Published online Feb 15, 2018. doi: 10.4291/wjgp.v9.i1.8
Peer-review started: March 24, 2017
First decision: May 4, 2017
Revised: November 25, 2017
Accepted: December 4, 2017
Article in press: December 5, 2017
Published online: February 15, 2018
To evaluate prognostic pathological factors associated with early metachronous disease and adverse long-term survival in these patients.
Clinical and histological features were analysed retrospectively over an eight-year period for prognostic impact on recurrent disease and overall survival in patients undergoing curative resection of a primary colorectal cancer.
A total of 266 patients underwent curative surgery during the study period. The median age of the study cohort was 68 year (range 26 to 91) with a follow-up of 7.9 years (range 4.6 to 12.6). Resection was undertaken electively in 225 (84.6%) patients and emergency resection in 35 (13.2%). Data on timing of surgery was missing in 6 patients. Recurrence was noted in 67 (25.2%) during the study period and was predominantly early within 3 years (82.1%) and involved hepatic metastasis in 73.1%. Emergency resection (OR = 3.60, P = 0.001), T4 stage (OR = 4.33, P < 0.001) and lymphovascular invasion (LVI) (OR = 2.37, P = 0.032) were associated with higher risk of recurrent disease. Emergency resection, T4 disease and a high lymph node ratio (LNR) were strong independent predictors of adverse long-term survival.
Emergency surgery is associated with adverse disease free and long-term survival. T4 disease, LVI and LNR provide strong independent predictive value of long-term outcome and can inform surveillance strategies to improve outcomes.
Core tip: Despite increasing uptake of national bowel cancer screening programme in the United Kingdom, majority of patients with colorectal cancer are diagnosed following the urgent 2-wk referral or present as an emergency (53%). Emergency resection surgery for colorectal cancer is associated with a high post-operative morbidity and mortality and adverse long-term survival compared to elective surgery. Although immediate survival may be affected by factors associated with provision of emergency surgery and critical care, long-term disease recurrence and survival is dictated by presence of adverse clinical and histological factors which can guide post-operative surveillance for recurrent disease.
- Citation: Littlechild J, Junejo M, Simons AM, Curran F, Subar D. Emergency resection surgery for colorectal cancer: Patterns of recurrent disease and survival. World J Gastrointest Pathophysiol 2018; 9(1): 8-17
- URL: https://www.wjgnet.com/2150-5330/full/v9/i1/8.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v9.i1.8
Colorectal cancer is the fourth most common cancer in the United Kingdom with over 40000 cases diagnosed each year[1]. At presentation, synchronous hepatic metastases are present in 20%-25% of patients with metachronous hepatic metastases developing in 40%-50%[2,3]. Up to 80% of patients who develop metachronous disease do so within the first 3 years[4,5]. About 10% of patients who have resection of the primary tumour with curative intent will develop metachronous lung metastases[6] with local recurrence having been reported to occur in 4%-10% of patients[7,8]. Recent decades have seen a significant improvement in early post-operative survival in patients undergoing lung or liver resection surgery for colorectal metastasis[9,10]. Intensive post-operative surveillance has shown to improve 5-year survival with recognition of early, asymptomatic recurrent disease[11]. Five-year survival has further improved with the advent of effective chemotherapy, especially for patients with resectable liver disease, seeing a rise from 35% to 50%[10,12]. Three meta-analyses[13-15] have shown improved, albeit modest, survival with aggressive surveillance, which has been proven to be within the National Health Service’s threshold of cost acceptability[16].
Despite the widespread use of screening programs for detection of colorectal cancer, a large number of cases in England are diagnosed in the acute or urgent setting either as an emergency presentation (26%) or following an urgent 2-wk referral (27%)[17]. Compared to elective resection, emergency surgery is associated with adverse postoperative outcomes (post-operative mortality 4.6% vs 16%), disease-free and overall long-term survival[18-20]. This may represent a multifactorial basis due to altered physiology, immunosuppression, advanced disease and aggressive tumour biology[21,22].
Surveillance after resection of the colorectal primary in the form of colonoscopy and computed tomography (CT) imaging with or without adjuncts such as positron emission tomography (PET/PET-CT) is generally considered the standard of care[4]. There is wide variation in follow-up protocols in randomised trials with no clear consensus about follow-up intensity[23]. The Association of Coloproctologists of Great Britain and Ireland (ACPGBI) recommends a CT scan within the first 2 years after resection of the primary tumour to detect metastases as part of the follow up of these patients[24]. Conversely, the American Society of Clinical Oncology (ASCO) has suggested an annual abdominal/chest CT scan for three years with more frequent scans for higher risk patients (defined as stage III or stage II with multiple high-risk features)[4]. The American National Comprehensive Cancer Network (NCCN) recommends an abdominal/pelvic and chest CT scan annually for up to five years[25]. Furthermore, it is clear that not all patients with colorectal cancer will benefit from intensive surveillance, incurring unnecessary costs. Identifying patients at high risk of developing early recurrence (within the first three years) will help to determine who would benefit from aggressive surveillance.
The aim of this study was to investigate the prognostic effects of histological factors on patterns of recurrent disease and survival in patients undergoing emergency resection surgery for colorectal cancer.
A retrospective audit of patients undergoing surgery for colorectal cancer was carried out after institutional approval. Patients undergoing consecutive curative resection for histologically proven, primary colorectal adenocarcinoma in the absence of synchronous metastatic disease on presentation were included the study covering an eight-year period from January 2001 to December 2008. The study population was identified using procedure codes from the hospital database.
Demographic data on age, sex and mode of presentation was recorded. In the emergency surgery setting the presence of bowel obstruction or perforation was recorded. Histological data was retrieved for site of tumour, TNM staging was based on postoperative histological findings along with degree of tumour differentiation, lymphovascular invasion (LVI), perineural invasion (PNI), resection margin status (R). Lymph node ratio (LNR) was calculated as the ratio of disease positive nodes to the total number of lymph nodes retrieved. Clinic letters and correspondences within the computerised hospital database were accessed to gain information on presentation, mode of surgery and neo-adjuvant and adjuvant therapies. Treatment decisions regarding neo-adjuvant and adjuvant therapies were carried out in multi-disciplinary team meetings based on patient fitness, symptoms, synchronous disease and post-operative recovery. Generally, patients with rectal tumour received neo-adjuvant therapy if the tumour was > T3b or in presence of nodal disease on cross-sectional imaging.
