Published online Aug 6, 2013. doi: 10.4292/wjgpt.v4.i3.61
Revised: April 24, 2013
Accepted: May 8, 2013
Published online: August 6, 2013
Processing time: 168 Days and 2.6 Hours
AIM: To study the outcomes of primary sclerosing cholangitis (PSC) patients with ulcerative colitis (UC) undergoing colectomy.
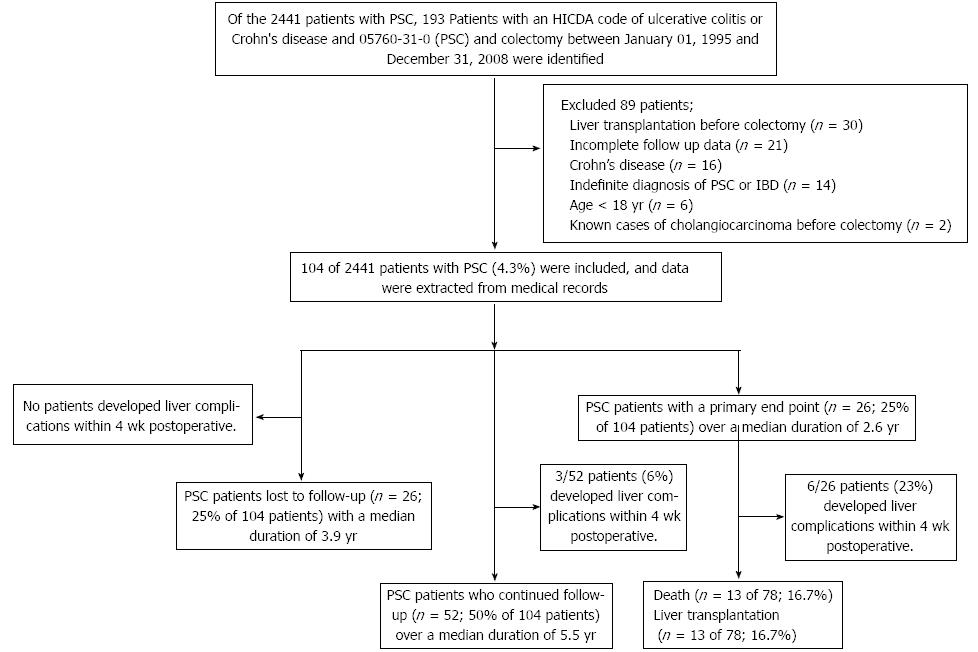
METHODS: We identified 193 patients with PSC and UC undergoing colectomy at the Mayo Clinic (Rochester, MN, United States), between January 1, 1995 and December 31, 2008 using a computerized record system. Eighty-nine patients were excluded due to unclear diagnosis, liver transplantation prior to colectomy, age less than 18 years, inadequate follow-up data or known cases of cholangiocarcinoma. We retrospectively reviewed data from patient medical records. Clinical information, date of colectomy, preoperative and follow-up liver tests and pathological findings of the colon were reviewed. The Mayo risk score at baseline was calculated to obtain survival estimates for up to 4 years of follow-up. The primary endpoint was defined by the presence of all-cause mortality and/or liver decompensation requiring liver transplantation. All patients who did not have a clinical note on December 31, 2008 were considered as patients with an incomplete follow-up unless they reached a study endpoint (death or underwent liver transplantation) prior to that date. The study was approved by the Institutional Review Boards of the Mayo Clinic.
RESULTS: Of the 2441 patients with PSC observed in this period, 104 patients (4.3%) had UC and underwent colectomy and were included. The median age was 43.2 years, and 67% were male. The leading indications for colectomy were severe colonic inflammation (49%), the presence of colonic dysplasia during routine surveillance (42%) and bowel perforation (3%). Twenty-six patients were lost to follow-up after a median duration of 3.9 years. The remaining 78 patients included 52 patients (66.7%) who were followed for a median duration of 5.5 years and 26 patients (33.3%) who developed primary endpoints including death (n = 13) or underwent liver transplantation (n = 13) with a median follow up of 2.6 years. For the secondary endpoint, the liver complications within 1 mo following the colectomy were found in 9 patients (8.6%) and included worsening liver tests (n = 3), liver failure requiring liver transplantation (n = 2), acute cholangitis (n = 3) and right hepatic vein thrombosis with hepatic infarct (n = 1). A multivariate logistic analysis demonstrated that only lower platelet count and lower albumin level preoperatively were significantly associated with more primary endpoints (OR = 0.99 and 0.05 respectively).
