Published online Dec 6, 2010. doi: 10.4292/wjgpt.v1.i6.123
Revised: October 31, 2010
Accepted: November 7, 2010
Published online: December 6, 2010
AIM: To investigate the role of Cupressus sempervirens (C. sempervirens) and Juniperus phoenicea (J. phoenicea) extracts as therapeutic effect against CCl4 with biochemical, histopathological evaluations.
METHODS: A single intraperitoneal dose of 10% CCl4 in olive oil (1 mL/kg body weight) was administered to a group of female Wister rats, sacrificed after 24 h (as the injury group). The other groups were given CCl4 as described above and divided as follows: two groups of ten rats each were orally administered either J. phoenicea extract or C. sempervirens extract three times per week for six weeks and a further group administered CCl4 was left for six weeks to allow self-recovery. At the end of experiment, the rats from all groups were sacrificed for sampling and for biochemical and histological analysis.
RESULTS: Remarkable disturbances were observed in the levels of all tested parameters. On the other hand, rats injected with the toxic agent and left for one and a half month to self recover showed moderate improvements in the studied parameters while, treatment with both medicinal herbal extracts ameliorated the levels of the disturbed biochemical parameters. The group treated with J. phoenicea extract showed a remarkable improvement in comparison to the CCl4 treated group. The C. sempervirens group revealing an even more remarkable effect showing histopathological liver& kidney profiles close to those of the control group.
CONCLUSION: C. sempervirens and J. phoenicea leaf extracts show a remarkable effect in enhancing liver and kidney functions and may thus be of therapeutic potential in treatment hepatotoxicity and nephrotoxicity.
-
Citation: Ali SA, Rizk MZ, Ibrahim NA, Abdallah MS, Sharara HM, Moustafa MM. Protective role of
Juniperus phoenicea andCupressus sempervirens against CCl4. World J Gastrointest Pharmacol Ther 2010; 1(6): 123-131 - URL: https://www.wjgnet.com/2150-5349/full/v1/i6/123.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v1.i6.123
Alternative drugs for treatment of liver and kidney disease have become a necessity to replace currently used drugs of doubtful efficacy and safety. Thus there is a world-wide trend to return to traditional medicinal plants to treat liver ailments[1]. Anti-fibrotics from natural products used in traditional medicine may reduce the risk of toxicity and maintain the therapeutic effectiveness when the drug is used clinically[2,3]. The use of natural products as a means for treatment and protection against diseases may be effective, less toxic and also less costly. The aim of the present study was to determine the potential role of the two plants extracts Juniperus phoenicea (J. phoenicea) and Cupressus sempervirens (C. sempervirens) in preventing or ameliorating the deleterious effects induced by experimental chemical hepatotoxins. In a previous study on the hepatoprotective effects of these two extracts, various biochemical determinants of liver function were evaluated including L-alanine aminotransaminase, aspartate aminotransaminase, and alkaline phosphatase, hight density lipoprotein low density lipoprotein, bilirubin, total cholesterol and triglycerides. Certain antioxidants were also measured, namely, glutathione, lipid peroxides and nitric oxide[4] and the study showed that treatment with both extracts ameliorated the levels of the disturbed biochemical parameters.
In this study we aimed to study the therapeutic effect of both extracts (J. phoenicea and C. sempervirens) against CCl4-induced hepatotoxicity in rats. This was achieved by measuring liver total protein and albumin. Serum total lactate dehydrogenase (LDH) was also measured and LDH-isoenzymes were analysed electrophoretically. Kidney functions were measured as serum urea and creatinine to evaluate the protective effect of both extracts against chemical toxicity. The histopathology of the architecture of liver and kidney was also investigated to investigate the role of the studied extracts on the architecture of injured liver and kidney cells.
All chemicals used in the present study were of high analytical grade products from Sigma, Aldrich (St. Louis, MO, USA), Merck (Germany), BDH (England), Riedal de Ha’en (Germany), Fluka (Switzerland), Randox (United Kingdom), and Bio-diagnostic (Egypt).
Adult female albino Wister rats weighing approximate 120 g, supplied from the animal house of National Research Center, Dokki, Egypt were used for experimental investigations. Animals were kept under constant environmental and nutritional conditions and were given food and water all throughout the period of the experiment. Appropriate anaesthetic and sacrifice procedures were followed ensuring that animals did not suffer at any stage of the experiments. Anaesthetic procedures complied with the legal ethical guidelines approved by the Ethical Committee of the Federal Legislation and National Institutes of Health Guidelines in USA and were approved by the ethical committee of the National Research Centre in Egypt. Animals were sacrificed under mild ether anesthesia. Blood was withdrawn and serum separated then liver and kidney samples were collected.
The dried powdered leaves (500 g) of either Juniperus phoenicea L. or Cupressus sempervirens L. were extracted in a Soxhlet apparatus with methyl alcohol. The methanolic extract was evaporated to dryness. The dried methanolic extract (approximate 28 g) was dissolved in a suitable amount of hot distilled H2O- MeOH (95:5 v/v, 200 mL) and partitioned between ethyl acetate and methanol. The ethyl acetate extract was partitioned between CHCl3 and EtOAc. Separation was then carried out by silica gel thin layer chromatography plates using solvent system C6H6-C6H5N-HCOOH. The isolated compounds were purified on Sephadex LH20 and were eluted with MeOH giving two major biflavonoid compounds from both plants. The methanolic fractions were chromatographed over Sephadex LH20 CC, eluted with H2O and finally with 50% MeOH. Five flavonoids were isolated from Cupressus sempervirens L. and four from Juniperus phoenicea L., while two phenolic acids were isolated from both plants. The isolated compounds were purified by paper chromatography using solvent system BuOH- Ac0H- H2O (4:1:5 v/v/v) upper layer. The isolation procedures for both plants were performed in Department of Pharmacognosy, National Research Centre, Dokki, and Giza, Egypt.
