Published online Oct 6, 2011. doi: 10.4292/wjgpt.v2.i5.36
Revised: September 20, 2011
Accepted: September 28, 2011
Published online: October 6, 2011
AIM: To study the role of capsaicin-sensitive afferent nerves in Helicobacter pylori (H. pylori) positive chronic gastritis before and after eradication.
METHODS: Gastric biopsy samples were obtained from corpus and antrum mucosa of 20 healthy human subjects and 18 patients with H. pylori positive chronic gastritis (n = 18) before and after eradication. Traditional gastric mucosal histology (and Warthin-Starry silver impregnation) and special histochemical examinations were carried out. Immunohistochemistry for capsaicin receptor (TRVP1), calcitonin gene-related peptide (CGRP) and substance P (SP) were carried out by the labeled polymer immunohistological method (Lab Vision Co., USA) using polyclonal rabbit and rat monoclonal antibodies (Abcam Ltd., UK).
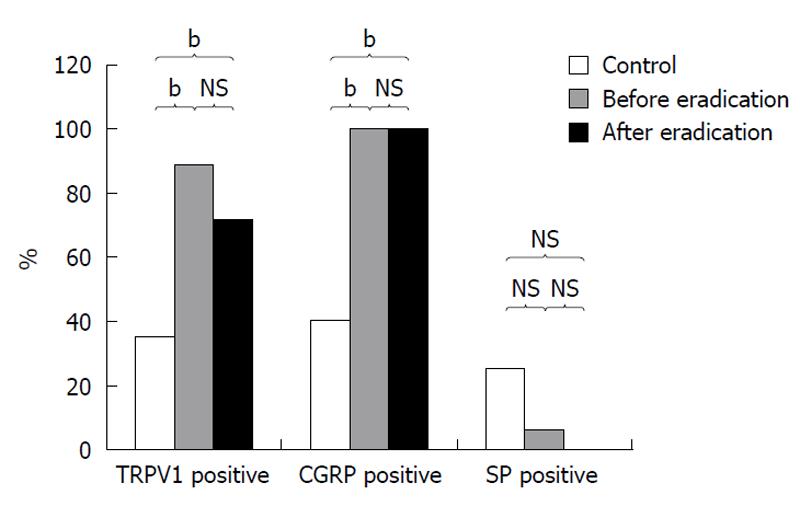
RESULTS: Eradication treatment was successful in 16 patients (89%). Seven patients (7/18, 39%) remained with moderate complaints, meanwhile 11 patients (11/28, 61%) had no complaints. At histological evaluation, normal gastric mucosa was detected in 4 patients after eradication treatment (4/18, 22%), and moderate chronic gastritis could be seen in 14 (14/18, 78%) patients. Positive immuno-staining for capsaicin receptor was seen in 35% (7/20) of controls, 89% (16/18, P < 0.001) in patients before and 72% (13/18, P < 0.03) after eradication. CGRP was positive in 40% (8/20) of controls, and in 100% (18/18, P < 0.001) of patients before and in 100% (18/18, P < 0.001) after eradication. The immune-staining of gastric mucosa for substance-P was positive in 25% (5/20) of healthy controls, and in 5.5% (3/18, P > 0.05) of patients before and in 0% of patients (0/18, P > 0.05) after H. pylori eradication.