CONCLUSION: One third of patients with PSC and UC undergoing colectomy died or underwent liver transplantation within 2.6 years. PSC patients with lower platelet counts and lower albumin levels were significantly more likely to have a poorer outcome.
Core tip: To study the outcomes of primary sclerosing cholangitis (PSC) patients with ulcerative colitis (UC) undergoing colectomy. We identified 193 patients with PSC and UC undergoing colectomy at the Mayo Clinic (Rochester, MN, United States), between January 1, 1995 and December 31, 2008. Eighty-nine patients were excluded. Of the 2441 patients with PSC, 104 patients (4.3%) had UC and underwent colectomy and were included. The median age was 43.2 years. One third of patients with PSC and UC undergoing colectomy died or underwent liver transplantation within 2.6 years. PSC patients with lower platelet counts and lower albumin levels were significantly more likely to have a poorer outcome.
- Citation: Treeprasertsuk S, Björnsson E, Sinakos E, Weeding E, Lindor KD. Outcome of patients with primary sclerosing cholangitis and ulcerative colitis undergoing colectomy. World J Gastrointest Pharmacol Ther 2013; 4(3): 61-68
- URL: https://www.wjgnet.com/2150-5349/full/v4/i3/61.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v4.i3.61
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease and is associated with inflammatory bowel disease (IBD) in 60%-80% of patients[1-3]. Ulcerative colitis (UC) is more commonly prevalent than Crohn’s disease (CD) in patients with PSC[4,5]. The colitis associated with PSC has unique findings and is usually extensive[4,6]. UC in patients with PSC is associated with an increased risk of colorectal neoplasia compared to patients with UC alone (OR = 4.8)[7]. The incidence of colorectal neoplasia at 5 years in PSC patients with IBD is significantly higher than in patients with UC alone (33% vs 13%, P = 0.054; borderline statistical significance by unmatched log rank test)[4].
A recent study reported that PSC was the third leading cause (15.4%) of abnormal liver tests among 545 patients with underlying IBD undergoing colectomy with ileal pouch-anal anastomosis (IPAA), after a transient elevation of liver tests (49%) and fatty liver (15.4%)[8]. Another study evaluated the progression of liver disease after proctocolectomy in patients with PSC and UC[9]. After proctocolectomy with IPAA, they found that 5 of 30 patients (16.7%) underwent liver transplantation at intervals of 1 to 11 years[9]. Previous studies have shown that patients with liver cirrhosis can experience worsening of their liver disease after surgery and poor outcomes[10,11]. Surgery may lead to severe complications such as decompensated liver disease, worsening of a pre-existing decompensation or even death. Very limited information exists on the prognosis of patients with PSC and UC undergoing colectomy[12]. We aimed to assess the outcomes and predictors of outcomes of PSC patients undergoing colectomy at the Mayo Clinic, Rochester, MN, United States.
This was a retrospective study using a computerized record system of patients who had been diagnosed with PSC and UC and were undergoing colectomy at the Mayo Clinic, Rochester, MN, United States, between January 1, 1995 and December 31, 2008. PSC was defined as present when all the following criteria were met: (1) chronic cholestatic disease of at least six months’ duration; (2) elevation of serum alkaline phosphatase (ALP) levels; (3) retrograde, operative, percutaneous or magnetic resonance cholangiography demonstrating intrahepatic and/or extrahepatic biliary duct obstruction, beading or narrowing consistent with PSC; and (4) exclusion of secondary sclerosing cholangitis or other causes of cholestatic liver diseases[3].
A diagnosis of PSC was made using the Hospital International Classification of Disease Adaptation (HICDA) codes of 05760310. A diagnosis of IBD was based on the following HICDA codes: cholangitis, sclerosing (05760310); disease, Crohn’s, nos (05630110); enteritis, regional, nos (05630111); ileitis (regional)-see also enteritis (05630112); colitis, Crohn’s (05630113); disease, Crohn’s, recurrent (05630120); enteritis, regional, recurrent (05630121); colitis, ulcerative, chronic-cuc (05631110); colitis, ulcerative, nos (05631120); colitis, thrombo-ulcerative (05631121); colitis, ulcerative, acute (05631130); colitis, granulomatous (05632110); disease, granulomatous, colon (05632111); disease, inflammatory bowel, nos (05639212). HICDA is an adaptation of the International Classification of Diseases (ICD)-8 for hospital morbidity, which was used at Mayo Clinic to maintain continuity of the Medical Index and the Rochester Epidemiology Project for on-going longitudinal studies[13]. Of the 2441 patients with PSC, we identified 193 PSC patients with IBD (7.9%) who had undergone colectomy and retrospectively reviewed data from their medical records.