Fifty adult female albino Wister rats were divided into five groups of ten rats each. The first untreated group served as control (Group 1). The second group which served as the cirrhotic control group received a single intraperitoneal (i.p) injection of 1 mL/kg body weight 10% CCl4 in olive oil), and was then sacrificed after 24 h and (Group 2)[5]. The remaining three groups were given CCl4 as described before and divided as follows: Group 3 - CCl4 treated rats, left for one and half months to self-recover; Group 4 - CCl4-treated rats administered J. phoenicea methanolic extract (E1) (300 mg/kg body weight) three times per weeks orally for one and half months; and Group 5 - CCl4-treated rats administered C. sempervirens methanolic extract (E2) (300 mg/kg body weight) three times per week orally for one and half months.
At the end of experiment, animals were fasted for 24 h, and blood was then withdrawn from the sublingual vein after anesthetizing with diethyl ether. Blood samples were centrifuged for ten minutes at 3000 r/min, and then serum was separated and stored in aliquots in eppendorf tubes at -20°C to be used for biochemical analyses.
Animals were then sacrificed, liver and kidney tissues was rapidly removed and cut into small sections which were put in 10% of formalin solution and left for histopathological analysis. Collected serum samples were subjected to the analytical methods.
Total proteins: Total protein reacts with Bradford reagent to give a blue complex, which is measured colorimetrically at 595 nm wavelength[6].
Determination of albumin level: This was performed according to Doumas et al[7] using Randox Diagnostic kits. In a buffered solution, bromocresel green forms a green colored complex with albumin the intensity of which is proportional to the amount of albumin present in the sample.
Determination of serum urea level: This was done according to the procedure of Patton and Crouch[8] using Diamond International Kits. In alkaline medium, the ammonium ions released by urease react with salicylate and hypochloride to form green indophenols. The absorbance of samples and standards were measured by spectrophotometer at 580 nm against a reagent blank. The concentration of urea (mg/dL) was determined.
Determination of serum creatinine level: This was done according to the procedure of Henry[9]. The rate of complex formation was measured photometrically at 492 nm, and the concentration of serum creatinine was measured as mg/dL.
Determination of LDH activity in serum: LDH activity was estimated by the method of Babson and Babson[10]. The reduction of NAD is coupled with the reaction of tetrazolium salt as Iodonitrotetrazolium chloride (INT) with phenazine methosulfate serving as an intermediateelectron carrier, resulting in the formation of formazan of INT. The developed color was measured at 503 nm and the activity was calculated as μmol/min per mg protein.
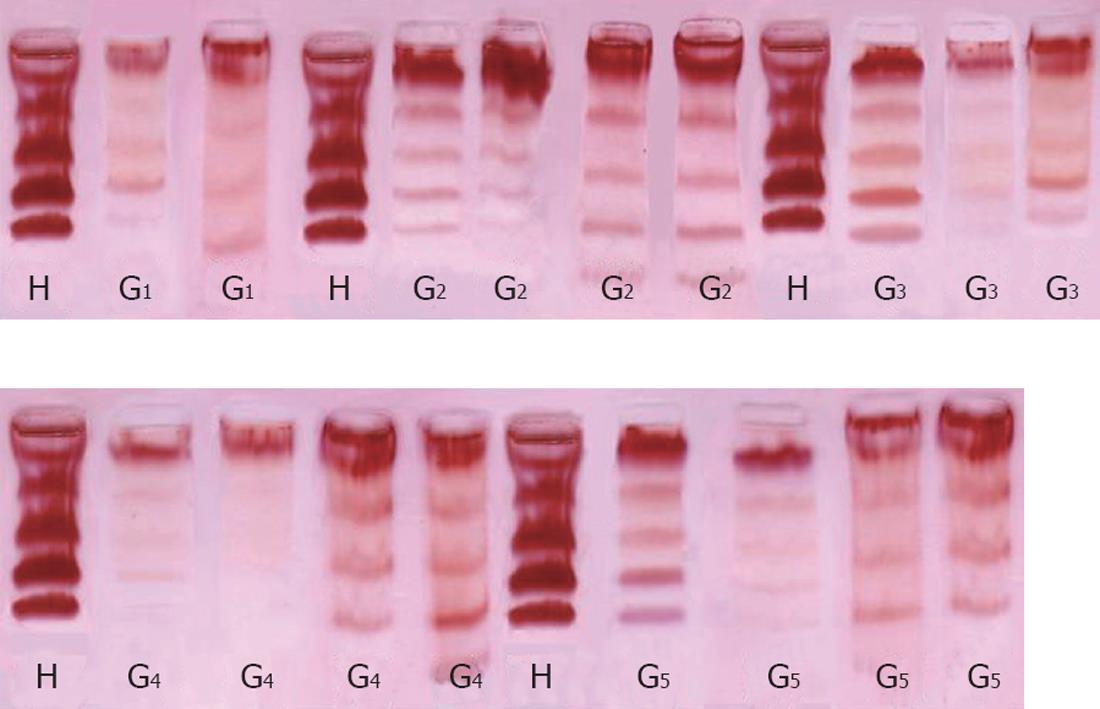
Electrophoretic separation of serum LDH-isoenzymes: The method of Dietz and Lubrano[11] was adopted for the preparation of ultra-thin layers of 5.5% polyacrylamide gel, for separation of LDH isoenzymes. The dry gel handles like a piece of paper[12] and can be stored for a long time without fading, distortion or cracking. The dry gel was scanned at 575 nm with an Ultrascan Laser Densitometer.
Histopathological analysis: Small pieces liver and kidney from the experimental animals were fixed in 10% neutral buffered-formalin prior to routine processing in paraffin-embedded blocks. Sections (4 μm thick) were cut and stained using hematoxylin-eosin (HE)[13].
Statistical analysis: All data obtained are expressed as the mean ± SD. Results were analyzed by a computerized statistical program. Values were compared by one-way analysis of variance and Fisher’s protected least significance difference for multiple comparisons as the post hoc test. A P-value < 0.05 was considered to be statistically significant[14].