CONCLUSION: Distibution of TRVP1 and CGRP is altered during the development of H. pylori positive chronic gastritis. The immune-staining for TRVP1, CGRP and SP rwemained unchanged before and after H. pylori eradication treatment. The capsaicin-sensitive afferentation is an independent from the eradication treatment. The 6 wk time period might not be enough time for the restituion of chronic H. pylori positive chronic gastritis. The H. pylori infection might not represent the main pathological factor in the development of chronic gastritis
-
Citation: Lakner L, Dömötör A, Tóth C, Szabó IL, Meczker &, Hajós R, Kereskai L, Szekeres G, Döbrönte Z, Mózsik G. Capsaicin-sensitive afferentation represents an indifferent defensive pathway from eradication in patients with
H. pylori gastritis. World J Gastrointest Pharmacol Ther 2011; 2(5): 36-41 - URL: https://www.wjgnet.com/2150-5349/full/v2/i5/36.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v2.i5.36
The integrity of gastric mucosa relies on the equilibrium between aggressive and defensive factors. The loss of this balance leads to the development of gastric disorders. Helicobacter pylori (H. pylori), one of the aggressive factors, is a widely spread bacterium. The urease positive, Gram negative and highly motile bacterium is one of the commonest pathogenic bacilli in humans and at least half of the world’s population is infected with this organism[1,2]. H. pylori - as a causative factor - increases the risk for development of human gastrointestinal disorders such as acute gastritis, chronic gastritis, gastro-duodenal ulcer, gastric mucosa-associated lymphoid tissue lymphoma, gastric adenocarcinoma and it has also been implicated in iron deficiency anemia and in extra-gastrointestinal disorders, like atherosclerosis, ischemic heart and cerebrovascular diseases[3-9]. The eradication of this organism has generally been associated with the histological improvement of gastritis[8].
Capsaicin-sensitive afferentation is one of the defensive mechanisms. These nerves have been shown to play a role in gastric mucosal protection by preventing drug-induced mucosal injury in animals[10-12] and by decreasing the amount of indomethacin (IND)-induced gastric microbleeding in healthy human subjects[13-15].
The capsaicin-sensitive afferent nerves have a temperature-gated nonselective cation channel called capsaicin receptor or transient receptor potential vanilloid 1 (TRVP1). This receptor is sensitive not only for capsaicin and other vanilloids, but also for protons, noxious heat and endogenous ligands, like anadarmide, N-oleodopamine or lypoxygenase products[16]. TRVP1 was detected in the area postrema and in the nucleus tractus solitary, where the afferent fibres of the vagus nerve taper. The vagus nerve consists of 10% efferent nerves, 90% afferents. Of these afferent nerves, about 10% are capsaicin-sensitive. The amount of efferent nerves and the capsaicin-sensitive afferent nerves is roughly equal in the vagus nerve. Capsaicin exposure exerts various responses in these afferent nerves depending on dose and exposure duration (excitation, sensory-blocking, long-term selective neurotoxic impairment and irreversible cell destruction)[17]. During exposure to small doses of capsaicin (from ng/kg to μg/kg body weight) neurotransmitters, such as: substance P (SP), calcitonin gene-related peptide (CGRP) and somatostatin, are released from the nerve endings[18-20]. These mediators are responsible for increasing mucosal blood flow by vasodilatation[21], activation of mast cells and other immune cells in the mucosa[22,23] and defense of gastrointestinal mucosa.
The presence of this receptor and of released neuorotransmitters has been studied in the development of human gastrointestinal disorders including gastritis, peptic ulcer, polyp, tumor and inflammatory bowel diseases by immunohistology[24-26]. In our recent work significant changes were observed in the presence of TRVP1, CGRP and SP in patients with chronic H. pylori positive gastritis and in histologically healthy subjects but no change could be detected between the patients who suffered from chronic gastritis without or with H. pylori infection[27]. The effect of omeprazole and omeprazole-like compounds have also been evaluated on changes of TRVP1, SP and CGRP immunodistribution in rats[28].
The aim of our present study was to investigate the changes in the immunohistological distribution of capsaicin receptor and released mediators (CGRP, SP) in the gastric mucosa of healthy subjects and patients with H. pylori positive chronic gastritis before and after H. pylori eradication therapy.
The observations were carried out in 38 persons, including 20 healthy subjects and 18 patients with H. pylori positive gastritis.
Ten males and 10 females with functional dyspepsia were examined at the First Department of Medicine, University of Pécs; no abnormalities were found by physical, laboratory, iconographic or histological methods. This group was taken as histologically healthy controls (age: 41-67 years; mean = 52.1 years).
