INTRODUCTION
The use of anesthetics can differentially affect a number of physiological parameters (cardiovascular, metabolic and hemodynamic) in experimental models[1-3]. As such, the use of certain anesthetics can affect the outcome of studies and different therapeutic interventions may be influenced by different anesthetic approaches. Ketamine is usually used as a short-acting anesthetic and analgesic agent that induces a trance-like anesthetic state known as dissociative anesthesia in both animals and humans[4]. Xylazine is considered safe when used alone or in combination with other anesthetics and analgesics such as ketamine or isoflurane in animal research. Co-administration of ketamine with xylazine (KX) is a routine anesthetic regimen for domestic and laboratory animals including mice and rats[5]. The sedative and muscle-relaxing properties of xylazine are beneficial in reducing side effects of ketamine such as tremor and muscle rigidity[6,7]. Recent research has revealed that KX may influence some physiological responses to surgical procedures or drug effects in small laboratory animals. In comparison to other anesthetics, it has been reported that KX increases the infarct size in cerebral ischemia[8], induces hyperglycemia in fed rodents[9], reduces TNF-α expression in rat spleen[10] and influences lipopolysaccharide-induced endotoxemia[11]. Some anesthetics have cardio- or renoprotective effects which may be relevant to designing ischemia-reperfusion protocols[12,13]. They can affect the systemic hemodynamic[14] and may induce moderate to severe tissue damage[15].
The laboratorial use of anesthetic agents such as ketamine associated with xylazine (KX) and sodium pentobarbitone (SP) could induce alterations of gastric blood flow (GBF) in portal hypertensive rats. In addition, special attention to GBF during anesthesia in the cirrhotic rats’ model could be important since the anesthetic agents could be worsening the gastric mucosa damage. This study aimed to evaluate the KX and SP anesthetic effects on GBF and ethanol (EtOH)-induced gastric damage in portal hypertensive rats.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (200-250 g) provided by the Animal House of the State University of Campinas were used in all experiments. They were housed in plastic cages and had free access to water and standard pellet chow. The experimental protocols were approved by the Ethical Principles in Animal Research adopted by the Brazilian College for Animal Experimentation.
Induction of portal hypertension
Portal hypertension was induced in male Sprague-Dawley rats anesthetized with either a mix of ketamine plus xylazine (100:10 mg/kg; ip) or SP (25-50 mg/kg; ip) using two procedures. Prehepatic portal hypertension was provoked by portal vein stenosis (PVS) according to Chojkier et al[16] (1981). The portal vein was isolated and a stenosis created by placing a single ligature of a 3-0 silk around both the portal vein and a 20-gauge blunt-tipped needle. The needle was then removed from the ligature, creating a calibrated constriction of the portal vein. In another experimental group, biliary cirrhosis with intrahepatic portal hypertension was induced by common bile duct ligation (BDL) as described by Lee et al[17] (1986). Briefly, BDL was performed by isolating the common bile duct and placing two 4-0 sutures proximal/distal to the porta hepatis. Next, a 5-10 mm segment between ligatures was resected. The abdomen was then sutured with 4-0 silk and the animals were allowed to recover. Controls had a sham operation. Experiments were performed 2[16] or 4[17] wk following surgery when pure portal hypertension and liver cirrhosis were established respectively.
Ethanol-induced gastric damage
Gastric resistance to a 40% EtOH-induced injury was investigated using an ex vivo gastric chamber preparation. Rats were fasted for 16 h prior to the procedure. Rats were anesthetized by SP or ketamine plus xylazine and placed over a heating pad connected to a rectal probe (Harvard Apparatus, Holliston, MS) to maintain body temperature at 37°C. The stomach was exposed following a midline laparotomy and opened with an incision along the greater curvature. It was pinned over a plexiglass platform and clamped with a plexiglass cylinder. The experiments consisted of six periods of 10 min each. The mucosa was bathed with phosphate buffered saline for two periods followed by 40% ethanol for one period and then HCl at pH 1.5 for three periods. All solutions were at 37°C when added to the chamber and continuously stirred at 200 r/m. The stomach was photographed at the end of experiments and damage (percent total glandular mucosa) analyzed using computerized planimetry by an observer blinded to the different treatment groups[18].
Gastric blood flow measurement
GBF was measured using laser-Doppler flowmetry and the ex vivo gastric chamber preparation[18]. A pencil probe (type N, penetration 1 mm, Transonic, Ithaca, NY) connected to a flowmeter (BLF 21D, Transonic, Ithaca, NY) was placed over the corpus. The preparation was equilibrated for 5 min. Basal GBF was then recorded for 5 min and experiments performed according to the protocol described previously[17]. GBF was recorded throughout the 60 min and maximum changes in flow were expressed as percentage change over basal[19].