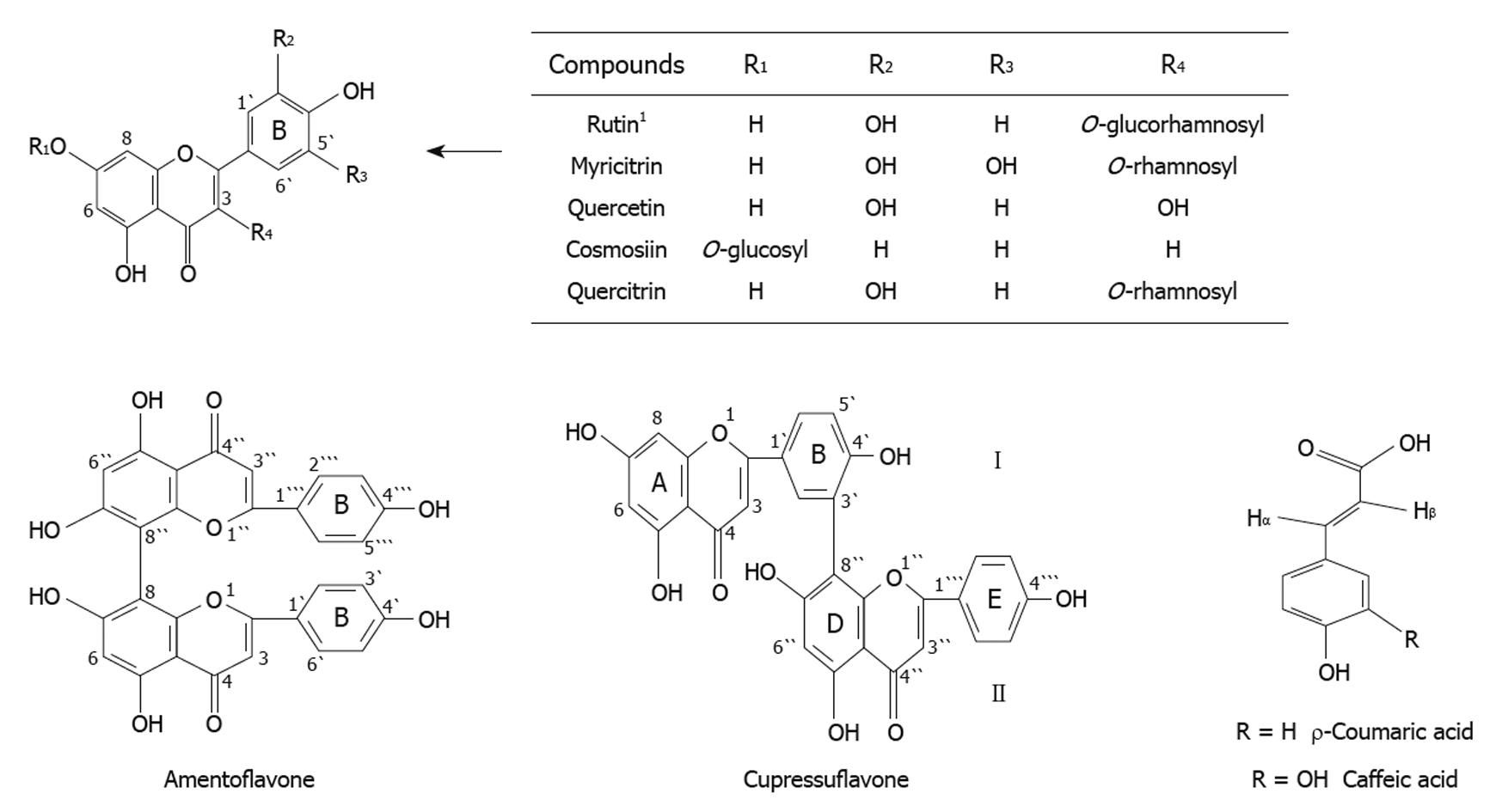
Chemical composition of the methanolic extracts of Cupressus sempervirens L. or Juniperus phoenicea L. was investigated by column chromatography followed by thin layer and paper chromatography. This revealed the presence of two major biflavonoid compounds; cupressuflavone and amentoflavone in the EtOAc fraction. Four flavonoid compounds namely, myricitrin, quercetin, cosmosin, quercitrin and two phenolic compounds; ρ-coumaric acid and caffeic acid were isolated from MeOH fraction of Juniperus phoenicea L. A further flavonoid, rutin was also identified in Cupressus sempervirens (Figure 1). Each isolated compound was identified by 1-HMR and 13C-NMR spectral analysis[15].
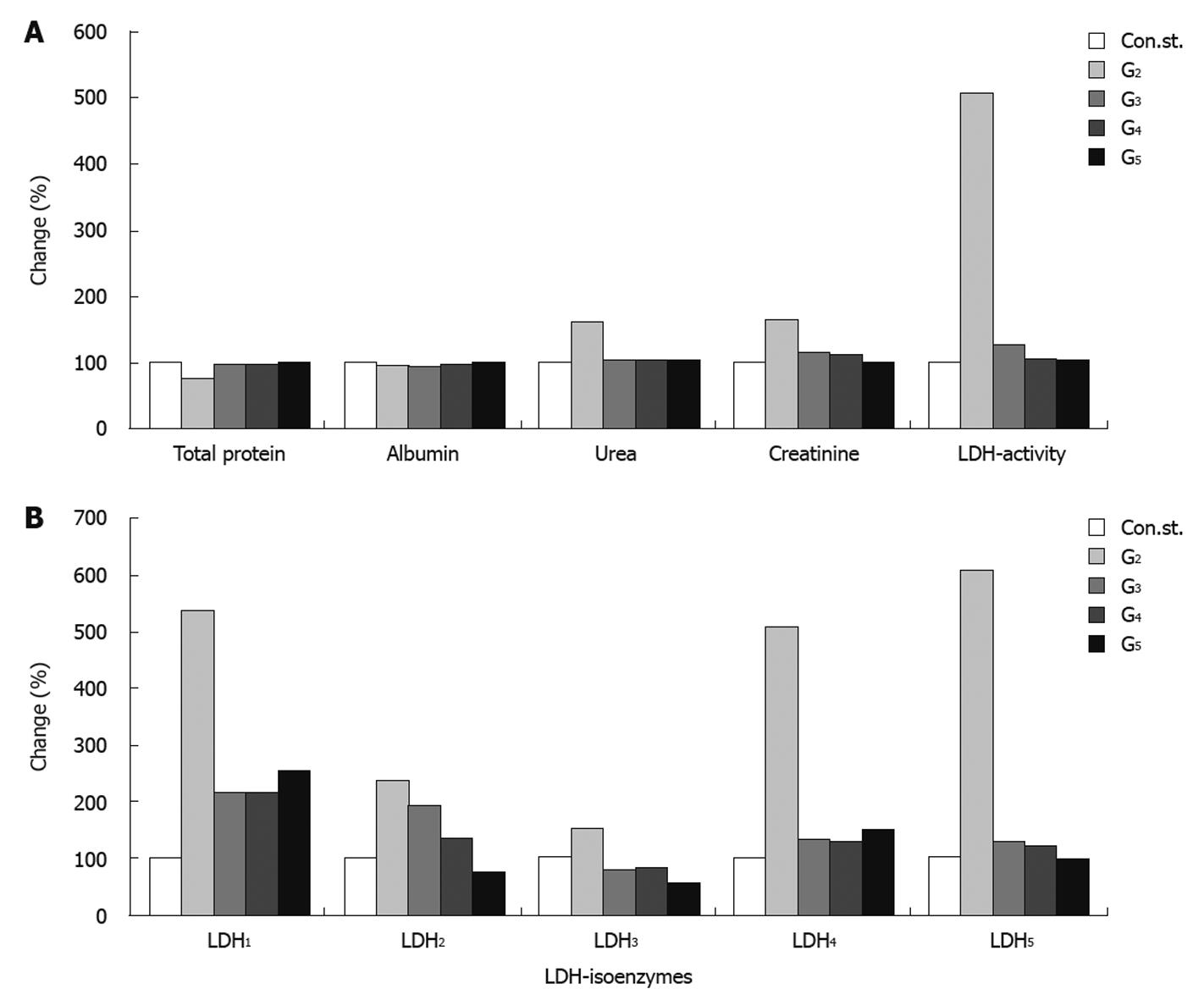
Serum total protein showed a pronounced decrease in the CCl4-intoxicated group, with a reduction of 25% compared to the normal healthy control group. Groups 3, 4 and 5 showed an improvement in the level of total protein amounting to 21%, 21% and 24% respectively compared to the CCl4-intoxicated group (G2). Concomitantly, a slight improvement was found in the level of serum albumin of 2%, 3% and 7% for groups 3, 4 and 5 respectively, compared to the CCl4-toxicated group (G2). The level of serum urea was significantly increased (62%) in the CCl4-toxicated group (G2) compared to the control healthy group. Administration of J. phenociea or C. sempervirens to the CCl4-toxicated group (G4 and G5 respectively) or without any treatment (G3) still showed a slight significant increase in urea level (2%, 3% and 2%) for groups G4, G5 and G3 respectively compared to the control healthy group. In addition, serum creatinine and total LDH activities were significantly increased