Eighteen patients with H. pylori positive chronic gastritis underwent physical, laboratory, ultrasonographic, endoscopic and histological examination at the Department of Medicine and Gastroenterology, Markusovszky Teaching Hospital, Szombathely (Hungary). The age of patients (6 males, 12 females) was 39 to 68 years (mean = 56.4 years) and a questionnaire was used to determine the patient’s symptoms. The gastric biopsies from patients with H. pylori positive chronic gastritis were collected from the hyperaemic areas of the gastric corpus and antrum by gastroscopy before and after eradication therapy. The time period between the first and control gastroscopy was 6 wk. Similar gastric biopsies were taken from the corpus and antrum of healthy subjects. H. pylori positive patients underwent 7 d eradication treatment with the combination of double dose proton-pump inhibitor (PPI; pantoprazole 2 × 40 mg/d), amoxycillin (1000 mg twice daily) and clarithromycin (500 mg twice daily) according to current European guidelines[29]. After this 1 wk combination therapy, patients continued to take a normal dose of PPI for another week. The H. pylori infection was detected before and after the eradication therapy using the [14C] urea breath test ([14C] UBT), rapid urease test, Warthin-Starry silver staining and specific histological examinations. The gastric tissue samples were analyzed in the Department of Pathology of Markusovszky Teaching Hospital, Szombathely (Hungary) and classified into different groups of chronic gastritis according to the Sydney’s System[30]. The groups of patients with chronic gastritis and histological healthy persons were established by an independent histopathologist.
The immunohistological studies were carried out on formalin fixed, paraffin embedded tissue samples of gastric mucosa using the peroxidase-labeled polymer method (Lab Vision Co., Fremont, USA). SP was detected by the NC1/34 HL rat monoclonal antibody, the TRVP1 receptor and CGRP were labeled using polyclonal rabbit antisera (all from Abcam Ltd., UK Cambridge) (Table 1). The immunohistochemical examinations were performed in the University of Pécs and in the Histopathology Ltd., Pécs (Hungary).
| Antisera | Abbreviation | Species | Dilution | Source |
| Calcitonin gene-related peptide | CGRP | Rabbit polyclonal | 1:100 | Abcam, Cambridge, UK |
| Substance P | SP | Rat monoclonal | 1:200 | Abcam, Cambridge, UK |
| Transient receptor potential vanilloid 1 | TRVP1 | Rabbit polyclonal | 1:1000 | Abcam, Cambridge, UK |
The human examinations were permitted by the Regional Ethical Committee of University Pécs, Hungary. Written informed consent was obtained from all participants.
TRVP1, SP and CGRP were statistically evaluated by χ2-probe. The results were taken to be significant, if P values were ≤ 0.05.
Before H. pylori eradication, the symptoms of patients with H. pylori positive chronic gastritis were unspecific: epigastrial pain (14/18, 77%), heartburn (13/18, 72%), nausea/vomiting (9/18, 50%) abdominal expansion (9/18, 50%), constipation (6/18, 38%).
Histopathological examination of gastric biopsies from patients with H. pylori positive chronic gastritis obtained before eradication treatment indicated moderate and severe inflammation in the gastric mucosa. The H. pylori eradication therapy was successful in 16 of 18 patients (89%).
After eradication, symptoms were found to remain moderate in seven patients (7/18, 39%) and 11 patients (11/18, 61%) had no complaints after treatment.
Gastroscopy along with gastric biopsies were carried out in all patients after H. pylori eradication. Histologically healthy mucosa could be detected only in 4 (4/18, 22%) patients; the gastric mucosa of the remaining patients (14/18, 78%) still showed moderate chronic gastritis.