We retrospectively reviewed data from the medical records. A detailed history and physical examination was recorded by a health care provider using standardized protocols. Clinical information, date of colectomy, preoperative and follow-up liver tests and pathological findings of the colon were reviewed. The Mayo risk score at baseline was calculated to obtain survival estimates through up to 4 years of follow-up. The Mayo risk score calculations can be accessed from the web site: http://www.mayoclinic.org/gi-rst/mayomodel3.html, and the MELD model/UNOS modification can be accessed from http://www.mayoclinic.org/ meld/mayomodel6.html.
We included PSC patients who underwent colectomy and had results of preoperative liver tests and at least one post-operatively. Colectomy cases included open or laparoscopic colectomy. All included patients must have had at least one follow-up visit after the colectomy. The liver tests included total bilirubin, direct bilirubin, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and ALP levels in the serum.
Of the 193 patients with PSC and UC undergoing colectomy, we excluded the patients with the followings: underwent liver transplantation prior to colectomy, inadequate follow-up data, CD, age less than 18 years and known cases of cholangiocarcinoma.
The primary endpoint was defined as the presence of all-cause mortality and/or liver decompensation requiring liver transplantation and it has been measured at 1 mo and at the end of follow-up. All causes of death listed on the death certificates or pathological findings (underlying, intermediate, immediate and other major conditions) were recorded using the ICD-10 revision.
The secondary end point was defined as the presence of liver complications post-operatively occurred within 1 mo which included ascites, variceal bleeding, clinical hepatic encephalopathy or liver failure and required hospitalization. The worsening liver tests were defined as increases in AST, ALT or total bilirubin to at least 2-fold greater than the baseline values. Other information including the the length of hospital stay and general postoperative complications were recorded.
All patients who did not have a clinical note on December 31, 2008 (the end of follow up in this study) were considered as patients with incomplete follow up unless they developed an endpoint (death or underwent liver transplantation) prior to that date. The study was approved by the Institutional Review Boards of the Mayo Clinic, and all participants provided permission for their medical information to be used for research.
Statistical analyses were performed with SPSS version 15.0 software. Subjects were categorized by the presence or absence of primary endpoints. Continuous variables were presented as the mean ± SD or median [interquartile range (IQR)] as appropriate. Comparisons between the two groups were performed using independent t tests if values were normally distributed or by the Wilcoxon rank sum test if the distribution was not normal. Categorical data were presented as numbers (percentage) and were compared by Fisher’s exact test or the χ2 test where appropriate. All tests were two sided, and the chosen level of significance was P < 0.05. A logistic regression analysis was used to identify factors significantly associated with the presence of primary endpoints in PSC patients with UC undergoing colectomy. Only variables with a P value < 0.1 in a univariate analysis were included in the multivariate analysis. We estimated receiver operating characteristic (ROC) curves of related variables for detection of the primary endpoints in patients with PSC to maximize the area under the curve (AUC).
Of the 2441 patients with PSC, 193 patients with PSC and UC undergoing colectomy were identified. Eighty-nine patients were excluded due to liver transplantation prior to colectomy (n = 30), inadequate follow-up data (n = 35), CD (n = 16), age less than 18 years (n = 6) and known cases of cholangiocarcinoma (n = 2). The remaining of 104 patients (4.3% of 2441 PSC patients) were included in this study. The median age was 43.2 years, and 67% were male. The demographic and biochemical data of the 104 patients are shown in Table 1. The median (IQR) Mayo risk score was -0.05 (-0.7, 1.1) while the median (IQR) MELD score was 9 (6, 12). The leading indications for colectomy were severe colonic inflammation (49%), colonic dysplasia observed during routine surveillance (42%) and bowel perforation (3%). Most of the preoperative total bilirubin, direct bilirubin and albumin levels were within normal range, while the mean ALP value was two fold greater than normal.