in group 2 compared to the control group. Treatment with the two extracts showed a significant decrease in the levels of creatinine compared with the CCl4-intoxicated group (G2) with a percentage change of 15%, 11% in G4, G5 respectively (Table 1 and Figure 2A).
| Parameters | Control | CCl4-toxicated | CCl4-self recovery | Treated groups | Improvement (%) | ANOVA (P) | |||
| Ext1 | Ext2 | ||||||||
| G1 | G2 | G3 | G4 | G5 | G3 | G4 | G5 | ||
| Total protein (TP) | 7.28 ± 0.61 (2) | 5.458 ± 0.55 (1,3,4,5) | 6.95 ± 0.28 (2) | 6.94 ± 0.43 (2) | 7.18 ± 0.42 (2) | 20.5 | 20.83 | 23.7 | < 0.0001 |
| Albumin (Alb) | 2.86 ± 0.45 (NS) | 2.64 ± 0.11 (NS) | 2.69 ± 0.12 (NS) | 2.73 ± 0.31 (NS) | 2.84 ± 0.24 (NS) | 1.74 | 3.41 | 7.0 | < 0.348 |
| Urea | 0.606 ± 0.071 (2) | 0.9809 ± 0.15 (1,3,4,5) | 0.620 ± 0.053 (2) | 0.619 ± 0.1102 (2) | 0.613 ± 0.0759 (2) | 59.6 | 59.7 | 59.7 | < 0.0001 |
| Creatinine | 0.293 ± 0.079 (2) | 0.480 ± 0.08 (1,3,4,5) | 0.338 ± 0.07 (2) | 0.353 ± 0.021 (2) | 0.294 ± 0.065 (2) | 48.7 | 52.73 | 63.6 | < 0.0001 |
| Activity (LDH) | 0.098 ± 0.028 (2) | 0.495 ± 0.242 (1,3,4,5) | 0.1237 ± 0.019 (2) | 0.104 ± 0.0412 (2) | 0.100 ± 0.017 (2) | 479.6 | 499.59 | 503.8 | < 0.0001 |
The levels of LDH1 was greatly increased in the CCl4-intoxicated group (G2) with a percentage change of more than 439.11% compared to normal healthy control group, while G3, G4 and G5 showed a lowering in the levels of LDH1 of 325%, 323% and 287% resp. LDH2, LDH3, LDH4 and LDH5 are also significantly increased in the CCl4-intoxicated group compared to the control group. Treatment with the two extracts induced a highly significant decrease in the levels of the four isoenzymes, as illustrated in Table 2, Figure 2B and Figure 3.