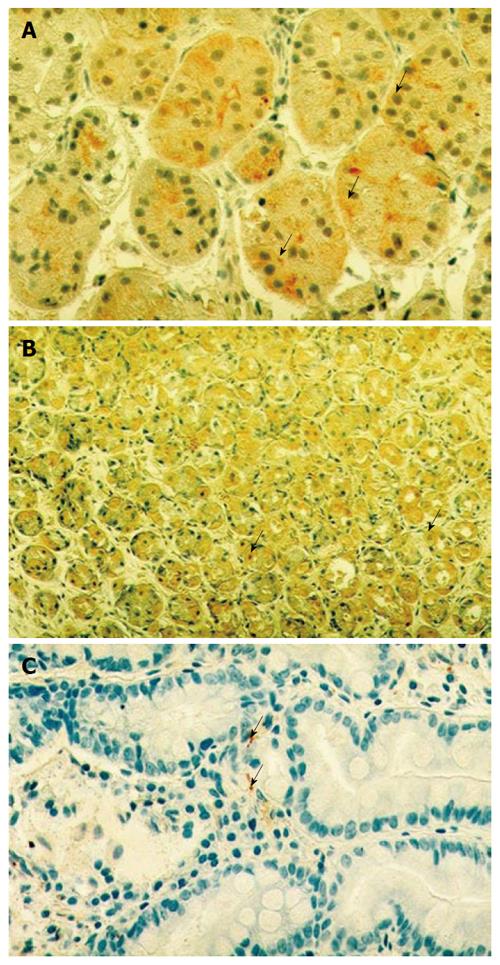
TRVP1 immune-staining indicated a fine cytoplasmic granulation in the epithelial cells of gastric mucosa (Figure 1A). TRVP1 was detected in 37% (7/20) of healthy subjects, whereas H. pylori positive gastritis patients were 89% (16/18, P < 0.001) positive before and 72% (13/18 P < 0.03) positive after eradication therapy.
CGRP staining showed fine positively stained cytoplasmic granules distributed in both the epithelial cells of gastric mucosa of patients with H. pylori positive chronic gastritis and in histologically healthy persons (Figure 1B). Immunohistochemistry for CGRP was positive in 100% (18/18, P < 0.001) of patients both before and after eradication (18/18, P < 0.001). In the mucosa of healthy individuals this staining was found to be positive in 40% (8/18) of controls.
SP was detected as small granular spot-like signals localized along the mucosal blood vessels (Figure 1C). The SP immune-staining was positive in 25% (3/20) of control persons, and in 5.5% (1/18, P > 0.05) before and 0% (0/18, P > 0.05) after eradication in the gastric mucosa.
The results of the immunohistological examinations for TRVP1 receptor and mediators (CGRP and SP) are summarized in Table 2. Interestingly, when compared to normal mucosa, the presence of SP decreased in the mucosa obtained from patients with H. pylori positive chronic gastritis before and after eradication treatment (Figure 2).
| TRVP1 | CGRP | Substance P | ||||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Before eradication (n = 18) | 16 (88.89) | 2 (11.11) | 18 (100) | 0 (0) | 1 (5.56) | 17 (94.44) |
| After eradication (n = 18) | 13 (72.22) | 5 (17.78) | 18 (100) | 0 (0) | 0 (0) | 18 (100) |
| Control group (n = 20) | 7 (35) | 13 (65) | 8 (40) | 12 (60) | 15 (75) | 5 (25) |
The possible role(s) of the capsaicin-sensitive afferent vagal nerve has been studied by our team since l980 under physiological and different pathological conditions in animal experiments[11,12,24], healthy subjects[31], and in patients with different gastrointestinal disorders[26,27].
The presence of TRPT1, CGRP significantly increased in H. pylori positive chronic gastritis compared with gastric mucosa of healthy subjects. The data suggested that the TRVP1, CGRP could be involved in the development of human chronic gastritis; however no significant changes were obtained after classical H. pylori eradication treatment. SP levels decreased in patients with H. pylori chronic gastritis and its value was not changed by eradication treatment.
Histologically healthy gastric mucosa could be detected in only 22% of patients at 6 wk after classical eradication treatment. It was interesting to note, however, that the distribution of gastric mucosal TRVP1, CGRP and SP did not change in H. pylori positive gastritis after classical eradication treatment.
How can we explain this unchanged distribution?