Surveillance following curative colorectal cancer resection incorporated 6 monthly clinical assessments for 3 years followed by annual reviews for a total of 5 years for patients without recurrent disease. This assessment was supplemented by 6 monthly CEA levels (Carcinoembroyic Antigen), CT of chest, abdomen and pelvis within 2 years of surgery and 5-yearly surveillance colonoscopy (following completion colonoscopy within a year where indicated) until the age of 75 years (or longer if life expectancy deemed longer than 10 years at this stage). Detection of new symptoms, increased CEA levels, and abnormal CT or colonoscopy findings warranted multidisciplinary team review during follow-up. Patients developing an extra-colonic primary malignancy during the course of follow-up were excluded from the study to minimise bias. Survival data was obtained as all-cause mortality using the Demographics Batch Service (DBS) to access the national electronic database of the United Kingdom NHS.
Primary outcome was development of early recurrence disease within three years of curative resection. This was subdivided into liver, lung and local recurrence. Secondary outcomes were overall recurrence, three-year and overall survival.
Statistical analysis was performed using SPSS® version 20.0 (IBM, New York, NY, United States) using non-parametric tests (χ2 test and logistic regression). Continuous data in the text are reported as median (range), unless stated otherwise. P < 0.050 was regarded as significant. Variables with P > 0.100 on univariate analysis were excluded from multiple regression predictive model analyses. Models with multiple variables were assessed for interactions. Receiver operating characteristic (ROC) curves were plotted for continuous variables to estimate threshold values that differentiated groups. Survival analysis was carried out using the Kaplan-Meier method. Differences in survival curves were assessed using the log rank method.
The database identified 266 patients meeting the inclusion criteria during the study period. Median period of follow-up was 7.9 years (4.6 to 12.6 years). The median age at the time of surgery was 68 years (26 to 91 years) with a male to female ratio of 1.5:1.1. Surgery was undertaken as an elective procedure in 225 patients (84.6%) and as an emergency in 35 (13.2%) patients and remained unclear for 6 (2.2%) patients. The indication for emergency surgery was not identified in 3 patients. Bowel obstruction was the predominant indication for emergency surgery, undertaken in 28 patients compared to 9 patients with a diagnosis of perforation on presentation.
Of the total 266 patients, 151 were diagnosed with tumours situated in the colon and 115 within the rectum. Data on neo-adjuvant therapy was missing in 12 patients (7 colonic and 5 rectal cancers). None of the remaining patients with colon cancer received neo-adjuvant therapy before surgery. Of 115 patients with rectal cancer, 39 (33.91%) received pre-operative radiotherapy, 10 (8.69%) received pre-operative chemo-radiotherapy and 5 (4.31%) had pre-operative chemotherapy alone. Postoperatively, amongst patients with tumour sited in the colon, only 53 (35.10%) received adjuvant therapies (data missing in 6 patients). Amongst patients with rectal cancer, 54 (46.96%) received neo-adjuvant therapy and 14 of these patients proceeded to have further adjuvant therapies post-operatively. Overall, 30 (26.09%) patients with rectal cancer had adjuvant therapies post-operatively (missing data in 3).
The site and histological features of the primary colorectal tumour in all 266 patients are shown in Table 1. TNM staging is presented from postoperative histological assessment. Pre-operative radiological changes in staging before and after neo-adjuvant therapy were not collected. Although T stage was not recorded for 18 patients positive residual disease was identified in 16 of these patients. Lymph node status was missing in 17 patients.
| Tumour site | |
| Rectum | 115 (43.23) |
| Recto-sigmoid junction | 2 (0.75) |
| Sigmoid | 64 (24.06) |
| Descending/splenic flexure | 7 (2.63) |
| Transverse colon | 14 (5.26) |
| Ascending/hepatic flexure | 15 (5.64) |
| Caecum | 49 (18.42) |
| Tumour size1 | |
| T1 | 8 (3.00) |
| T2 | 54 (20.30) |
| T3 | 121 (45.49) |
| T4 | 66 (24.81) |
| Missing | 18 (6.77) |
| Nodal status and invasion1 | |
| N0 | 154 (57.89) |
| N1 | 65 (24.43) |
| N2 | 30 (11.28) |
| LVI (data missing in 15) | 60 (22.56) |
| PNI (data missing in 15) | 14 (5.26) |
| Resection margin (data missing in 16) | |
| R0 | 229 (86.09) |
| R1 | 14 (5.26) |
| R2 | 7 (2.63) |
| Tumour differentiation (data missing in 17) | |
| Well | 22 (8.27) |
| Moderately | 176 (66.17) |
| Poor | 51 (19.17) |
Complete data was available from 35 patients undergoing emergency colorectal cancer resection. Detailed characteristics of patients undergoing emergency and elective colorectal resection for cancer are shown in Table 2. No significant differences were noted between the two groups in age, sex, tumour grade, nodal disease, LNR, LVI, PNI or resection margin status on univariate regression analysis. Right colon was more commonly the site of primary cancer in the emergency group and associated with higher rate of obstruction. More patients undergoing emergency surgery had T4 disease stage on univariate analysis (OR = 4.33, 95%CI: 2.03-9.25, P < 0.001) and a higher proportion received adjuvant therapies post-resection compared to elective resection (45.7% vs 28.8%, OR = 2.10, 95%CI: 1.01-4.37, P = 0.047). Multivariate modelling was not carried out as no other histological parameter reached significance of P < 0.100.