| Patient characteristics1 | Total (n = 104) | Patients who continued follow-up or developed primary endpoints (n = 78) |
| Baseline characteristics data | ||
| Age at colectomy (yr) | 43 (30-53) | 42 (28-52) |
| Gender, male | 70 (67) | 56 (72) |
| Race, Caucasian | 98 (94) | 76 (97) |
| Presence of advanced liver fibrosis at baseline | 27 (32) | 24 (39) |
| Mayo risk score at baseline | -0.05 (-0.7, 1.1) | -0.001 (-0.8, 1.4) |
| BMI (kg/m2) | 25 (22.6, 28.6) | 24.7 (22, 27.4) |
| Previous use of immunosuppressive drugs | 38 (36.5) | 29 (37) |
| History of receiving ursodeoxycholic acid | 33 (31.7) | 26 (33.3) |
| Diabetes mellitus or impaired glucose tolerance | 7 (7) | 6 (7.6) |
| History of current smoking | 2 (2) | 2 (2.6) |
| Indication for colectomy | ||
| Severe colonic inflammation | 51 (49) | 37 (47) |
| Colonic dysplasia | 44 (42) | 36 (46) |
| Bowel perforation | 3 (2.8) | 3 (3.8) |
| Other indications | 6 (5.7) | 2 (2.6) |
| Laboratory tests at baseline | ||
| ALT (< 40 U/L) | 70 (43, 113) | 73 (43, 135) |
| AST (< 40 U/L) | 50 (30, 96) | 55 (32, 100) |
| Albumin (g/dL) | 3.9 (3.5, 4.2) | 3.9 (3.5, 4.2) |
| Total bilirubin (mg/dL) | 0.7 (0.5, 1.5) | 0.8 (0.5, 1.9) |
| Direct bilirubin (mg/dL) | 0.2 (0.1, 0.7) | 0.3 (0.1, 0.9) |
| ALP (U/L) | 359 (194, 657) | 385 (197, 839) |
| Glucose (mg/dL) | 93 (86, 106) | 93.5 (86, 107) |
| Creatinine (mg/dL) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) |
| CA 19-9 (normal < 55 U/mL) | 17.2 (8.8, 51) | 16.8 (8.5, 51) |
| Platelet (× 109/L) | 289 (201, 350) | 289 (205, 351) |
| INR | 1 (0.9, 1.1) | 1 (0.9, 1.1) |
| Clinical outcomes at the end of follow-up | ||
| Undergoing Ileal pouch-anal anastomosis | 78 (75) | 58 (74) |
| Length of hospital stay (d) | 7 (6, 11) | 8 (5, 12) |
| New diagnosis of malignancy after colectomy | 32 (30.8) | 26 (33.4) |
| Colorectal cancer | 19 (18.3) | 13 (16.7) |
| Other malignancy | 13 (12.5) | 13 (16.7) |
| Pathological findings | ||
| Colonic inflammation | 62 (60) | 45 (57.7) |
| Presence of colonic dysplasia | 24 (23) | 21 (27) |
| Colon cancer | 19 (18.3) | 12 (15.4) |
| Post-operative general complications within 1 mo | 36 (34.6) | 34 (43.6) |
| Post-operative liver complications within 1 mo | 9 (8.7)2 | 9 (11.5) |
| Results of follow-up | ||
| All-cause mortality | 13 (12.5) | 13 (16.7) |
| Liver transplantation | 13 (12.5) | 13 (16.7) |
| Continued follow-up | 52 (50) | 52 (66.6) |
| Lost to follow-up | 13 (25.0) | - |
Table 1 summarizes the postoperative clinical outcomes of the 104 patients with a median (IQR) hospital stay of 7 d (6, 11). Of 104 patients with PSC and UC, 26 were lost to follow-up after a median duration of 3.9 years. The remaining 78 patients included 52 patients (66.7%) who continued follow up, with a median duration of 5.5 years, and 26 patients developed primary endpoints including death or underwent liver transplantation (33.3%), with a median follow up of 2.6 years (Figure 1). The causes of death of the 13 patients were liver-related complications: hepatocellular carcinoma, hepatic renal syndrome and/or liver failure (n = 4), metastatic cancer to the liver (n = 5), acute cholangitis (n = 1), amyloidosis (n = 1) and unknown causes (n = 2). Two patients died at 10 and 20 d following colectomy. For the secondary endpoint, the liver complications within 1 mo following the colectomy were found in 9 patients (8.6%) and included worsening liver tests (n = 3), liver failure requiring liver transplantation (n = 2), acute cholangitis (n = 3) and right hepatic vein thrombosis with hepatic infarct (n= 1) (Table 2). General postoperative complications were found in 36 patients (34.6%) within 1 mo. The most common complications were anemia or blood loss requiring blood transfusion (n = 11; 10.6%), intra-abdominal abscess requiring drainage (n = 4; 3.8%), acute bowel obstruction requiring re-exploration (n = 4; 3.8%), bowel ileus (n = 4; 3.8%), high ileostomy output (n = 3; 2.8%), wound infection or delayed wound healing (n = 3; 2.8%) and other complications (n = 8; 7.7%) including urinary retention (n = 3), fever with unknown causes (n = 2), acute pancreatitis (n = 1), abdominal pain with unknown causes (n = 1) and portal vein thrombosis (n = 1).