We might need to start with the observed facts: (1) TRVP1, CGRP and SP can be immunohistologically detected in rat and human gastric mucosa under healthy and different pathological circumstances; (2) The changes in expression of TRVP1, CGRP and SP are a consequence of activation in capsaicin-sensitive afferent nerves; (3) The presence of H. pylori was proved in all patients with chronic gastritis in our study; (4) The eradication of H. pylori was successfully carried out, and that was associated with a significant decrease in patients’ complaints; (5) The gastric mucosa normalized in 25% of patients; and 75% of patients demonstrated only moderate histological signs of gastritis in their gastric mucosa after eradication treatment; and (6) Independently from the proof that chronic gastritis is associated with H. pylori infection, the histological picture of gastric mucosa indicates only a moderate remission.
We are able to explain the unchanged immunohistochemical distribution of TRVP1, CGRP and SP of H. pylori positive chronic gastritis after eradication treatment as follows: (1) A 6-wk time period after eradication is not enough time for the complete healing of chronic gastritis. Although patients’ complaints decreased and the eradication treatment was principally successful, despite the trend seen in gastrointestinal mucosal histology showing lower infection, successful eradication treatment differs from the traditional and specific immunhistological distribution of TVPV1, CGRP and SP; (2) A 6-wk time period (after eradication) might not be long enough for the complete histological recovery and healing of chronic H. pylori (in terms of histology and immunohistology) in patients; (3) H. pylori as etiological factor represents only one of the factors causing chronic gastritis (at least in terms of histology); (4) The immunohistological distribution of TRPR1, CGRP and SP seems to be independent of the chronic gastritis produced by different physical, chemical, bacteriological, immunological agents. Histologically healthy gastric mucosa could be detected only in 4 cases of the control biopsies and in 14 cases the appearance of chronic gastritis was just moderated. Our data showed that gastric mucosa presented less inflammation after eradication treatment. Changes in the immunohistological picture of gastric mucosa (before and after eradication treatment) suggest that the time period (6 wk) was too short for healing of chronic gastritis by eradication treatment. That fact is confirmed by the repeated histological examinations; (5) It can be suggested that other permanent factors (stress, drugs), not responding to the H. pylori eradication therapy, also play a part in the development of chronic gastritis; this is in accordance with our further observations. The presence and distribution of TRVP1, CGRP and SP is not altered by different factors (H. pylori, drugs, etc.) evoking chronic gastritis; and (6) A low percentage of the participants was refractory to the eradication therapy so that the persistent H. pylori infection, before and after eradication, could maintain the same immunohistochemical appearance and the same inflammatory response.
Earlier, we demonstrated that the amount of vanilloid receptor, CGRP and SP increased in patients with chronic gastritis; however, no differences were obtained in their presence in gastric mucosa of H. pylori positive and H. pylori negative patients[27]. The results presented clearly indicate that the immunohistochemical distribution of vanilloid receptor, CGRP and SP during the classical eradication treatment in patients with H. pylori positive chronic gastritis is independent from the eradication treatment. This suggests that the function of capsaicin-sensitive afferent nerves is also independent. Capsaicin is able to reduce the IND-induced gastric microbleeding in human healthy subjects. The involvement of TRVP1, CGRP and SP in different gastrointestinal disorders shows the importance of continuing such studies to better understand gastric defensive mechanisms in humans[15]. Similar conclusions were obtained from the results of animal experiments, when we applied drugs (substances) acting on both efferent and afferent vagal nerves[2], and received a summary of both actions. The results of these observations led us to conclude that capsaicin-sensitive afferent nerves are the most important physiological regulators of gastric basal acid secretion (BAO) and of chemical-induced gastric mucosal damage in human healthy subjects[15,31,32]. The most important message of this study is that gastric capsaicin-sensitive afferentation has a permanent defensive role in the gastric mucosa against injury produced by different noxious agents. Consequently the modification of the function of capsaicin-sensitive afferent nerves offers new possibilities in the field of human medical therapy.
The unchanged functional state of capsaicin-sensitive afferentation, before and after classical eradication treatment in patients, also elucidates the important defensive regulatory function of TRVP1, CGRP and SP in the gastric mucosa of patients with H. pylori chronic gastritis during the eradication treatment. This is in comparison with the results obtained in patients with H. pylori positive gastritis vs healthy persons.
The integrity of gastric mucosa relies on the equilibrium between the aggressive and defensive factors. The loss of this balance leads to development of different gastric disorders. Helicobacter pylori (H. pylori) is one of aggressive factors, which is widely spread bacterium. At least half of the world’s population is infected with H. pylori and it has been taken as causative factor for development of human gastrointestinal diseases such as acute and chronic gastritis, gastro-duodenal ulcer, gastric mucosa-associated lymphoid tissue lymphoma and different extra-gastrointestinal disorders (atherosclerosis, ischemic heart and cerebrovascular diseases). The eradication of this bacteria by application of proton pump inhibitors and antibiotics has been generally carried out in these patients. The presence of H. pylori can be detected by using [14C] urea breath test, rapid urease test, Warthin-Starrry silver and specific histological staining from biopsy samples obtained from antrum and corpus of the stomach. These observations were obliquate before the eradication treatment; meanwhile the success of eradication therapy can be easily checked by [14C] urea breath test. However, the presence of H. pylori infection can be detected by the clinically accepted (requirement) methods in so-called in human healthy persons (without any medically detectable diseases), in whom the eradication treatment is not medically indicated.
The vagus nerve consists of 10 percent of efferent nerves and 90 percent of afferents. These afferent nerves are capsaicin-sensitive in about 10%. Capsaicin-sensitive afferentation is one of the defensive mechanisms. These nerve have been shown to play role in gastric mucosal protection by preventing the drug-induced gastric mucosal damage in animals and decrease the indomethacin-induced gastric mucosal microobleeding in humans. The capsaicin-sensitive nerves having a temperature-gated non-selective cation channel called capsaicin receptor or transient potential receptor vanilloid 1 (TRVP1) mediate the releases of calcitonin gene-related (CGRP), substance P (SP) and somatostatin, which are responsible for increased mucosal blood flow, activation of mast cells and other immune cells in the in the gastric mucosa and defence of gastrointestinal tract.
TRVP1, CGRP and SP are demonstrated by immunohistochemical methods in the gastric mucosa. The immunohistochemical studies were carried out on formalin fixed, paraffin embedded tissue samples of gastric mucosa using the peroxidase-labeled polymer method (Lab Vision Co., Fremont, USA). SP was detected by NC1/34HL rat monoclonal antibody, the TRVP1 receptor and CGRP were labelled using polyclonal antisera (all from Abcam Ltd.,Cambridge, UK). The systematic examinations of above mentioned immunohistochemical studies inform us about the functional state of capsaicin-sensitive afferent nerves. The TRVP1, CGRP and SP can be detected well in the healthy human subjects, meanwhile the expressions of TRVP1 and CGRP significantly incease in patients with chronic H. pylori positive and negative gastritis in association with the decrease of SP. In the present study, these immunohistochemical examinations were carried out before and 6 wk after the eradication treatment. The eradication was successful in 89% percent; the histologically intact mucosa was obtained in 22%. It was surprising to note, that no changes could be detected in the extents of expressions of TRVP1, CGRP and SP in patients with H. pylori infection- induced chronic gastritis before and after eradication treatment.
The capsaicin-sensitive afferent nerves have a preventive role in the gastrointestinal tract. That role is an independent from the eradication treatment in patients with chronic H. pylori positive gastritis. The stimulation of capsaicin-sensitive afferent nerves by small doses of capsaicin might offer a new possibility in the treatment of patients with H. pylori positive chronic gastritis.
It is an interesting study presenting new information. This report provides data that capsaicin-sensitive afferent nerves take place in the gastric defence mechanism in treatment of H. pylori and H. pylori negative chronic gastritis.