| Variables | Emergency surgery | Elective surgery | P2 |
| n | 35 | 225 | |
| Median age in years | 70 (41-85) | 68 (26-91) | NS |
| Sex (males/females) | 20/15 | 128/97 | NS |
| Site of CRC | - | ||
| Right colon | 17 (48.6%) | 56 (24.8%) | < 0.001 |
| Left colon | 15 (42.9%) | 58 (25.8%) | NS |
| Rectum | 3 (8.6%) | 111 (49.3%) | < 0.001 |
| Obstruction | 26 (74.3%) | 21 (0.9%) | < 0.001 |
| Right colon | 15 (42.9%) | 1 (0.4%) | - |
| Left colon | 10 (28.6%) | 0 | - |
| Rectum | 1 (2.9%) | 1 (0.4%) | - |
| Perforation | 9 (25.7%) | 0 | < 0.001 |
| Right colon | 2 (5.7%) | 0 | - |
| Left colon | 5 (14.3%) | 0 | - |
| Rectum | 2 (5.7%) | 0 | - |
| Neoadjuvant therapy | 1 (2.9%) | 53 (23.6%) | 0.021 |
| Adjuvant therapy | 16 (45.7%) | 65 (28.8) | 0.047 |
| Poorly differentiated | 5 (14.3%) | 44 (19.6%) | NS |
| T4 disease | 18 (51.4%) | 46 (20.4%) | < 0.001 |
| Positive resection margin | 2 (5.7%) | 19 (8.4%) | NS |
| N2 disease | 15 (42.9%) | 78 (34.7%) | NS |
| Liver metastasis | 16 (42.1%) | 30 (13.3%) | < 0.001 |
| Early (< 3 yr) | 14 (40.0%) | 26 (11.6%) | - |
| Lung metastasis | 3 (8.6%) | 10 (4.4%) | NS |
| Early (< 3 yr) | 3 (8.6%) | 8 (3.6%) | - |
| Local metastasis | 1 (2.9%) | 9 (4.0%) | NS |
| Early (< 3 yr) | 1 (2.9%) | 6 (2.7%) | - |
| Overall recurrence | 17 (48.6%) | 47 (21.3%) | 0.001 |
| Median recurrence in days | 280 (112-1155) | 420 (70-1855) | NS |
| Median DFS in days | 1120 (105-3892) | 2302 (704522) | 0.004 |
| Median survival in days | 1913 (105-3892) | 2359 (194-4522) | 0.040 |
Emergency resection was also positively associated with increased risk of recurrent disease on univariate analysis (48.57% vs 20.89%, OR = 3.60, 95%CI: 1.69-7.64, P = 0.001) with liver being the commonest site of early recurrent disease (40.0% vs 11.6%, OR = 5.78, 95%CI: 2.66-12.55, P < 0.001). A subset analysis demonstrated a greater proportion of patients undergoing emergency resection received adjuvant therapies compared to elective resection (45.7% vs 28.9%). In the emergency surgery group, patients with T4 disease were likely to received adjuvant therapies (P = 0.004). These patients receiving adjuvant therapies demonstrated an increased trend towards risk of liver metastasis but it failed to reach significance (P = 0.089). No differences were seen in recurrence rates for lung and local disease in the two groups. Although, median period to recurrence in days was shorter in the emergency resection group, this did not reach significance (OR = 0.99, 95%CI: 0.99-1.00, P = 0.181).
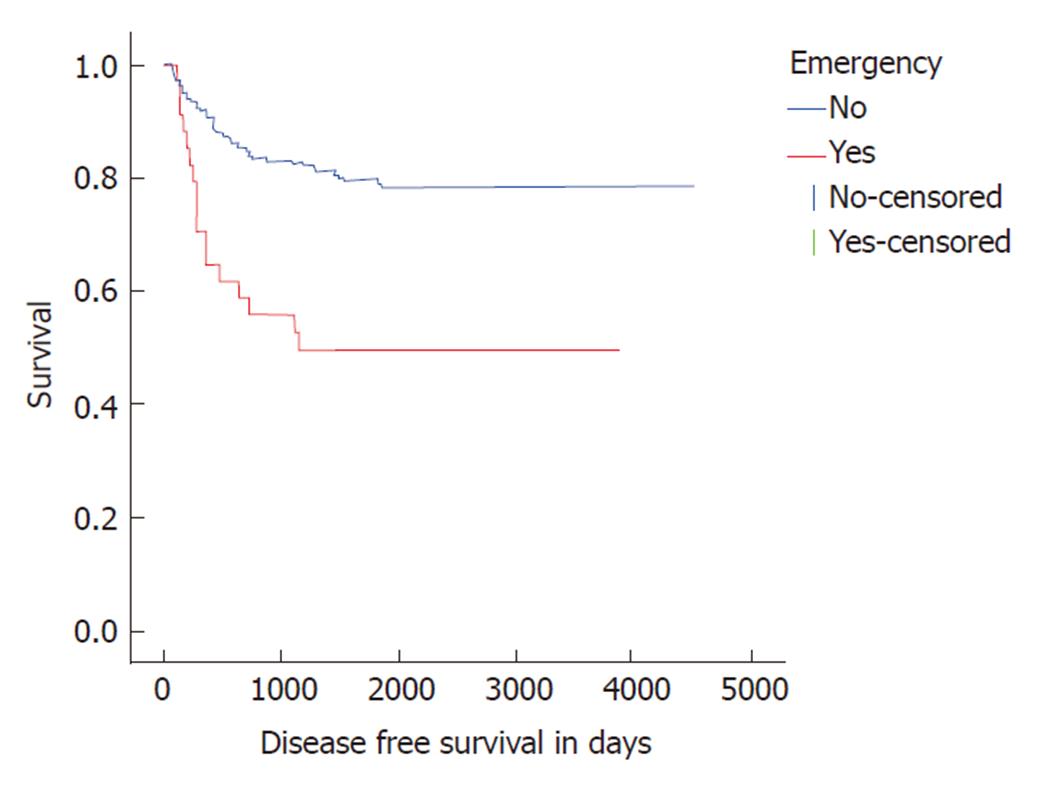
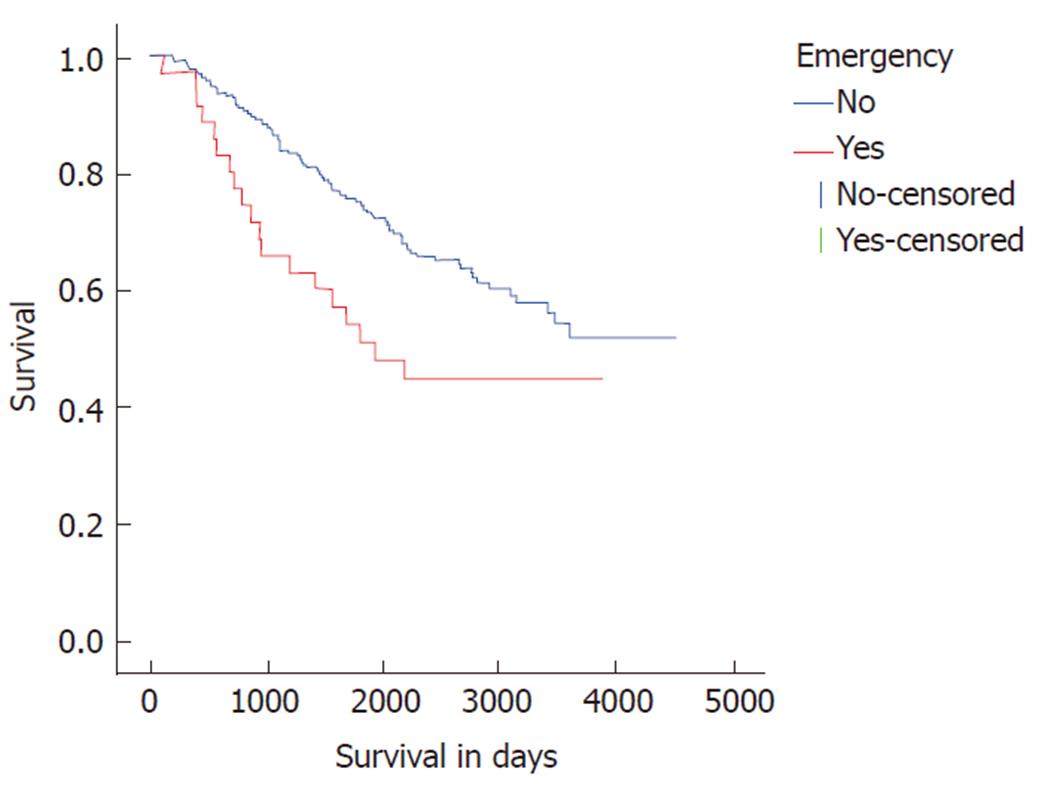
Patients undergoing emergency resection also displayed adverse disease free survival (DFS) and overall survival compared to elective resections. Median DFS and overall survival were poorer in the emergency resection at 1155 days (Log rank P < 0.001, Figure 1) and 1931 d (Log rank P = 0.027, Figure 2) respectively.