| Case, Sex | Age at colectomy (yr) | Presence of advanced liver fibrosis | Pathological findings in the colon | Blood loss requiring transfusion | Liver complications | Other complications | Length of stay (d) | Presence of primary endpoints | Duration of follow up (yr) |
| 1, M | 28 | Yes | Moderate inflammation | No | Worsening liver tests | Abdominal pain, Dehydration | 18 | No | 8.5 |
| 2, M | 28 | Yes | Transverse colon cancer grade 3/4 T3N2 | Yes; 2 units | Worsening liver tests | High ileostomy output | 15 | Death; colon cancer metastasis to liver | 1.2 |
| 3, F | 52 | Yes | Mild inflammation | No | Worsening liver tests | Delayed wound healing, Blood loss | 8 | Death; liver failure | 0.3 |
| 4, M | 32 | No | Moderate inflammation | No | Acute cholangitis | None | 9 | Liver transplant | 1.1 |
| 5, M | 33 | Yes | Moderate inflammation | No | Acute cholangitis | None | 6 | No | 8.3 |
| 6, F | 54 | No | Mild inflammation | Yes; 6 units | Liver failure | Severe blood loss, shock | 8 | Liver transplant; liver failure | 0.3 |
| 7, F | 21 | Yes | Necrotized distal ileum with perforation | Yes; 9 units | Liver failure | DIC, Respiratory failure, GI-bleeding | 30 | Death; liver failure | 0.03 (12 d) |
| 8, F | 21 | No | Moderate inflammation | Yes; 2 units | SMV and hepatic vein thrombosis | Anemia, | 15 | Death; liver failure | 8 |
| 9, M | 41 | No | Moderate inflammation | No | Acute cholangitis | Wound infection | 10 | No | 3.6 |
By the end of the follow-up of patients with PSC and UC who underwent colectomy, 13 patients developed colorectal cancer (15%) and 13 patients (16.7%) were diagnosed with other malignancies. The primary location of the malignancies were cholangiocarcinoma (n = 6), hematologic malignancy (n = 4), gallbladder cancer (n = 1), hepatocellular carcinoma (n = 1) and intradural extramedullary spinal cord tumor (n = 1). Colonic dysplasia was found in 21 patients (21.2%) including low-grade dysplasia in 16 and high grade dysplasia in 5.
Table 3 shows the comparison of clinical characteristics of the 78 PSC patients with UC who underwent colectomy based on the presence of primary endpoints. Table 4 shows the results of 3 models from the multivariate analysis to identify the best-fit model for predictors of primary endpoints. Model 1 was the best-fit model, which found that only a higher platelet count and higher albumin level preoperatively were significantly associated with fewer primary endpoints (OR = 0.99 and 0.05, respectively; P < 0.05). Using the ROC curves for the detection of primary endpoints, we found that a preoperative platelet count of 194 × 109/L was the best cutoff value based on a sensitivity of 46%, a specificity of 88.5%, a positive predictive value (PPV) of 66.7%, and an negative predictive value (NPV) of 76.7% with an AUC of 0.67. The best cutoff value of the preoperative albumin level for the presence of primary endpoints was 3.7 g/dL with a sensitivity of 73%, a specificity of 82%, a PPV of 70%, an NPV of 84%, and an AUC of 0.80.
| Clinical characteristics1 | Without primary endpoints (n = 52) | With primary endpoints (n = 26) | P value2 |
| Gender, %female | 11 (21) | 11 (42) | 0.052 |
| Age at colectomy (yr) | 38.7 (27.8, 51.6) | 45.8 (29.4, 52) | 0.40 |
| Presence of advanced liver fibrosis | 12 (23) | 12 (46) | 0.032 |
| Pre-operative Mayo risk score | -0.1 (-0.9, 0.9) | 1.3 (-0.2, 2.2) | 0.012 |
| Pre-operative MELD score | 7 (6, 9) | 14 (11, 18) | < 0.0012 |
| History of anemia or blood loss requiring a post-operative blood transfusion within 1 mo | 4 (7.7) | 7 (27) | 0.022 |
| Post-operative liver complications within 1 mo | 3 (5.8) | 6 (23) | 0.022 |
| Length of hospital stay (d) | 7 (5, 10) | 9 (7, 15) | 0.07 |
| Hemoglobin (g/dL) | 13.1 (11.9, 14.4) | 10.8 (9.8, 13.1) | < 0.0012 |
| Platelet count (× 109/L) | 296 (247, 357) | 244 (126, 337) | 0.022 |
| INR | 0.9 (0.9, 1.0) | 1.1 (1.1, 1.3) | < 0.0012 |
| Total bilirubin (mg/dL) | 0.7 (0.5, 1.3) | 2.3 (0.6, 5.0) | 0.0012 |
| Direct bilirubin (mg/dL) | 0.2 (0.1, 0.4) | 0.9 (0.2, 3.5) | 0.0022 |
| ALP (U/L) | 352 (180, 494) | 709 (276, 1232) | 0.0032 |
| AST (U/L) | 44 (31, 90) | 80 (36, 129) | 0.09 |