| Parameters | Control | CCl4-toxicated | CCl4-self recovery | Treated groups | Improvement (%) | ANOVA (P) | |||
| Ext1 | Ext2 | ||||||||
| G1 | G2 | G3 | G4 | G5 | G3 | G4 | G5 | ||
| LDH1 | 0.0036 ± 0.0009 (2) | 0.0193 ± 0.017 (1,3,4,5) | 0.00767 ± 0.0028 (2) | 0.00775 ± 0.0052 (2) | 0.00904 ± 0.0056 (2) | 324.86 | 322.63 | 286.596 | < 0.037 |
| LDH2 | 0.012 ± 0.004 (2) | 0.0279 ± 0.0179 (1,5) | 0.0165 ± 0.0039 (NS) | 0.0161 ± 0.0131 (NS) | 0.0086 ± 0.006 (2) | 96.61 | 100 | 163.56 | < 0.044 |
| LDH3 | 0.0131 ± 0.0075 (NS) | 0.0199 ± 0.010 (3,4,5) | 0.0100 ± 0.0025 (2) | 0.0106 ± 0.0094 (2) | 0.00727 ± 0.0014 (2) | 75.57 | 70.99 | 96.41 | < 0.057 |
| LDH4 | 0.00813 ± 0.00479 (2) | 0.0411 ± 0.0314 (1,3,4,5) | 0.0106 ± 0.0035 (2) | 0.0102 ± 0.0063 (2) | 0.0123 ± 0.0054 (2) | 375.15 | 380.07 | 354.24 | < 0.003 |
| LDH5 | 0.0673 ± 0.0186 (2) | 0.409 ± 0.217 (1,3,4,5) | 0.0866 ± 0.0189 (2) | 0.081 ± 0.008 (2) | 0.066 ± 0.019 (2) | 479.02 | 487.19 | 510.37 | < 0.0001 |
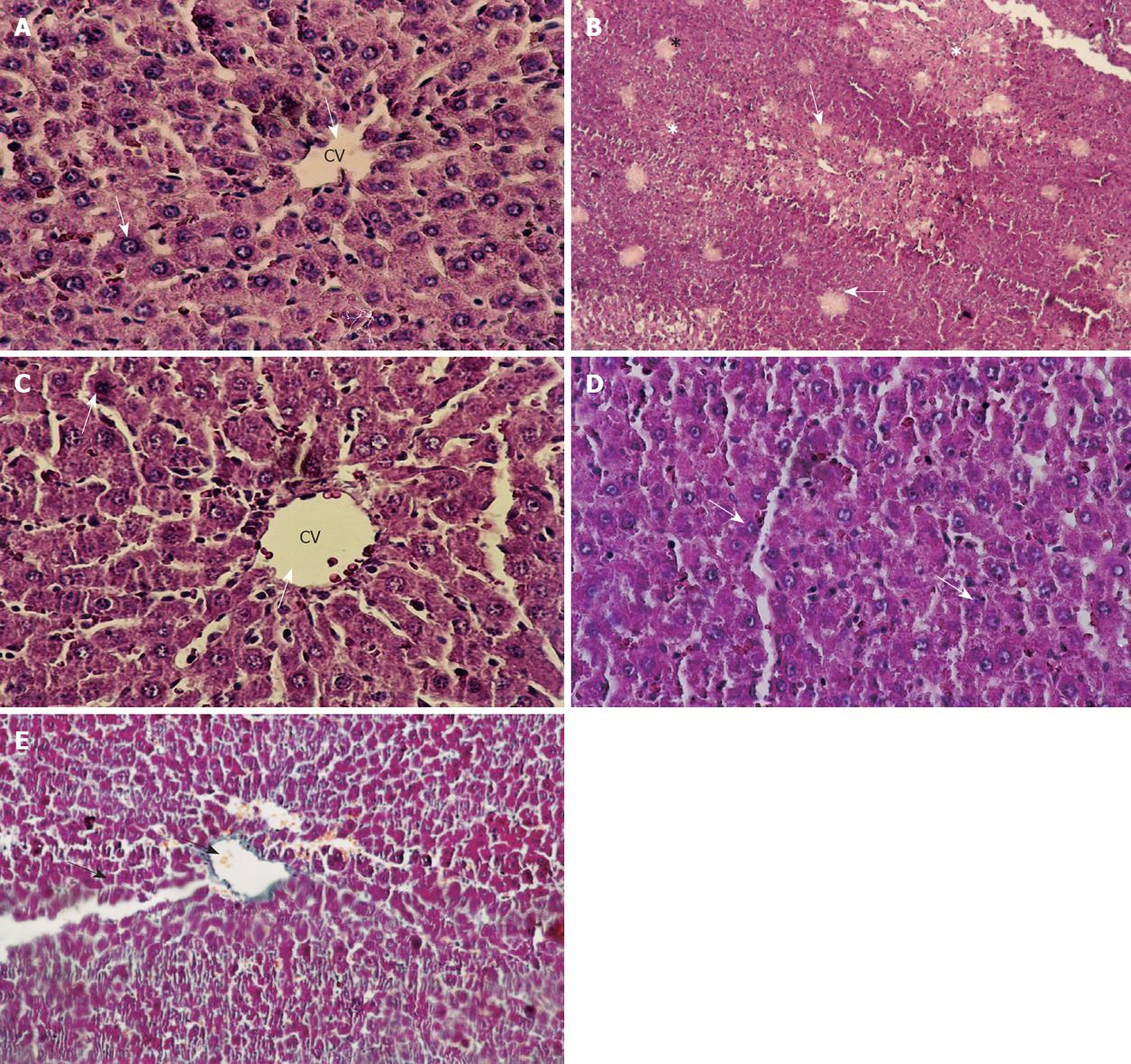
Histologically, control livers stained with HE staining showed normal parenchyma architecture (Figure 4A). After CCl4 treatment, significant liver damage was observed with classic histology of cirrhosis, coagulative necrosis, massive fibrosis, fatty degeneration and formation of regenerative nodules (Figure 4B). The group treated with J. phoenicea extract showed a remarkable improvement compared to the CCl4-treated group (Figure 4C). The C. sempervirens group showed an even more remarkable effect with values close to those of the control group (Figure 4D). In the group of rats intoxicated with CCl4 and left without further treatment for 6 wk to allow self-recovery, the liver parenchyma still showed massive fibrosis and micronodular cirrhosis as well as moderate fatty change and regenerative nodules surrounded by fibrous connective tissue extending between portal regions, similar to animals treated with CCl4 (G2) (Figure 4E). The regression in these alterations was slower than the groups treated with both extracts.