Recurrent disease was observed in 67 out of 264 patients with available data (25.38%) during the median follow-up period of 7.9 years (range 4.6 to 12.6 years). Amongst patients with recurrence, liver was the commonest site in 49 out of 67 (73.13%) patients with isolated liver disease in 41 patients. This was followed by lung disease in 14 (20.90%) patients with isolated disease in 4. Local recurrence was seen in 10 (14.93%) patients and 6 of whom had isolated local disease. Ten patients developed recurrence at multiple sites; 7 (10.45%) with concomitant liver and lung and 3 (4.58%) patients had lung metastasis with local recurrence.
Recurrence was predominantly early (< 3 years) affecting 55 patients (Table 3). The liver remained the commonest site of early recurrent disease with 35 out of 41 patients presenting with isolated liver disease. Early multi-site disease was seen in a small proportion of patients with liver disease (4 patients with liver and lung disease, 2 with liver and lung and local disease). In contrast, isolated lung disease was noted in only 3 out of 11 patients with early disease (4 patients had lung and liver disease, 2 had lung disease with local recurrence, 2 had lung, liver and local recurrence). Local recurrence was seen in 7 patients and was isolated in 3.
| Site of recurrence | Early recurrence (n = 55) | Colonic cancer (n = 151) | Rectal cancer (n = 115) | P1 |
| Liver | 41 (74.55%) | 25 | 16 | 0.554 |
| Lung | 11 (20.00%) | 6 | 5 | 0.879 |
| Local | 7 (12.73%) | 4 | 3 | 0.984 |
| > 1 site | 8 (14.55%) | 4 | 4 | 0.695 |
Recurrence between 3 to 5 years following curative resection represented 14.92% (10 patients) of overall recurrent disease. The liver remained the predominant site with 5 patients demonstrating isolated liver disease and 1 with liver and lung metastasis. Two patients presented with lung disease (1 isolated and 1 combined lung and liver disease) and 3 patients presented with isolated late local recurrence. Beyond the 5-year follow-up period, 1 patient developed isolated liver disease and 1 had liver and lung metastasis. No local recurrence was noted beyond 5-year follow-up.
Age, sex, tumour location, tumour differentiation and neo-adjuvant therapy did not show correlation with early all-site recurrent disease. Significant histological markers of early recurrence on univariate analyses included T4 stage (OR = 3.61, 95%CI: 1.88-6.96, P < 0.001), N2 nodal status (OR = 4.18, 95%CI: 1.89-9.24, P < 0.001), LVI (OR = 3.40, 95%CI: 1.79-6.46, P < 0.001), PNI (OR = 4.54, 95%CI: 1.51-13.60, P < 0.007) and R1 resection margin (OR = 4.51, 95%CI: 1.50-13.53, P = 0.007). LNR provided a strong correlation for early all-site recurrence on univariate analyses (OR = 25.55, 95%CI: 4.52-144.32, P < 0.001) with an optimal cut-off at 0.015 (52.7% sensitivity and 68.4% specificity). In a multivariate predictive model for early all-site recurrence, emergency surgery with perforation was the strongest predictor amongst factors including T4 stage and LVI.
For early liver disease, no correlation was noted with age, sex, site of tumour or tumour differentiation on univariate analysis. Emergency surgery was associated with a higher risk of recurrent disease in the liver (OR = 5.13, 95%CI: 2.33-11.30, P < 0.001), with an adverse outcome in the presence of perforation than obstruction alone (OR 4.78 vs 3.74). Histological factors demonstrating significance on univariate analysis included T4 stage (OR = 2.76, 95%CI: 1.32-5.76, P = 0.007), N2 stage (OR = 3.32, 95%CI: 1.42-7.76, P = 0.006), LVI (OR = 3.02, 95%CI: 1.49-6.10, P = 0.002), PNI (OR = 3.71, 95%CI: 1.17-11.80, P = 0.026) and R1 resection margin (OR = 3.70, 95%CI: 1.16-11.74, P = 0.027). LNR (OR = 11.69, 95%CI: 1.94-70.24, P = 0.007) provided AUC of 0.61 (95%CI: 0.51-0.71, P = 0.023) with the optimal cut-off at 0.015 (53.7% sensitivity and 67.3% specificity). A multivariate predictive model of presentation and histological features showed that perforation at the time of surgery was the strongest independent predictor of early liver recurrence amongst other markers of predictive value (Table 4).
| Early liver recurrence | B | SE | OR | 95%CI | |
| T4 | 0.453 | 0.440 | 0.303 | 1.574 | 0.664-3.729 |
| N2 | 0.706 | 0.540 | 0.191 | 2.025 | 0.703-5.831 |
| LVI | 0.974 | 0.456 | 0.033 | 2.648 | 1.084-6.468 |
| PNI | 0.735 | 0.700 | 0.294 | 2.085 | 0.529-8.214 |
| R1 | 0.954 | 0.726 | 0.189 | 2.597 | 0.626-10.777 |
| Obstruction | 1.385 | 0.514 | 0.007 | 3.995 | 1.457-10.949 |
| Perforation | 1.958 | 0.781 | 0.012 | 7.086 | 1.533-32.749 |
No independent predictors were identified for early lung recurrence. Early local recurrence represented a very small number of patients (7) for detailed analyses.