| Albumin (g/dL) | 4.1 (3.4, 4.3) | 3.5 (3.3, 3.9) | < 0.0012 |
| Duration of follow up from colectomy to the last follow-up (yr) | 5.5 (3.8, 8.8) | 2.6 (0.8, 5.6) | 0.0072 |
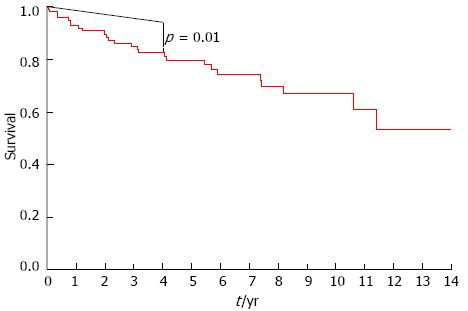
Figure 2 shows the survival curve of the 104 patients with PSC and UC who underwent colectomy. The smooth line represents median survival estimates calculated from the Mayo risk scores at baseline, and the stepped line corresponds to survival per the Kaplan-Meier method. The two survival curves were found to significantly differ over this time period (P = 0.01) which indicated that PSC patients with UC who underwent colectomy died or required liver transplantation more often than those PSC patients with UC who had no colectomy regarding to the same baseline calculated Mayo risk scores.
Our study indicates that one third of PSC patients with UC who underwent colectomy died or required liver transplantation within an average interval of 2.6 years. This result was similar to a previous study from the Cleveland Clinic showing that 38% of cirrhotic patients with PSC who underwent colectomy experienced early postoperative death compared to 0% of non-cirrhotic patients[14]. However, the previous study was limited by the small number of included patients with PSC who underwent colectomy (n = 24) and the need for a preoperative diagnosis of cirrhosis. The present study builds on previous reports from our center regarding the risk of colectomy in patients with PSC and UC[15,16].
However, three previous studies reported that proctocolectomy had little effect on the progression of liver disease in patients with PSC and UC and there was no significant difference in the survival of patients undergoing colectomy compared to unoperated patients[17-20]. A study from England showed that PSC patients who underwent colectomy prior to or concurrent with liver transplantation (n = 17) had a mortality rate of 12%, and they concluded that colectomy was a relatively safe procedure and believed that considering colectomy pre-, during, or shortly after liver transplantation in selected patients with risk factors for colorectal cancer would reduce the risk of colorectal cancer[19]. The low colectomy rate of 4% in our study might reflect the usually quiescent colitis in PSC. The majority of our patients were the large duct PSC which might have an impact on the poorer outcome from liver complications[9,21,22]. Recently, the outcomes after elective colectomy in patients with cirrhosis were examined and showed that cirrhotic patients undergoing colectomy had a 3.7-fold increased risk of death (HR of 3.7; 95%CI: 2.6-5.2)[23]. The in-hospital mortality (6%), length of stay (9 d), and total expenses of cirrhotic patients were significantly higher than for those without cirrhosis[23,24].
Our results showed that a lower platelet count and lower albumin level preoperatively were associated with poorer outcomes. Thus, using simple preoperative blood test screening may provide useful information for monitoring patients pre-operatively. The timing of colectomy is an important issue. Recently, a study from Italy[20] showed that eight of 16 patients with PSC and UC post-liver transplantation had active colitis despite immunosuppressive medications with a median interval from liver transplantation to colectomy of 6.5 years. Few studies showed that the colitis condition in PSC patients with UC remained inactive or under controlled of at least 60% of cases after orthotopic liver transplantation[25,26]. Another studied revealed that liver transplantation for PSC independently reduced the need for colectomy (HR = 0.43; 95%CI: 0.25-0.75; P = 0.003)[11] Additionally, the presence of colon carcinoma and high grade dysplasia were more frequent in the non liver transplantation group and this group of patient had increased inflammation of the colonic mucosa at histology (P = 0.011)[10]. Thus, the patients with severe progressive PSC requiring liver transplantation should proceeded for supportive care of colitis and listed for liver transplantation which might reduced the disease activity of UC and the need for colectomy[10,11].