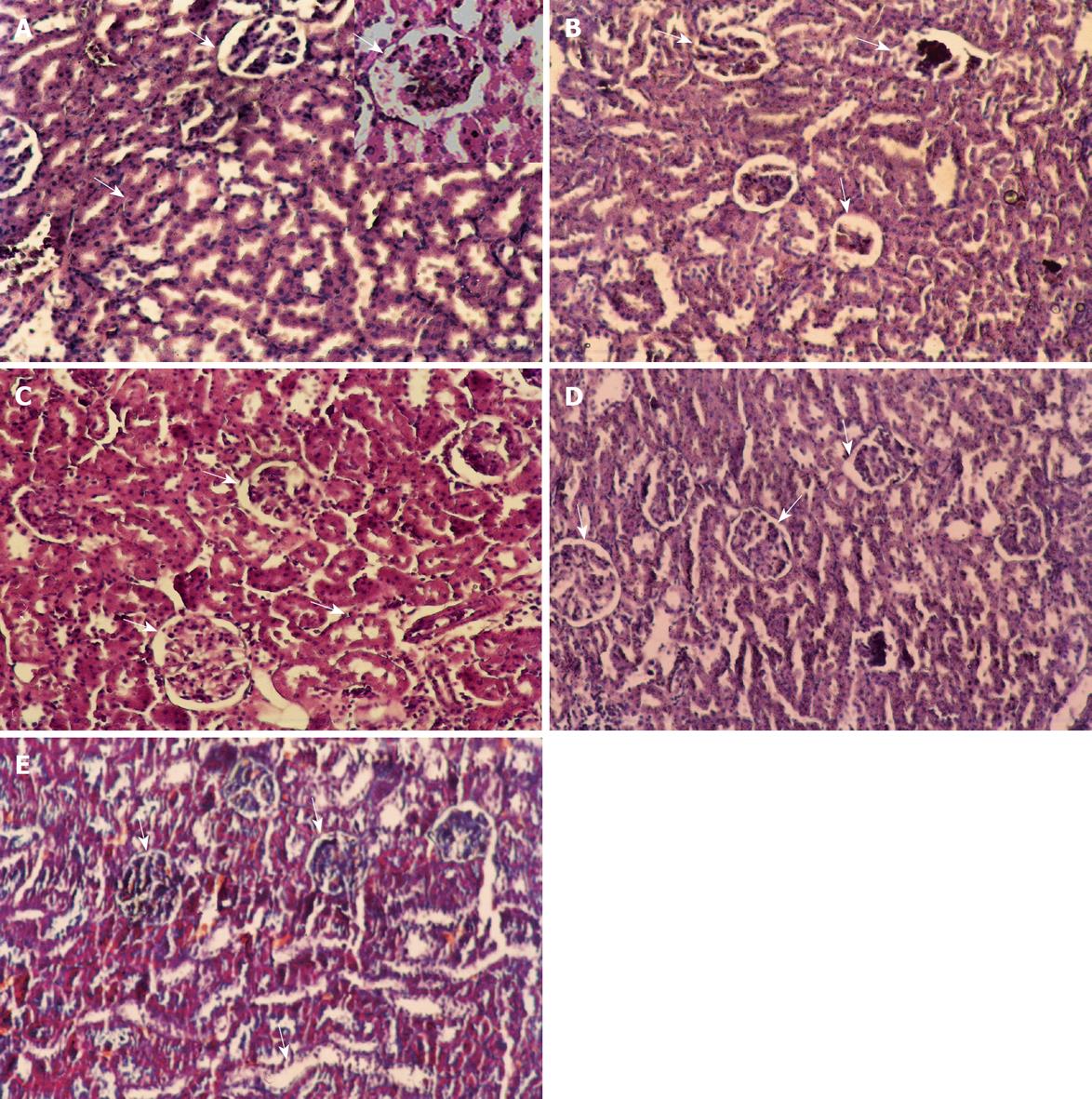
Microscopically, kidney stained with HE staining showed Glomeruli and tubules with apparently normal histological features (Figure 5A). However in the CCl4 (24 h) group extensive cortical damage was observed (Figure 5B). Focal glomerular necrosis was detected in this group and the affected glomeruli showed hypocellularity and shrinkage. Most of the cortical tubules showed morphologic changes, some of them being dilated and lined with flattened epithelial cells. In the group of rats protected with C. sempervirens or J. phoenicea to CCl4-toxicated group, the glomeruli were normal, although sparse tubular changes were observed. Treatment with C. sempervirens (Figure 5C) showed a more remarkable improvement than J. phoenicea (Figure 5D). In the group of rats intoxicated with CCl4 and left for one and half month without any treatment to allow self-recovery (Figure 5E) the kidney glomeruli still showed a few necroses with some epithelial cells showing vacuolization, atrophy and detachment of tubular epithelial cells. The regression in theses alteration was slower than in the groups treated with both extracts.
In CCl4-induced injury, the brunt of the damage falls on hepatocellular membranes. The relative loss of phospholipids moieties results in an alteration of the membrane cholesterol:phospholipids ratio leading to abnormal transmembrane losses of fluid, abnormal transmembrane signal transmission and ultimately to cell injury, fibrosis or death[16]. It should be pointed out that treatment of liver diseases by various synthetic drugs is costly and may induce undesirable side- effects. An alternative approach to the use of chemically synthesized drugs for the treatment of liver disorders is the use of natural plant extracts, some of which have been used by traditional medical practitioners for centuries. The potency of these extracts will open new areas for the development of a safe and cheap hepatoprotective drugs from natural sources for treatment of a wide range of liver diseases[17].
The phytochemical investigation of the methanolic extract of J. phoenicea and C. sempervirens revealed the presence of flavonoids and phenolic acids, which possess significant antioxidant and thereby anti-hepatotoxic properties[18]. Previously, the free radical scavenging properties against DPPH0 of flavonoids from the two plants under investigation were measured using ESR techniques in comparison with α-tocopherol as standard antioxidant. A high antioxidant activity for quercetin, rutin, caffeic acid, and p-coumaric acid were reported[19]. Leaf extracts of C. sempervirens play an important role in traditional herbal medicine and are used as antiseptic, antirheumatic, antihemorrhoidal, antidiarrheic, vasoconstrictive agents, for cough, colds, parasitic infections, inflammation and as strong hair tonic. It is also used for treatment of gastrointestinal disorders (diarrhea) and against dermatosis[20,21].