Long-term survival data was available from 262 patients with a median survival of 9.9 years. One-, three- and five-year survival in the study cohort was 96%, 82% and 72% respectively. Emergency surgery was associated with poorer survival (log rank P = 0.027) with 1, 3 and 5 year survival at 90%, 65% and 50% respectively (Figure 2). Multivariate Cox regression demonstrated T4 status to be a stronger predictor of adverse outcome than emergency surgery (HR 1.96, 95%CI: 1.27 to 3.02, P = 0.003). No significant difference in long-term survival was noted in patients with colonic or rectal disease (log rank P = 0.217).
Amongst histological features, T4 disease (HR = 1.65, 95%CI: 1.05-2.60, P = 0.031), PNI (HR = 3.20, 95%CI: 1.72-5.97, P < 0.001) and LNR were independent predictors of adverse outcome in a multivariate Cox regression model. LNR was associated with the greatest risk of early death (HR = 11.87, 95%CI: 3.98-35.36, P < 0.001).
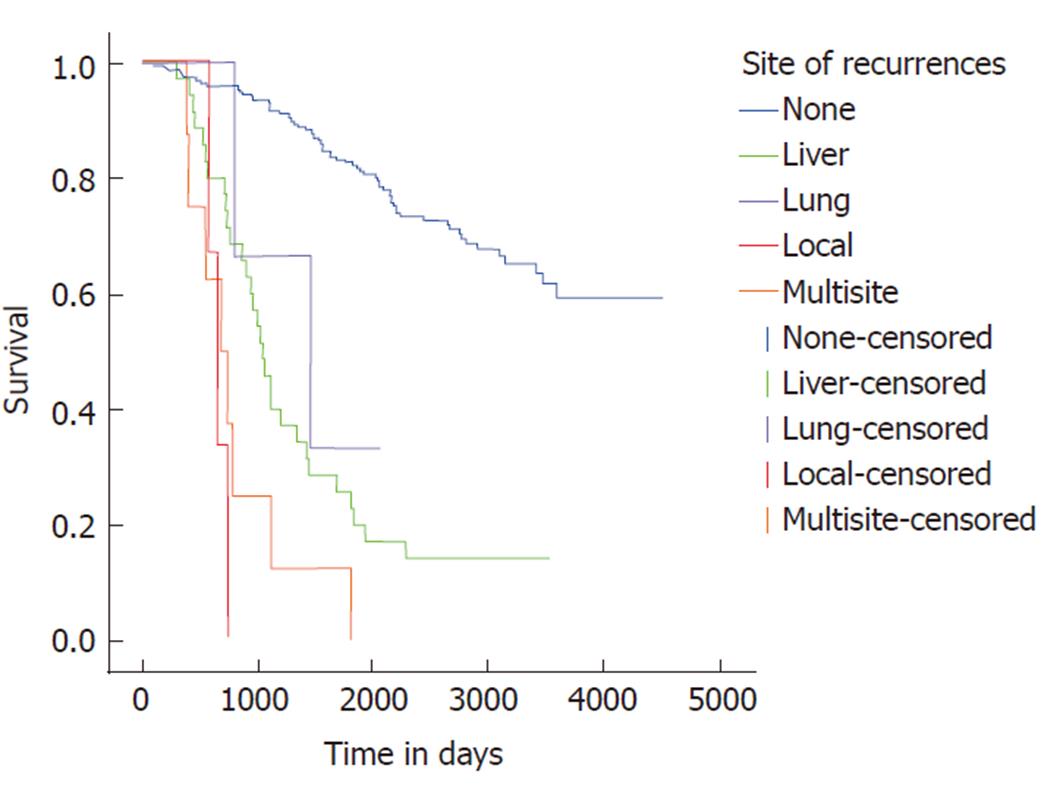
Median survival with early recurrence was 2.62 years (95%CI: 1.92-3.31). In the presence of early liver recurrence, median survival was 2.78 years (95%CI: 2.27-3.39); early lung disease was 2.17 years (95%CI: 1.82-2.53) and early local recurrence was 1.80 years (95%CI: 1.27-2.33). For overall recurrence, isolated liver or lung only metastatic disease showed a better survival pattern than multi-site or local recurrent disease (Figure 3). Median survival in isolated liver disease was 2.87 years (95%CI: 2.39-3.35), which was not significantly different compared to 3.98 years (95%CI: 1.12-6.85) for isolated lung recurrence. Multi-site disease and local disease were associated with significantly adverse survival compared to isolated liver disease.
Emergency resection for colorectal cancer presents a high risk group with increased risk of early recurrence and adverse long-term survival. Right colonic tumours and T4 stage are prognostic predictors for emergency colorectal cancer resection. T4 stage, LVI and emergency resection also offer a strong prognostic prediction for development of recurrent disease identifying high-risk patients for aggressive surveillance or adjuvant therapies.
Emergency colorectal resection constituted 13.2% of patients undergoing curative surgery and did not constitute all emergency presentations with symptomatic colorectal cancer. This is smaller than the reported national average of up to 30%[17,26] and may be the result of patient selection practices and the emerging role of self-expanding metal stent for acute malignant large bowel obstruction. Emergency surgery in our cohort was associated with poorer early and long-term survival. Hogan et al[20] concluded similarly that emergent surgery was associated with higher rates of local recurrence and poorer disease-free survival. Patients presenting in an emergency setting with advanced disease may have a distinct, aggressive tumour with unfavourable biology[22] and therefore these patients may be more prone to early recurrence. Furthermore, in the emergency setting patients have altered physiology and are immunosuppressed so that tumour dissemination in these circumstances occurs more easily[21]. Correlation between the increased morbidity of emergency surgery and timely or successful progression to adjuvant therapies was not specifically evaluated. The use of self-expanding colonic stents offers to address the associated high morbidity and mortality with emergency surgery[27,28].
Lymph node involvement, along with depth of bowel wall invasion, has represented an important prognostic indicator for metachronous metastasis[29]. This is in broad correlation with our study. Nodal status was noted to be an independent predictor in early all-site recurrence, but no independent predictive value was noted for early liver and lung recurrent disease. In the setting of emergency surgery, where adequate lymph node yield may be compromised, LNR may prove to be a more useful prognostic tool since the patient’s physiology might not tolerate prolonged surgery in order to attain a good lymph node yield.