Drugs/chemicals
All drugs were of analytical grade. SP (Hypnol®, Cristalia), Xylazine (Rompun®, Bayer) and ketamine (Ketalar®, Parke-Davis) were used as clinically available preparations.
Statistical analysis
Data are expressed as mean ± SEM and comparisons among groups (n = 5) were analyzed by one-way ANOVA followed by the Student’s Newman-Keul’s test for multiple comparisons. Statistical significance was considered when P < 0.05.
RESULTS
Effects of anesthetics on ethanol-induced injury in rats with portal hypertension
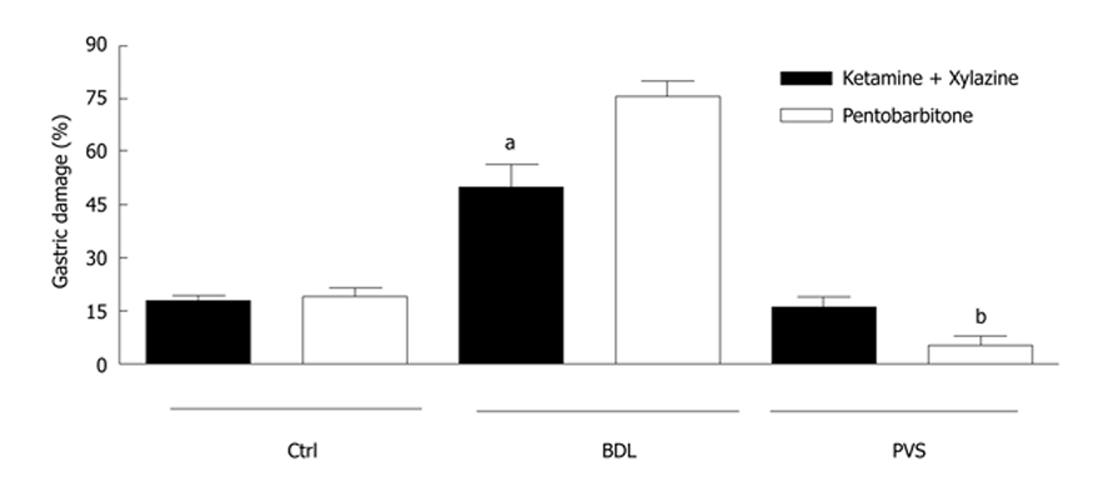
In control animals under SP or KX anesthesia, topical application of ethanol produced minimal gastric damage (20% ± 3%; n = 5, Figure 1). This was associated with a reduction in GBF followed by a hyperemic response when the mucosa was bathed with HCl (Figure 2A).
Figure 1 Gastric damage in control and portal hypertensive rats.
Ethanol (EtOH)-induced gastric damage was significantly increased in cirrhotic bile duct ligation (BDL) rats compared to controls when sodium pentobarbitone (SP) anesthetic was administered. In BLD rats, ketamine/xylazine (KX) anesthesia partially restored the resistance of the portal hypertensive gastric mucosa to ethanol-induced damage. An inverse effect was obtained from KX and SP anesthetics in portal vein stenosis rats. Asterisks denote significant differences between the two anesthesia treatments in BDL and portal vein stenosis rats (n = 5; aP < 0.001; bP < 0.05). PVS: Portal vein stenosis.
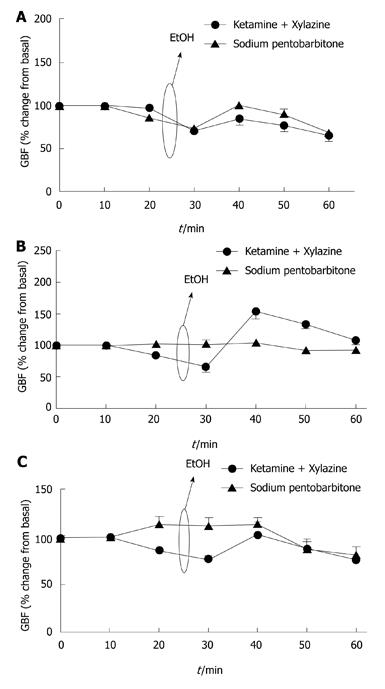
Figure 2 Gastric blood flow responses by topical application of ethanol (40%) in control (A), bile duct ligation (B) and portal vein stenosis (C) rats.
A: Under both sodium pentobarbitone and ketamine/xylazine anesthesia, a reduction in gastric blood flow (GBF) was observed in control rats followed by hyperemic response of the gastric microcirculation (n = 5); B: GBF responses were absent in bile duct ligation rats under sodium pentobarbitone anesthesia whereas ketamine/xylazine anesthesia partially restored GBF responses to ethanol (n = 5); C: GBF rates increased in portal vein stenosis rats under sodium pentobarbitone anesthesia while under ketamine/xylazine anesthesia was reduced (n = 5).