Elevated serum levels of liver-specific enzymes as well as alterations in several other liver parameters and reduction in the levels of serum total proteins may indicate liver or kidney disease[22]. The present data showed that rats treated with a single dose of CCl4 developed significant hepatic damage as observed from the decreased level of total protein. The decline in protein content may be due to defects in protein biosynthesis as well as disruption and disassociation of polyribosomes from endoplasmic reticulum following administration of CCl4[23]. The improvements in the level of total protein after treatment with the natural products may be due to the promotion of ribosome assembly on endoplasmic reticulum which facilitates uninterrupted protein biosynthesis[24]. In the present study, a significant reduction in serum albumin was detected in the CCl4-intoxicated rats while a slight improvement occurred after treatment with the two test extracts. These results are in agreement with Ohta et al[25] who reported that, serum albumin concentrations decrease in rats with chronic CCl4-intoxication at an advanced state of liver cirrhosis.
CCl4-intoxicated rats showed a significant increase in the levels of serum urea and creatinine due to altered kidney function. This is in agreement with the findings of Ronis et al[26] who noted that administration of CCl4 causes increases in plasma creatinine levels in rats. Administration of J. phoenicea or C. sempervirens extracts to cirrhotic rats induced a significant decrease in the levels of serum urea and creatinine in comparison to rats left to recover without any treatment.
LDH is a key enzyme in glycolysis which catalyzes the process of lactate production[27]. The results obtained in the present study revealed significant increases in the levels of total LDH in CCl4-intoxicated rats. This correlates with the findings of Hung et al[28] who noted that exposure of rats to CCl4 increased the serum levels of LDH. However, treatment of cirrhotic rats with J. phoenicea or C. sempervirens extracts resulted in decreased levels of total LDH. Our results also coincide with those of Gupta et al[29] who noted a significant increase in serum levels of all LDH the isoenzymes. Analysis of each tissue revealed characteristic changes in LDH isoenzyme patterns indicating organ-specific tissue damage. These alterations in LDH and its isoenzymes, may be directly or indirectly related to the mechanism(s) of the toxic action, and also provide insight into the site/organ(s) of toxicity[29]. Levels of all LDH-isoenzymes were improved after treatment with both extracts. An excellent enhancement in LDH5 was also noticed after treatment with the two extracts. It was clearly noticed that, as predominating isoenzymes, both LDH4 and LDH5 were more or less at normal levels.
Furthermore, CCl4 treatment induces the necrosis of hepatocytes around centrilobular veins, and the accumulation of inflammatory cells[30,31]. In the present study, histopathological changes we observed indicating liver damage after CCl4 administration. It has been reported that CCl4 causes necrosis, fibrosis, steatosis and foamy degeneration of hepatocytes and cirrhosis in liver. It is worth noting that the present biochemical findings correlated with the histological observations in the liver and kidney which clearly revealed that the hepatic cells, central vein, and portal triad and kidney normal histologic features Glomeruli and tubules are almost normal in J. phoenicea L or C. sempervirens groups.
The result of this study, confirmed by histological observation, show that methanolic extracts from both plants under investigation have preventive action on CCl4 induced hepatotoxicity. This phenomenon was due to flavonoid compounds namely myricitrin, quercetin, cosmosin and quercitrin as well as the phenolic compounds ρ-coumaric acid and caffeic acid. The role of these extracts in restoring different enzymatic activities and in ameliorating the toxic and hazardous disorders induced on the liver and kidney may be due to a high antioxidant activity for these flavonoids, especially rutin, which present in C. sempervirens leaves. The potency of the extracts will open new areas for the development of a safe and cheap hepatoprotective drug from natural wealth for the treatment of a wide range of liver diseases.
The methanolic extracts of the leaves of two traditional medicinal plants, namely, Cupressus sempervirens (C. sempervirens) and Juniperus phoenicea (J. phoenicea) were evaluated for their therapeutic effect.
The phytochemical investigation of the methanolic extracts of J. phoenicea and C. sempervirens revealed the presence of flavonoids and phenolic acids, which possess significant antioxidant and thereby anti-hepatotoxic properties.
The flavonoid compounds, myricitrin, quercetin, cosmosin, quercitrin and the phenolic compounds ρ-coumaric acid and caffeic acid. The role of these extracts in restoring different enzymatic activities and in ameliorating the toxic and hazardous disorders induced on the liver and kidney.
The levels of serum biochemical parameters including total protein, albumin, total lactate dehydrogenase activity, lactate dehydrogenase isoenzymes were determined. In addition urea and creatinine were estimated as measures of kidney function in experimentally CCl4 induced liver injury in rats. The histopathological liver and kidney profiles were also studied.
The two extracts under study possess potent activities against CCl4 toxicity due to the high antioxidant activity of the flavonoids, especially rutin, which are present in C. sempervirens leaves. The potency of the extracts will open new areas for the development of a safe and cheap hepatoprotective drug from natural wealth for the treatment of a wide range of liver diseases.