Keum et al[30] concluded that high risk factors for recurrence include rectal cancer, T2 stage and an infiltrative growth pattern. However, this study only looked at patients who underwent resection for stage I colorectal cancer which may explain their finding of T2 stage compared with ours of T4 stage. It has been stated previously that rectal tumours and younger age at presentation have a higher recurrence risk[31]. However, this was not the case in our study for reasons that are not clear to us.
LVI was also found to be an independent predictor of early all-site recurrence in a similar study by Huh et al[32] however they classified early recurrence as less than 1 year after operation. Lim et al[33] found that after attempted curative resection, patients with LVI-positive tumours had a higher rate of all-site recurrence than those without LVI. This is in correlation with our study.
Up to 50% of patients develop hepatic metastases within the first three years after curative resection[3]. In our study, overall recurrence occurred in 25% of the patient population, with 82% of the recurrence diagnosed within three years after resection. This is commensurate with the reported literature[4,34]; Meyerhardt et al[4] reports 80% of recurrences occur within the first three years. The liver was by far the most common site for early recurrence with 74% of cases occurring there. This is due to haematogenous spread via the portal venous system[35]. Pietra et al[36] also reported that 65% of recurrence occurred in the liver, in keeping with our observation.
Median survival of patients with early liver recurrence was 2.78 years. This compares favourably with patients presenting with early local recurrence who had a median survival of 1.8 years. This improved outcome may be a reflection of a more aggressive approach of isolated liver disease with the advent of improved liver parenchyma sparing techniques and advances in surgical techniques and perioperative care. The 5-year survival rate for patients that undergo curative metachronous resection of four or less hepatic lesions is 24%-58%[37-40]. A 5-year survival of 24% has also been reported for curative metachronous resection of > 8 hepatic lesions[41]. Detection of metachronous disease as early as possible is imperative in limiting the extent of resection and improving survival. Renehan et al[15] highlighted the value of intensive follow-up by demonstrating a high detection of early metachronous disease by aid of CEA and computed tomography. This has delivered an opportunity for early detection and planning of definitive intervention. This may allow for more liver parenchymal-sparing techniques in the form of metastectomies and also improve the success of redo-hepatectomies[42]. The earlier the diagnosis is made, the more likely it is going to be resectable[43].
The lungs are the second most common location for metastatic spread of colorectal cancer[44]. It occurs in 5%-15% of patients and not all of these have concurrent liver metastases[45]. Only 4.1% of patients with synchronous pulmonary metastases are resectable, whereas 14.8% of patients with metachronous pulmonary metastases are resectable[46] with a 36%-40% 5-year survival rate in resected patients[47]. In our study, 14 (5.2%) patients developed lung metastases in the follow-up period with a median survival period of 2.17 years. Nodal status and Dukes stage were predictive of early lung recurrence on univariate analysis but not multivariate analysis. This result should be treated with caution due to the limited number of patients who developed lung metastases. This is in contrast to a similar study by Kim et al[48] that reported a 3 year overall survival rate of 54.6%, although their study included 105 patients. Our study only contained 14 patients who developed lung metastases and it is possible that increased survival may have occurred with more patients in this category.
Negative predictive features when considering a patient for pulmonary metastatectomy follow a similar pattern to metastasis elsewhere and include an unresectable primary tumour, extra-pulmonary metastases, resection margins (R1/2) and mediastinal lymph node disease[49]. Blackmon et al[50] concluded that more than three lung metastases present at the first metastatectomy and a preoperative disease free survival of less than three years predicts recurrence. This suggests that early pickup of metastases means more patients can be considered for a successful resection. However, due to a small number of patients with lung disease in our cohort, no meaningful analyses of multiple clinical and pathological factors could be carried out.
The value of early detection of metastatic disease in offering an absolute reduction in mortality is clear[15]. Predictive tools can aid the clinician in identifying patients at higher risk of early metachronous metastasis as not all patients will benefit from aggressive surveillance. Our results suggest that there may be a subgroup of patients who would benefit from more intensive follow-up. As most tumours recur within the first three years after resection[34,51], it is imperative that the focus on follow-up occurs during this time frame. However, there is a lack of specific guidance for patients who may be at increased risk.
Current surveillance protocols consist of a combination of CEA testing, CT scans, and colonoscopy. Emergency surgery and the presence of specific histological features can inform the selection of patients at high-risk of early recurrence and should be factored into surveillance strategies. There is a substantial range in intensity of follow-up with the United Kingdom having noticeably less intensive follow-up in comparison to American collaborators[4,21,22]. A recent United Kingdom study of 1202 participants who had undergone curative surgery for primary colorectal cancer found no survival benefit from combined intensive monitoring groups compared to follow-up only if symptoms recurred[52]. This study was a randomised trial and so highlights the need to target the patients that would benefit from an intensive follow-up regimen. This has benefits for all stakeholders; earlier detection of recurrence in high-risk patients and a reduction of unnecessary investigations in the low-risk group. Exciting developments in the availability of other prognostic markers in the future may enhance the efficacy of a risk-adapted follow-up strategy[53]. The overexpression of vascular endothelial growth factor[54] and interleukin-8[55] in colorectal carcinoma cells are two such examples.
Our study is limited, foremost by the retrospective approach of data gathering using the hospital coding process. The weaknesses of such a design are well known[56]. Secondly, relevant data on neoadjuvant and adjuvant therapies and cause of death was not available to afford reliable assessment of correlation. More patients in the emergency resection group received postoperative adjuvant therapies which may have been the result of a higher tumour stage (T4), early recurrence disease or palliative treatment. Indication for post-operative therapies and cause of death outcomes were not collected and survival was calculated on basis of all-cause mortality. The majority of recurrent disease occurred in the liver, leading to more reliable statistical conclusions here. However, this was limited in number for both lung and local recurrent disease reflecting differences in association of histological and clinical predictive features. Furthermore, low event rates in neo-adjuvant therapies and positive resection margins, missing data on total number of lymph nodes harvested, limited reliable evaluation of these factors in overall outcomes.