About 17% of our PSC patients with UC developed colorectal cancer, which was similar to a previous report from England showing that the cumulative risks of developing colorectal cancer in patients with an intact colon and IBD were 14% and 17% after 5 and 10 years, respectively[19]. Recent study showed that the colonic neoplasms that developed in PSC-UC patients were spread throughout the colon on colonoscopy and they were found predominantly on right sided colon[5]. Thus, surveillance colonoscopy and biopsies should be performed in patients with PSC and UC at 1-year to 2-year intervals[3].
The main strengths of our study are the inclusion of a large number of PSC patients with PSC and UC and the available clinical data and pathological findings, which were useful for outcome assessment. However, our study is limited by its retrospective nature in a tertiary center, and it contains data derived from multiple physicians from 1995 to 2008, which may have resulted in a selection bias. Additionally, surgeons excluded the colectomy procedure for all patients with poor liver conditions. Second, we included all PSC patients who underwent colectomy and had results from preoperative liver tests and at least one post-operative test, which may explain the small number of patients with liver complications. Thus, further multicenter prospective studies of post-operative liver complications and poor outcomes in patients with PSC and UC undergoing colectomy should be performed to provide clearer guidance for the selection of patients to be referred for a liver transplantation and colectomy rather than colectomy alone.
Unfortunately, we had to exclude a number of patients (10%) who had incomplete data because they were lost to follow-up. Additionally, the Mayo risk score and MELD score could not be calculated annually from our retrospective data therefore the colectomy might changed the progression of the PSC severity which cannot be concluded. Last, we had only a small number of patients with liver complications, and we can therefore not draw a firm conclusion regarding the association between liver complications and poor outcomes.
In conclusion, one third of PSC patients with UC who underwent colectomy died or underwent liver transplantation within an average interval of 2.6 years. PSC patients with advanced liver fibrosis (lower platelet count and lower albumin level) and UC who underwent colectomy were associated with significantly poorer outcomes.
The authors thank Barbara A. Abbott, from Biomedical Statistics and Informatics, Jill Keach, Diaa Elfaki, MD, and Imam, Mohamad, MBBS for providing the list of patients.
The colitis associated with primary sclerosing cholangitis (PSC) has unique findings and is usually extensive. Ulcerative colitis (UC) in patients with PSC is associated with an increased risk of colorectal neoplasia compared to patients with UC alone. Previous studies have shown that patients with liver cirrhosis can experience worsening of their liver disease after surgery and poor outcomes. Surgery may lead to severe complications such as decompensated liver disease, worsening of a pre-existing decompensation or even death. Very limited information exists on the prognosis of patients with PSC and UC undergoing colectomy.
Authors aimed to assess the outcomes and predictors of outcomes of PSC patients undergoing colectomy at the Mayo Clinic, Rochester, MN, United States.
One third of patients with PSC and UC undergoing colectomy died or underwent liver transplantation within 2.6 years. PSC patients with lower platelet counts and lower albumin levels were significantly more likely to have a poorer outcome.
PSC patients with UC who underwent colectomy died or required liver transplantation more often than those PSC patients with UC who had no colectomy regarding to the same baseline calculated Mayo risk scores.
The primary endpoint was defined as the presence of all-cause mortality and/or liver decompensation requiring liver transplantation and it has been measured at 1 mo and at the end of follow-up. All causes of death listed on the death certificates or pathological findings (underlying, intermediate, immediate and other major conditions) were recorded using the International Classification of Diseases-10 revision. The secondary end point was defined as the presence of liver complications post-operatively occurred within 1 mo which included ascites, variceal bleeding, clinical hepatic encephalopathy or liver failure and required hospitalization To concisely and accurately describe, define or explain the specific, unique terms that are not familiar to majority of the readers, but are essential for the readers to understand the article.
Few studies showed that the colitis condition in PSC patients with UC remained inactive or under controlled of at least 60% of cases after orthotopic liver transplantation. Another studied revealed that liver transplantation for PSC independently reduced the need for colectomy. Additionally, the presence of colon carcinoma and high grade dysplasia were more frequent in the non liver transplantation group and this group of patient had increased inflammation of the colonic mucosa at histology. Thus, the patients with severe progressive PSC requiring liver transplantation should proceeded for supportive care of colitis and listed for liver transplantation which might reduced the disease activity of UC and the need for colectomy.