In BDL rats under SP anesthesia, the EtOH-induced damage increased over the control value to 76% ± 4% (n = 5; Figure 1). However significant (P < 0.001) protection in gastric damage was obtained when BDL rats were anesthetized with KX (50% ± 6%; n = 5; Figure 1). This was associated with the development of a hyperemic response to ethanol in BDL.
In contrast to SP anesthesia, higher EtOH-induced gastric damage was observed in PVS rats under KX anesthesia (16% ± 3% for KX vs 5% ± 2 % for SP; n = 5; Figure 1).
Effects of anesthetics on gastric blood flow
The effects of anesthetics on GBF responses submitted to 40% EtOH-induced injury were investigated in control and portal hypertensive rats. Control animals under SP or KX anesthesia did not show a difference in hyperemic responses (n = 5, Figure 2A). In BDL rats under SP anesthesia, any changes in GBF were observed throughout the experiments. On the other hand, KX anesthesia partially restored GBF dysfunction in BDL rats, contributing to the reduced gastric damage observed (n = 5, Figure 2B).
In contrast, KX anesthesia lowered GBF in PVS rats. This was associated with reduced hyperemic response to topical application of 40% ethanol over the gastric mucosa. However, under SP anesthesia GBF was increased in PVS rats (n = 5, Figure 2C).
DISCUSSION
This study demonstrates that sodium pentobarbitone (SP) and Ketamine/Xylazine (KX) anesthetics have significantly different effects on GBF in fasted portal hypertensive rats. The cirrhotic rats (e.g. BDL rats) treated under KX anesthesia had less gastric mucosal damage from ethanol stimulus. While the specific process by which KX anesthetics affect GBF is unknown, previous studies demonstrate that glucose levels are improved under treatment with KX anesthetics viaα2- adrenoreceptor activation[19] and in turn the glucose acts to impair vagal activity in the stomach[20]. Therefore, it suggests that the increase of glucose serves to increase the pressure of GBF.
Physiological changes in the blood glucose concentration have been reported to modulate gastrointestinal motor function[21,22], particularly stomach motor function[20]. For example, hyperglycemia has been documented to exert an inhibitory effect on gastric function under a number of experimental conditions and in a wide range of species.
The mechanism responsible for hyperglycemia-induced inhibition of gastric motility remains unclear. In terms of mechanism, it has been proposed by several investigators that glucose acts to impair vagal activity to the stomach[23-25]. In addition, there are some studies showing that systemically administered glucose produces a decrease in efferent activity of the gastric vagus nerve[20,26,27]. Moreover, it has been proposed that glucose acts in the hepatic portal area to inhibit hepatic afferent nerve traffic to the brain which results in a reflex reduction in efferent activity of the gastric vagus nerve[26,28]. It has also been proposed that glucose acts in the brain to alter control of vagal mediated gastric motility[25,27,29,30].
Xylazine is analogue of clonidine and its effect as a α2-adrenergic agonist is known. It has been reported that activation of α2-adrenoceptor can increase, decrease or not change the levels of glucagon. Increased glucagon levels associated with decreased insulin levels may account for hyperglycemic effect of α2-adrenoceptor agonist[31,32]. In rats, xylazine increases urine flow rate by activating of the α2-adrenergic receptors in hypothalamic paraventricular nucleus which in turn decreases vasopressin release[33]. Saha et al[9] (2005) demonstrated that KX anesthetic increases blood glucose in fasted rats. It suggests that the acute hyperglycemia effect of KX reflects, in part, α2-adrenoceptor-dependent changes of glucoregulatory hormones including insulin, growth hormone, adrenocorticotrophic hormone and corticosterone[34,35]. Ketamine also induces changes in GBF. Rodrigues et al[36] (2006) demonstrated that KX anesthesia reduced the constrictor effect of noradrenaline in mesenteric arterioles when compared to chloral hydrate anesthesia. The reduction may be explained by a direct effect of ketamine on vascular smooth muscle cells causing relaxation[37,38]. Hence, this inhibition of contraction can be caused by agonists. It has been demonstrated in isolated aorta[39] and in the mesenteric artery[40]. These effects are primarily caused by the reduction of calcium flow into cells by the inhibition of voltage dependent L-type calcium channels[41]. Besides, the effect of ketamine reduces the endothelial release of nitric oxide (NO)[42]. Despite noradrenaline promoting vasoconstriction, this effect was reduced in the KX-anesthetized rats. It occurred independent of the NO release reduction that it promoted by ketamine[36].
The data of the research suggest that administration of KX in BDL rats partially restored the hyperemic gastric response to topical ethanol administration. In contrast, when SP was administered in BDL rats, any changes in hyporesponsive GBF followed topical ethanol administration was observed.
The gastric mucosa of the PVS rats group responded differently to anesthetic treatments. Naturally, the PVS rats group has elevated GBF because of the presence of excessive vasoactive mediators[43]. However, under KX anesthesia it was observed that the GBF is reduced in PVS rats whereas the high GBF of PVS rats under SP anesthesia was not altered. The high GBF protected the gastric mucosa from damage stimulus caused by topical ethanol administration.
PVS and common BDL are models used to evaluate portal hypertension in rats. However, the effects of ethanol on portal hypertensive gastric mucosa are different between these models. GBF differs between the models under SP anesthesia. Thus it is suspected that links exist between liver damage, the pentobarbital administration and levels of vasodilator mediators. Pentobarbital has important vascular effects in rats with portal hypertension and cirrhosis.
Studies have shown different results under the two models of portal hypertensive gastropathy: in the BDL model there is evidence of alterations of basal hemodynamic parameters which augment the gastric lesions[17,19,44]; these lesions were reduced in the PVS model. The low damage resistance in the BDL group could be due to the fact that, at the same time, the liver is involved and the pentobarbital anesthesia was used.
In animal models, it is generally assumed that the physiological parameters under general anesthesia represent the basal state of the animal (before institution of the disease model). However, the anesthetic category can variably affect cardiovascular, neurohumoral and behavioral parameters. Similarly, if the fed and fasted states of animals are chosen arbitrarily, it may cause several changes in physiological parameters. Therefore, it is possible that the anesthetic category agents and/or fed and fasted states of animals used in the studies with different therapeutic interventions can influence the results.
This study suggests that are significant differences in the GBF and gastric damage after intraperitoneal injection of the anesthetic agents between PVS and common BDL models. In the latter model, the rats under SP anesthesia did not show any changes in GBF although it increased gastric damage. On the other hand, ketamine associated with xilazine (KX) anesthesia was able to partially restore GBF dysfunction in BDL rats, contributing to the reduced gastric damage.
The use of KX anesthesia in BDL model should be preferred to SP anesthesia, demonstrating that the choice of appropriate anesthetics should avoid misleading interpretation of experimental data.
Data from this research suggest that the choice of appropriate anesthetics in experimental models of portal hypertension present extreme relevance and should be taken in to consideration before the completion of these studies in order to avoid misleading interpretation of the data.
COMMENTS
Background
Portal vein obstruction can cause portal hypertension and other clinical disorders such as severe hemorrhage. So, it is very important to prevent hemorrhages in gastrointestinal surgical procedures. In addition, many kinds of drugs have vasoactive actions such as vasodilatation or vasoconstriction. Anesthetics are vasoactive drugs and should be used carefully in gastrointestinal surgical procedures due to side effects on gastric mucosal blood flow. In this work, the better anesthetic to use in surgery of the gastric mucosa of portal hypertensive rats was suggested.
Research frontiers
Ketamine/xylazine (KX) and sodium pentobarbitone (SP) are two anesthetics for experimental and medical use. In the area of preventing gastric hemorrhage and simultaneously reduce gastric damage, the effects of these anesthetics on gastric blood flow (GBF) response to 40% ethanol-induced injury were investigated in control and portal hypertensive rats using laser doppler flowmetry and gastric chamber techniques.
Innovations and breakthroughs
In order to reduce gastric damage and improve GBF from portal hypertensive rats, the rat gastric mucosa was submitted to ethanol stimulus under two different kinds of anesthetics. Bleeding and gastric damage in bile duct ligation (BDL) rats were reduced under KX anesthesia but not in pure portal hypertension. On the other hand, bleeding and gastric damage were increased in BDL rats under SP anesthesia while the GBF was reduced. The present study shows that the use of inappropriate anesthetic can aggravate gastric damage in portal hypertensive rats.
Applications
The results of this study suggest that the use of KX in experimental procedures involving cirrhotic rats is preferable to SP anesthesia, demonstrating that the choice of appropriate anesthetics should avoid misleading interpretations of experimental data.
Terminology
Portal Hypertensive Gastropathy (PHG) is a subclinical entity from portal hypertension and it can cause severe hemorrhage. BDL and portal vein stenosis are two experimental models to induce portal hypertension in rats. KX and SP are two anesthetics for experimental and medical use.
Peer review
This is a good descriptive study in which the authors analyze the effect of two different kinds of anesthetic drugs on gastric damage induced by ethanol in rats. The results are interesting and suggest that KX is a potential anesthetic that could be the better choice to reduce gastric hemorrhage in surgical procedures in PHG.