Although emergency presentation in the form of obstruction continues to represent a significant proportion of patients with initial diagnosis, self-expanding colonic metal stents are likely to play an increasingly important role in improving immediate postoperative outcomes, stoma rates and long-term outcomes without adverse oncological outcomes[28]. Proven survival benefit from primary and redo liver resections in isolated disease and emerging technologies for palliative control of local and distant metastatic disease mean that predictive clinicopathological markers could be used in a more intensive, targeted surveillance strategy to identify more patients with early recurrence. Emergency resection, tumour stage, lymphovascular invasion and lymph node ratio > 0.015 represent a high-risk of recurrent disease and can inform surveillance strategies to enable early interventions.
Colorectal cancer is the fourth most common cancer in the United Kingdom with over 40000 cases diagnosed each year. Despite the widespread use of screening programs, a large number of cases are diagnosed in the acute or urgent setting with adverse post-operative mortality, disease-free and overall long-term survival.
A large proportion of patients with colorectal cancer are diagnosed in the acute setting with an emergency presentation (26%) or following an urgent 2-wk referral (27%). Compared to elective resection, emergency surgery is associated with adverse postoperative outcomes (post-operative mortality 4.6% vs 16%), disease-free and overall long-term survival. The basis for this is multifactorial and may include altered physiology, immune-suppression, adverse tumour biology, advanced disease, peri-operative complications and lower progression to adjuvant therapies.
Adverse predictive factors for survival in colorectal cancer include emergency presentation with obstruction or perforation and histo-pathological features such as T4 disease, advanced nodal disease. Lymph node ratio offers to a new representation of nodal disease. Although it is affected directly but the lymph node yield, its utility as a predictive tool for recurrent disease has not been evaluated. We aimed to identify clinical and histological predictive factors for early recurrence disease and pattern to inform surveillance strategies and aid in early detection.
Following institutional approval, a retrospective study of clinical and histo-pathological parameters was carried out to study patterns of recurrence and survival in consecutive patients undergoing elective and emergency resection for colorectal cancer over an eight-year study period.
Outcomes were evaluated in 266 consecutive patients following curative surgery with a median follow-up of 7.9 years. The proportion of patients undergoing emergency resection was 13.2%. Recurrent disease was detected in 67 patients (25.2%) during follow-up with the majority identified early within 3 years (82.1%). Liver was the predominant site of metastatic disease (73.1%). Emergency resection (OR = 3.60, P = 0.001), T4 stage (OR = 4.33, P < 0.001) and lymphovascular invasion (LVI) (OR = 2.37, P = 0.032) were associated with higher risk of recurrent disease. Emergency resection, T4 disease and a high lymph node ratio (LNR) were strong independent predictors of adverse long-term survival.
Our study reaffirms the independent predictive potential of histological and clinical features for recurrent disease in patients undergoing emergency resection for colorectal cancer. Furthermore, it introduces the independent utility of lymph node ratio (LNR) alongside T stage and lympho-vascular invasion in identifying patients with high risk of recurrent disease.
Modified surveillance strategies should be evaluated in presence of adverse clinical and histological factors to improve early detection of recurrent disease in high-risk patients to offset adverse disease-free and overall long-term survival.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Mayol J, Nishida T, Zhu X S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Cancer Research UK. Bowel cancer incidence statistics. [accessed 2014 Jun]. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/incidence/. [Cited in This Article: ] |
| 2. | Tan EK, Ooi LL. Colorectal cancer liver metastases - understanding the differences in the management of synchronous and metachronous disease. Ann Acad Med Singapore. 2010;39:719-715. [PubMed] [Cited in This Article: ] |
| 3. | Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, Primrose JN, Parks RW. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55 Suppl 3:iii1-iii8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, Petrelli NJ, Ryan K, Schrag DH, Wong SL. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31:4465-4470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 5. | Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 622] [Cited by in F6Publishing: 645] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 6. | Nozawa H, Sunami E, Nakajima J, Nagawa H, Kitayama J. Synchronous and metachronous lung metastases in patients with colorectal cancer: A 20-year monocentric experience. Exp Ther Med. 2012;3:449-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Landmann RG, Weiser MR. Surgical management of locally advanced and locally recurrent colon cancer. Clin Colon Rectal Surg. 2005;18:182-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Read TE, Mutch MG, Chang BW, McNevin MS, Fleshman JW, Birnbaum EH, Fry RD, Caushaj PF, Kodner IJ. Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J Am Coll Surg. 2002;195:33-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, Johnston M, McCormack P, Pass H, Putnam JB Jr; International Registry of Lung Metastases. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1072] [Cited by in F6Publishing: 1019] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 10. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1478] [Cited by in F6Publishing: 1383] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 11. | Bruinvels DJ, Stiggelbout AM, Kievit J, van Houwelingen HC, Habbema JD, van de Velde CJ. Follow-up of patients with colorectal cancer. A meta-analysis. Ann Surg. 1994;219:174-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 244] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR, D’Angelica MI. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744-752, 752-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 334] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 13. | Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007;CD002200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Figueredo A, Rumble RB, Maroun J, Earle CC, Cummings B, McLeod R, Zuraw L, Zwaal C; Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer. 2003;3:26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 291] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 15. | Renehan AG, Egger M, Saunders MP, O’Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 436] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | Renehan AG, O’Dwyer ST, Whynes DK. Cost effectiveness analysis of intensive versus conventional follow up after curative resection for colorectal cancer. BMJ. 2004;328:81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | National Cancer Intelligence Network data briefing. Routes to diagnosis. NCIN, November 2010. [accessed 2015 Mar]. Available from: http://www.ncin.org.uk/databriefings. [Cited in This Article: ] |