P- Reviewers Braden B, Lakatos PL, Manesis EK, Trastulli S, Yoshida EM S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 544] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 2. | Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, Fleming TR, Fisher LD, Beaver SJ, LaRusso NF. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 426] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 4. | Loftus EV, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 517] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 5. | Navaneethan U, Venkatesh PG, Lashner BA, Remzi FH, Shen B, Kiran RP. Temporal trends in colon neoplasms in patients with primary sclerosing cholangitis and ulcerative colitis. J Crohns Colitis. 2012;6:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Broomé U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006;26:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 383] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 8. | Navaneethan U, Remzi FH, Nutter B, Fazio VW, Shen B. Risk factors for abnormal liver function tests in patients with ileal pouch-anal anastomosis for underlying inflammatory bowel disease. Am J Gastroenterol. 2009;104:2467-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Lepistö A, Kivistö S, Kivisaari L, Arola J, Järvinen HJ. Primary sclerosing cholangitis: outcome of patients undergoing restorative proctocolecetomy for ulcerative colitis. Int J Colorectal Dis. 2009;24:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Marelli L, Xirouchakis E, Kalambokis G, Cholongitas E, Hamilton MI, Burroughs AK. Does the severity of primary sclerosing cholangitis influence the clinical course of associated ulcerative colitis. Gut. 2011;60:1224-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Navaneethan U, Venkatesh PG, Mukewar S, Lashner BA, Remzi FH, McCullough AJ, Kiran RP, Shen B, Fung JJ. Progressive primary sclerosing cholangitis requiring liver transplantation is associated with reduced need for colectomy in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Lindberg J, Stenling R, Palmqvist R, Rutegård J. Early onset of ulcerative colitis: long-term follow-up with special reference to colorectal cancer and primary sclerosing cholangitis. J Pediatr Gastroenterol Nutr. 2008;46:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Melton LJ. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1115] [Cited by in RCA: 1189] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 14. | Post AB, Bozdech JM, Lavery I, Barnes DS. Colectomy in patients with inflammatory bowel disease and primary sclerosing cholangitis. Dis Colon Rectum. 1994;37:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Kartheuser AH, Dozois RR, LaRusso NF, Wiesner RH, Ilstrup DM, Schleck CD. Comparison of surgical treatment of ulcerative colitis associated with primary sclerosing cholangitis: ileal pouch-anal anastomosis versus Brooke ileostomy. Mayo Clin Proc. 1996;71:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Kartheuser AH, Dozois RR, Wiesner RH, LaRusso NF, Ilstrup DM, Schleck CD. Complications and risk factors after ileal pouch-anal anastomosis for ulcerative colitis associated with primary sclerosing cholangitis. Ann Surg. 1993;217:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Martin FM, Rossi RL, Nugent FW, Scholz FJ, Jenkins RL, Lewis WD, Gagner M, Foley E, Braasch JW. Surgical aspects of sclerosing cholangitis. Results in 178 patients. Ann Surg. 1990;212:551-556; discussion 551-556;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Cangemi JR, Wiesner RH, Beaver SJ, Ludwig J, MacCarty RL, Dozois RR, Zinsmeister AR, LaRusso NF. Effect of proctocolectomy for chronic ulcerative colitis on the natural history of primary sclerosing cholangitis. Gastroenterology. 1989;96:790-794. [PubMed] |
| 19. | Vera A, Gunson BK, Ussatoff V, Nightingale P, Candinas D, Radley S, Mayer A, Buckels JA, McMaster P, Neuberger J. Colorectal cancer in patients with inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Transplantation. 2003;75:1983-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Bosso MC, Marchesa PE, Ricchiuti A, Giacardi A, Cocchis D, Campi M, Palisi M, Salizzoni M. Proctocolectomy for ulcerative colitis after liver transplantation for primary sclerosing cholangitis. Transplant Proc. 2009;41:1390-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Tischendorf JJ, Geier A, Trautwein C. Current diagnosis and management of primary sclerosing cholangitis. Liver Transpl. 2008;14:735-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Tanaka A, Takamori Y, Toda G, Ohnishi S, Takikawa H. Outcome and prognostic factors of 391 Japanese patients with primary sclerosing cholangitis. Liver Int. 2008;28:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Csikesz NG, Nguyen LN, Tseng JF, Shah SA. Nationwide volume and mortality after elective surgery in cirrhotic patients. J Am Coll Surg. 2009;208:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Meunier K, Mucci S, Quentin V, Azoulay R, Arnaud JP, Hamy A. Colorectal surgery in cirrhotic patients: assessment of operative morbidity and mortality. Dis Colon Rectum. 2008;51:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Navaneethan U, Choudhary M, Venkatesh PG, Lashner BA, Remzi FH, Shen B, Kiran RP. The effects of liver transplantation on the clinical course of colitis in ulcerative colitis patients with primary sclerosing cholangitis. Aliment Pharmacol Ther. 2012;Mar 19; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Gelley F, Miheller P, Péter A, Telkes G, Nemes B. Activity of ulcerative colitis before and after liver transplantation in primary sclerosing cholangitis: the Hungarian experience. Transplant Proc. 2012;44:2164-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |