-Sawaf G, Rezza G, Ensoli B, Nicastri E, Ventura L, Ercoli L, Sarmati L, Rocchi G. High seroprevalence of antibodies to human herpesvirus-8 in Egyptian children: evidence of nonsexual transmission. J Natl Cancer Inst. 1999;91:465-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Dilnur P, Katano H, Wang ZH, Osakabe Y, Kudo M, Sata T, Ebihara Y. Classic type of Kaposi’s sarcoma and human herpesvirus 8 infection in Xinjiang, China. Pathol Int. 2001;51:845-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic kaposi sarcoma: epidemiology and risk factors. Cancer. 2000;88:500-517. [PubMed] [Cited in This Article: ] |
| 18. | Schulz TF. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8): epidemiology and pathogenesis. J Antimicrob Chemother. 2000;45 Suppl T3:15-27. [PubMed] [Cited in This Article: ] |
| 19. | Penn I. Kaposi’s sarcoma in transplant recipients. Transplantation. 1997;64:669-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 166] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Montagnino G, Bencini PL, Tarantino A, Caputo R, Ponticelli C. Clinical features and course of Kaposi’s sarcoma in kidney transplant patients: report of 13 cases. Am J Nephrol. 1994;14:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Lesnoni La Parola I, Masini C, Nanni G, Diociaiuti A, Panocchia N, Cerimele D. Kaposi’s sarcoma in renal-transplant recipients: experience at the Catholic University in Rome, 1988-1996. Dermatology. 1997;194:229-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Tappero JW, Conant MA, Wolfe SF, Berger TG. Kaposi’s sarcoma. Epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 225] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Errihani H, Berrada N, Raissouni S, Rais F, Mrabti H, Rais G. Classic Kaposi’s sarcoma in Morocco: clinico-epidemiological study at the National Institute of Oncology. BMC Dermatol. 2011;11:15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Friedman SL, Wright TL, Altman DF. Gastrointestinal Kaposi’s sarcoma in patients with acquired immunodeficiency syndrome. Endoscopic and autopsy findings. Gastroenterology. 1985;89:102-108. [PubMed] [Cited in This Article: ] |
| 25. | Taccogna S, Crescenzi A, Stasi R, Turrini L, Gallo A, Rossi Z. Kaposi sarcoma of the stomach: a case report. BMJ Case Rep. 2009;. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | López de Blanc S, Sambuelli R, Femopase F, Luna N, Gravotta M, David D, Bistoni A, Criscuolo MI. Bacillary angiomatosis affecting the oral cavity. Report of two cases and review. J Oral Pathol Med. 2000;29:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Parfitt JR, Rodriguez-Justo M, Feakins R, Novelli MR. Gastrointestinal Kaposi’s sarcoma: CD117 expression and the potential for misdiagnosis as gastrointestinal stromal tumour. Histopathology. 2008;52:816-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Barillari G, Buonaguro L, Fiorelli V, Hoffman J, Michaels F, Gallo RC, Ensoli B. Effects of cytokines from activated immune cells on vascular cell growth and HIV-1 gene expression. Implications for AIDS-Kaposi’s sarcoma pathogenesis. J Immunol. 1992;149:3727-3734. [PubMed] [Cited in This Article: ] |
| 29. | Jacobson LP, Yamashita TE, Detels R, Margolick JB, Chmiel JS, Kingsley LA, Melnick S, Muñoz A. Impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma and non-Hodgkin’s lymphomas among HIV-1-infected individuals. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1999;21 Suppl 1:S34-S41. [PubMed] [Cited in This Article: ] |
| 30. | Nasti G, Martellotta F, Berretta M, Mena M, Fasan M, Di Perri G, Talamini R, Pagano G, Montroni M, Cinelli R. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440-2446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Volberding PA, Abrams DI, Conant M, Kaslow K, Vranizan K, Ziegler J. Vinblastine therapy for Kaposi’s sarcoma in the acquired immunodeficiency syndrome. Ann Intern Med. 1985;103:335-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 100] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Lassoued K, Clauvel JP, Katlama C, Janier M, Picard C, Matheron S. Treatment of the acquired immune deficiency syndrome-related Kaposi’s sarcoma with bleomycin as a single agent. Cancer. 1990;66:1869-1872. [PubMed] [Cited in This Article: ] |
| 33. | Remick SC, Reddy M, Herman D, Grace C, Harper G, Willis K, Candon B, Horton J, Ruckdeschel JC. Continuous infusion bleomycin in AIDS-related Kaposi’s sarcoma. J Clin Oncol. 1994;12:1130-1136. [PubMed] [Cited in This Article: ] |
| 34. | Gill PS, Rarick M, McCutchan JA, Slater L, Parker B, Muchmore E, Bernstein-Singer M, Akil B, Espina BM, Krailo M. Systemic treatment of AIDS-related Kaposi’s sarcoma: results of a randomized trial. Am J Med. 1991;90:427-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 116] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, Kaplan LD, Du Mond C, Mamelok RD, Henry DH. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16:2445-2451. [PubMed] [Cited in This Article: ] |
| 36. | Stewart S, Jablonowski H, Goebel FD, Arasteh K, Spittle M, Rios A, Aboulafia D, Galleshaw J, Dezube BJ. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J Clin Oncol. 1998;16:683-691. [PubMed] [Cited in This Article: ] |
| 37. | Martín-Carbonero L, Palacios R, Valencia E, Saballs P, Sirera G, Santos I, Baldobí F, Alegre M, Goyenechea A, Pedreira J. Long-term prognosis of HIV-infected patients with Kaposi sarcoma treated with pegylated liposomal doxorubicin. Clin Infect Dis. 2008;47:410-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Gill PS, Tulpule A, Espina BM, Cabriales S, Bresnahan J, Ilaw M, Louie S, Gustafson NF, Brown MA, Orcutt C. Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi’s sarcoma. J Clin Oncol. 1999;17:1876-1883. [PubMed] [Cited in This Article: ] |
| 39. | Medveczky MM, Horvath E, Lund T, Medveczky PG. In vitro antiviral drug sensitivity of the Kaposi‘s sarcoma-associated herpesvirus. AIDS. 1997;11:1327-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Kedes DH, Ganem D. Sensitivity of Kaposi’s sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J Clin Invest. 1997;99:2082-2086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 227] [Article Influence: 8.4] [Reference Citation Analysis (0)] |