| 18. | Wong SK, Jalaludin BB, Morgan MJ, Berthelsen AS, Morgan A, Gatenby AH, Fulham SB. Tumor pathology and long-term survival in emergency colorectal cancer. Dis Colon Rectum. 2008;51:223-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Morris EJ, Taylor EF, Thomas JD, Quirke P, Finan PJ, Coleman MP, Rachet B, Forman D. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut. 2011;60:806-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 20. | Hogan J, Samaha G, Burke J, Chang KH, Condon E, Waldron D, Coffey JC. Emergency presenting colon cancer is an independent predictor of adverse disease-free survival. Int Surg. 2015;100:77-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Vallejo R, Hord ED, Barna SA, Santiago-Palma J, Ahmed S. Perioperative immunosuppression in cancer patients. J Environ Pathol Toxicol Oncol. 2003;22:139-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Ghazi S, Berg E, Lindblom A, Lindforss U; Low-Risk Colorectal Cancer Study Group. Clinicopathological analysis of colorectal cancer: a comparison between emergency and elective surgical cases. World J Surg Oncol. 2013;11:133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Baca B, Beart RW Jr, Etzioni DA. Surveillance after colorectal cancer resection: a systematic review. Dis Colon Rectum. 2011;54:1036-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | The Association of Coloproctologists of Great Britain and Ireland. Guidelines for the Management of Colorectal Cancer. Third edition. 2007;. [Cited in This Article: ] |
| 25. | Benson AB, Arnoletti P, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Dilawari RA, Engstrom PF, Enzinger PC. Clinical practice and guidelines in oncology (National Comprehensive Cancer Network Guidelines) Colon Cancer NCCN Version 3, 2014. [Cited in This Article: ] |
| 26. | Scott N, Hill J, Smith J, Kelly S, Fearnhead N. National Bowel Cancer Audit Annual Report. Association of Coloproctologist of Great Britain and Ireland. 2013;. [Cited in This Article: ] |
| 27. | Tan CJ, Dasari BV, Gardiner K. Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg. 2012;99:469-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 28. | Hill J, Kay C, Morton D, Magill L, Handley K, Gray R. CREST Trial Collaborative Group CREST: Randomised phase III study of stenting as a bridge to surgery in obstructing colorectal cancer-Results of the UK ColoRectal Endoscopic Stenting Trial (CREST). J Clin Oncol. 2016;34:Abstr 3507. [Cited in This Article: ] |
| 29. | Liang JL, Wan DS, Pan ZZ, Zhou ZW, Chen G, Li LR, Lu ZH, Wu XJ. [Multivariate regression analysis of recurrence following curative surgery for colorectal cancer]. Ai Zheng. 2004;23:564-567. [PubMed] [Cited in This Article: ] |
| 30. | Keum MA, Lim SB, Kim SA, Yoon YS, Kim CW, Yu CS, Kim JC. Clinicopathologic factors affecting recurrence after curative surgery for stage I colorectal cancer. J Korean Soc Coloproctol. 2012;28:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Hellinger MD, Santiago CA. Reoperation for recurrent colorectal cancer. Clin Colon Rectal Surg. 2006;19:228-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Huh JW, Kim CH, Lim SW, Kim HR, Kim YJ. Early recurrence in patients undergoing curative surgery for colorectal cancer: is it a predictor for poor overall survival? Int J Colorectal Dis. 2013;28:1143-1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Lim SB, Yu CS, Jang SJ, Kim TW, Kim JH, Kim JC. Prognostic significance of lymphovascular invasion in sporadic colorectal cancer. Dis Colon Rectum. 2010;53:377-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, Labianca R, Seitz JF, O’Callaghan CJ, Francini G. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664-8670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 491] [Cited by in F6Publishing: 504] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 35. | Bar-Gil Shitrit A, Shitrit D, Bakal I, Braverman D, Kramer MR. Endobronchial metastases from colon cancer without liver metastases: report of eight cases. Dis Colon Rectum. 2007;50:1087-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Pietra N, Sarli L, Costi R, Ouchemi C, Grattarola M, Peracchia A. Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study. Dis Colon Rectum. 1998;41:1127-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 241] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, Cottier B, Poston G. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97:1110-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 266] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 38. | de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 534] [Cited by in F6Publishing: 555] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 39. | Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg. 2004;240:438-447; discussion 447-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 421] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 40. | Jamison RL, Donohue JH, Nagorney DM, Rosen CB, Harmsen WS, Ilstrup DM. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg. 1997;132:505-510; discussion 511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 245] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 41. | Malik HZ, Hamady ZZ, Adair R, Finch R, Al-Mukhtar A, Toogood GJ, Prasad KR, Lodge JP. Prognostic influence of multiple hepatic metastases from colorectal cancer. Eur J Surg Oncol. 2007;33:468-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Allen PJ, Jarnagin WR. Current status of hepatic resection. Adv Surg. 2003;37:29-49. [PubMed] [Cited in This Article: ] |
| 43. | Adam R, Lucidi V, Bismuth H. Hepatic colorectal metastases: methods of improving resectability. Surg Clin North Am. 2004;84:659-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | McCormack PM, Attiyeh FF. Resected pulmonary metastases from colorectal cancer. Dis Colon Rectum. 1979;22:553-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 111] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Tan KK, Lopes Gde L Jr, Sim R. How uncommon are isolated lung metastases in colorectal cancer? A review from database of 754 patients over 4 years. J Gastrointest Surg. 2009;13:642-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 2010;59:1383-1388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 248] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 47. | Fiorentino F, Hunt I, Teoh K, Treasure T, Utley M. Pulmonary metastasectomy in colorectal cancer: a systematic review and quantitative synthesis. J R Soc Med. 2010;103:60-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 48. | Kim CH, Huh JW, Kim HJ, Lim SW, Song SY, Kim HR, Na KJ, Kim YJ. Factors influencing oncological outcomes in patients who develop pulmonary metastases after curative resection of colorectal cancer. Dis Colon Rectum. 2012;55:459-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Treasure T, Milošević M, Fiorentino F, Macbeth F. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax. 2014;69:946-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 50. | Blackmon SH, Stephens EH, Correa AM, Hofstetter W, Kim MP, Mehran RJ, Rice DC, Roth JA, Swisher SG, Walsh GL. Predictors of recurrent pulmonary metastases and survival after pulmonary metastasectomy for colorectal cancer. Ann Thorac Surg. 2012;94:1802-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Berman JM, Cheung RJ, Weinberg DS. Surveillance after colorectal cancer resection. Lancet. 2000;355:395-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, George S, Mant D; FACS Trial Investigators. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 319] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 53. | Rose J, Augestad KM, Cooper GS. Colorectal cancer surveillance: what’s new and what’s next. World J Gastroenterol. 2014;20:1887-1897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Toiyama Y, Inoue Y, Saigusa S, Okugawa Y, Yokoe T, Tanaka K, Miki C, Kusunoki M. Gene expression profiles of epidermal growth factor receptor, vascular endothelial growth factor and hypoxia-inducible factor-1 with special reference to local responsiveness to neoadjuvant chemoradiotherapy and disease recurrence after rectal cancer surgery. Clin Oncol (R Coll Radiol). 2010;22:272-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Lurje G, Zhang W, Schultheis AM, Yang D, Groshen S, Hendifar AE, Husain H, Gordon MA, Nagashima F, Chang HM. Polymorphisms in VEGF and IL-8 predict tumor recurrence in stage III colon cancer. Ann Oncol. 2008;19:1734-1741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 56. | Audit commission. PbR Data assurance Framework 2007/2008. London: Audit Commission; . [Cited in This Article: ] |