Peer reviewers: Mario Guslandi, MD, FACG, Professor, Gastroenterology Unit, S.Raffaele University Hospital, Via Olgettina 60, 20159 Milan, Italy; Giedrius Barauskas, Professor, Department of Surgery, Kaunas University of Medicine, Kaunas LT-50009, Lithuania; Omar Mohamed Abdel-Salam, Professor, Department of Toxicology and Narcotics, Medical Division-National Research Centre, Tahrir Street, Dokki, Cairo 1231, Egypt
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
| 1. | Logan RP, Walker MM. ABC of the upper gastrointestinal tract: Epidemiology and diagnosis of Helicobacter pylori infection. BMJ. 2001;323:920-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Höcker M, Hohenberger P. Helicobacter pylori virulence factors--one part of a big picture. Lancet. 2003;362:1231-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 77] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 3. | Pakodi F, Abdel-Salam OM, Debreceni A, Mózsik G. Helicobacter pylori. One bacterium and a broad spectrum of human disease! An overview. J Physiol Paris. 2000;94:139-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Parsonnet J. The incidence of Helicobacter pylori infection. Aliment Pharmacol Ther. 1995;9 Suppl 2:45-51. [PubMed] [Cited in This Article: ] |
| 5. | Janulaityte-Gunther D, Kucinskiene R, Kupcinskas L, Pavilonis A, Labanauskas L, Cizauskas A, Schmidt U, Wadström T, Andersen LP. The humoral immuneresponse to Helicobacter pylori infection in children with gastrointestinal symptoms. FEMS Immunol Med Microbiol. 2005;44:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Mitani K, Tatsuta M, Iishi H, Yano H, Uedo N, Iseki K, Narahara H. Helicobacter pylori infection as a risk factor for gastric ulceration. Hepatogastroenterology. 2004;51:309-312. [PubMed] [Cited in This Article: ] |
| 7. | Peng H, Ranaldi R, Diss TC, Isaacson PG, Bearzi I, Pan L. High frequency of CagA+ Helicobacter pylori infection in high-grade gastric MALT B-cell lymphomas. J Pathol. 1998;185:409-412. [PubMed] [Cited in This Article: ] |
| 8. | Salih BA, Abasiyanik MF, Saribasak H, Huten O, Sander E. A follow-up study on the effect of Helicobacter pylori eradication on the severity of gastric histology. Dig Dis Sci. 2005;50:1517-1522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Zhang C, Yamada N, Wu YL, Wen M, Matsuhisa T, Matsukura N. Comparison of Helicobacter pylori infection and gastric mucosal histological features of gastric ulcer patients with chronic gastritis patients. World J Gastroenterol. 2005;11:976-981. [PubMed] [Cited in This Article: ] |
| 10. | Zhang C, Yamada N, Wu YL, Wen M, Matsuhisa T, Matsukura N. Helicobacter pylori infection, glandular atrophy and intestinal metaplasia in superficial gastritis, gastric erosion, erosive gastritis, gastric ulcer and early gastric cancer. World J Gastroenterol. 2005;11:791-796. [PubMed] [Cited in This Article: ] |
| 11. | Abdel-Salam OM, Debreceni A, Mózsik G, Szolcsányi J. Capsaicin-sensitive afferent sensory nerves in modulating gastric mucosal defense against noxious agents. J Physiol Paris. 1999;93:443-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Mózsik G, Abdel-Salam OME, Szolcsányi J. Capsaicin-sensitive afferent nerves in gastric mucosal damage and protection. Budapest: Akadémiai Kiadó 1997; 13. [Cited in This Article: ] |
| 13. | Kang JY, Yeoh KG, Chia HP, Lee HP, Chia YW, Guan R, Yap I. Chili--protective factor against peptic ulcer? Dig Dis Sci. 1995;40:576-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Mózsik G, Belágyi J, Szolcsányi J, Pár G, Pár A, Rumi G, Rácz I. Capsaicin-sensitive afferent nerves and gastric mucosal protection in the human healthy subjects. A critical overview. Mediators in Gastrointestinal Protection and Repair. Kerala: Research Signpost 2004; 43-62. [Cited in This Article: ] |