Peer reviewer: Martin J Ronis, Professor, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, Arkansas Children’s Nutrition Center, Little Rock, AR 72202, United States
S- Editor Wang JL L- Editor Hughes D E- Editor Lin YP
| 1. | Ali SA, Hamed MA. Effect of Ailanthus altissima and Zizyphus spina Christi on bilharzial infestation in mice: histological and histopathological Studies. J Applied Sci. 2006;6:1437-1446. [Cited in This Article: ] |
| 2. | Ali SA, El-Rigal N, Rizk MZ. Nutritional supplementation with Ailanthus altissimia and Ziziphus spina Christi to compensate for some metabolic disorders in Schistosoma mansoni infected mice. Pak J Biol Sc. 2006;9:1700-1706. [Cited in This Article: ] |
| 3. | El-Ansary AK, Ahmed SA, Aly SA. Antischistosomal and liver protective effects of Curcuma longa extract in Schistosoma mansoni infected mice. Indian J Exp Biol. 2007;45:791-801. [Cited in This Article: ] |
| 4. | Rizk MZ, Abdallah MS, Sharara HM, Ali SA, Ibrahim NA, Mousstafa MM. Efficiency of Cupressus sempiverens L. and Juniperus phoenicea against carbon tetrachloride hepatotoxicity in rats. Trends Med Res. 2007;2:83-94. [Cited in This Article: ] |
| 5. | Nishida K, Ohta Y, Kongo M, Ishiguro I. Response of endogenous reduced glutathione through hepatic glutathione redox cycle to enhancement of hepatic lipid peroxidation with the development of acute liver injury in mice intoxicated with carbon tetrachloride. Res Commun Mol Pathol Pharmacol. 1996;93:198-218. [Cited in This Article: ] |
| 6. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [Cited in This Article: ] |
| 7. | Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromocresel green. Clin Chem Acta. 1971;31:87-96. [Cited in This Article: ] |
| 8. | Patton CJ, Crouch SR. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Anal Chem. 1977;44:464-469. [Cited in This Article: ] |
| 9. | Henry RJ. Clinical Chemistry, Principles and Techniques. 2nd ed. Harper and Row. 2nd ed. New York: Academic press 1974; 545. [Cited in This Article: ] |
| 10. | Babson SR, Babson AL, An improved amylase assay using dyed amylopectin. Clin Chem Acta. 1973;44:193-197. [Cited in This Article: ] |
| 11. | Dietz AA, Lubrano T. Separation and quantitation of lactic dehydrogenase isoenzymes by disc electrophoresis. Anal Biochem. 1967;20:246-257. [Cited in This Article: ] |
| 12. | Juang RH, Chang YD, Sung HY, Su JC. Oven-drying method for polyacrylamide gel slab packed in cellophane sandwich. Anal Biochem. 1984;141:348-350. [Cited in This Article: ] |
| 13. | Hirsch C, Zouain CS, Alves JB, Goes AM. Induction of protective immunity and modulation of granulomatous hypersensitivity in mice using PIII, an anionic fraction of Schistosoma mansoni adult worm. Parasitology. 1997;115:21-28. [Cited in This Article: ] |
| 14. | Arkin H, Colton RR. Table for Statisticians. 2nd ed. New York: Barnes & Noble 1992; 115. [Cited in This Article: ] |
| 15. | Harborne JB. The flavonoids advance in research. London: Chapman and Hall 1993; 460-461. [Cited in This Article: ] |
| 16. | Das D, Pemberton PW, Burrows PC, Gordon C, Smith A, McMahon RF, Warnes TW. Antioxidant properties of colchicine in acute carbon tetrachloride induced rat liver injury and its role in the resolution of established cirrhosis. Biochim Biophys Acta. 2000;1502:351-362. [Cited in This Article: ] |
| 17. | El-Banhawey MA, Ashry MA, El-Ansary AK, Aly SA. Effect of Curcuma longa or parziquantel on Schistosoma mansoni infected mice liver--histological and histochemical study. Indian J Exp Biol. 2007;45:877-889. [Cited in This Article: ] |
| 18. | Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65:337-353. [Cited in This Article: ] |
| 19. | Ibrahim NA, Risk M. phytochemical investigation and hepatoprotective activity of Juniperus phoenicea leaves growing in Egypt. Bull Fac Pharm Cairo Univ. 2005;43:175-183. [Cited in This Article: ] |
| 20. | Uncini Manganelli RE, Camangi F, Tomei PE. Curing animals with plants: traditional usage in Tuscany (Italy). J Ethnopharmacology. 2001;78:171-191. [Cited in This Article: ] |
| 21. | Ibrahim NA, El-Seedi HR, Mohammed MM. Phytochemical investigation and hepatoprotective activity of Cupressus sempervirens L. leaves growing in Egypt. Nat Prod Res. 2007;21:857-866. [Cited in This Article: ] |
| 22. | Sharpe PC, McBride R, Archbold GP. Biochemical markers of alcohol abuse. QJM. 1996;89:137-144. [Cited in This Article: ] |
| 23. | Clawson GA. Mechanisms of carbon tetrachloride hepatotoxicity. Pathol Immunopathol Res. 1989;8:104-112. [Cited in This Article: ] |
| 24. | Rajesh MG, Latha MS. Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J Ethnopharmacol. 2004;91:99-104. [Cited in This Article: ] |
| 25. | Ohta Y, Sahashi D, Sasaki E, Ishiguro I. Alleviation of carbon tetrachloride-induced chronic liver injury and related dysfunction by L-tryptophan in rats. Ann Clin Biochem. 1999;36:504-510. [Cited in This Article: ] |
| 26. | Ronis MJ, Huang J, Longo V, Tindberg N, Ingelman-Sundberg M, Badger TM. Expression and distribution of cytochrome P450 enzymes in male rat kidney: effects of ethanol, acetone and dietary conditions. Biochem Pharmacol. 1998;55:123-129. [Cited in This Article: ] |
| 27. | Augoff K, Grabowski K. [Significance of lactate dehydrogenase measurements in diagnosis of malignancies]. Pol Merkur Lekarski. 2004;17:644-647. [Cited in This Article: ] |
| 28. | Hung MY, Fu TY, Shih PH, Lee CP, Yen GC. Du-Zhong (Eucommia ulmoides Oliv.) leaves inhibits CCl4-induced hepatic damage in rats. Food Chem Toxicol. 2006;44:1424-1431. [Cited in This Article: ] |
| 29. | Gupta RC, Goad JT, Kadel WL. In vivo alterations in lactate dehydrogenase (LDH) and LDH isoenzymes patterns by acute carbofuran intoxication. Arch Environ Contam Toxicol. 1991;21:263-269. [Cited in This Article: ] |
| 30. | Guyot C, Lepreux S, Combe C, Doudnikoff E, Bioulac-Sage P, Balabaud C, Desmoulière A. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135-151. [Cited in This Article: ] |
| 31. | Pradeep HA, Khan S, Ravikumar K, Ahmed MF, Rao MS, Kiranmai M, Reddy DS, Ahamed SR, Ibrahim M. Hepatoprotective evaluation of Anogeissus latifolia: in vitro and in vivo studies. World J Gastroenterol. 2009;15:4816-4822. [Cited in This Article: ] |