| 15. | Mózsik G, Szolcsányi J, Rácz I. Gastroprotection induced by capsaicin in healthy human subjects. World J Gastroenterol. 2005;11:5180-5184. [PubMed] [Cited in This Article: ] |
| 16. | Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6360] [Cited by in F6Publishing: 6414] [Article Influence: 237.6] [Reference Citation Analysis (0)] |
| 17. | Mózsik G, Vincze A, Szolcsányi J. Four response stages of capsaicin-sensitive primary afferent neurons to capsaicin and its analog: gastric acid secretion, gastric mucosal damage and protection. J Gastroenterol Hepatol. 2001;16:1093-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143-201. [PubMed] [Cited in This Article: ] |
| 20. | Szolcsányi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 2004;38:377-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Holzer P, Livingston EH, Saria A, Guth PH. Sensory neurons mediate protective vasodilatation in rat gastric mucosa. Am J Physiol. 1991;260:G363-G370. [PubMed] [Cited in This Article: ] |
| 22. | Stead RH. Innervation of mucosal immune cells in the gastrointestinal tract. Reg Immunol. 1992;4:91-99. [PubMed] [Cited in This Article: ] |
| 23. | Sipos G, Altdorfer K, Pongor E, Chen LP, Fehér E. Neuroimmune link in the mucosa of chronic gastritis with Helicobacter pylori infection. Dig Dis Sci. 2006;51:1810-1817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Mózsik G, Pár A, Pár G, Juricskay I, Figler M, Szolcsányi J. Insight into the molecular pharmacology to drugs acting on the afferent and efferent fibres of the vagal nerve in the gastric mucosal protection. Ulcer Research, Proceedings of the 11th International Conference. Bologna: Monduzzi 2004; 163-168. [Cited in This Article: ] |
| 25. | Vincze Á, Szekeres G, Király Á, Bódis B, Mózsik G. The immunohistochemical distribution of capsaicin receptor, CGRP and SP in the human gastric mucosa in patients with different gastric disorders. Ulcer Research, Proceedings of the 11th International Conference. Bologna: Monduzzi 2004; 149-153. [Cited in This Article: ] |
| 26. | Dömötör A, Peidl Z, Vincze A, Hunyady B, Szolcsányi J, Kereskay L, Szekeres G, Mózsik G. Immunohistochemical distribution of vanilloid receptor, calcitonin-gene related peptide and substance P in gastrointestinal mucosa of patients with different gastrointestinal disorders. Inflammopharmacology. 2005;13:161-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Dömötör A, Kereskay L, Szekeres G, Hunyady B, Szolcsányi J, Mózsik G. Participation of capsaicin-sensitive afferent nerves in the gastric mucosa of patients with Helicobacter pylori-positive or-negative chronic gastritis. Dig Dis Sci. 2007;52:411-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Mózsik G, Peidl Z, Szolcsányi J, Dömötör A, Hideg K, Szekeres G, Karádi O, Hunyady B. Participation of vanilloid/capsaicin receptors, calcitonin-gene-related peptide and substance P in gastric protection of omeprazole and omeprazole-like compounds. Inflammopharmacology. 2005;13:139-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1348] [Cited by in F6Publishing: 1286] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 30. | Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6:209-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 665] [Cited by in F6Publishing: 633] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 31. | Mózsik G, Szolcsányi J, Dömötör A. Capsaicin research as a new tool to approach of the human gastrointestinal physiology, pathology and pharmacology. Inflammopharmacology. 2007;15:232-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Mózsik Gy, Dömötör A, Past T, Vas V, Perjési P, Kuzma M, Blazics Gy, Szolcsányi J. Capsaicinoids: From the Plant Cultivation to the Production of the Human Medical Drug. Budapest: Akadémiai Kiadó 2009; . [Cited in This Article: ] |