Copyright
©The Author(s) 2016.
World J Gastrointest Pathophysiol. Feb 15, 2016; 7(1): 27-37
Published online Feb 15, 2016. doi: 10.4291/wjgp.v7.i1.27
Published online Feb 15, 2016. doi: 10.4291/wjgp.v7.i1.27
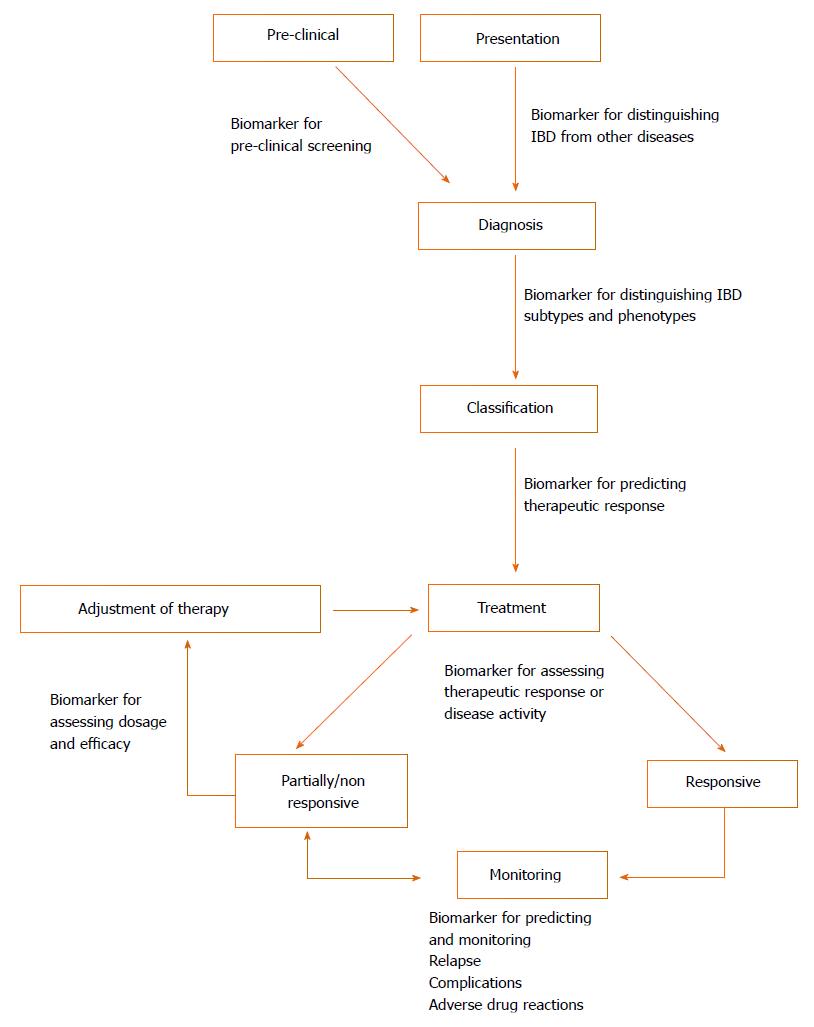
Figure 1 Potential application of biomarkers in inflammatory bowel disease in different stages of clinical management.
When presenting clinically, one important use of biomarkers could be in the diagnosis of IBD, as well as differentiating subtypes (e.g., UC vs CD) and phenotypes (e.g., fistulising). Whilst not currently part of management, preclinical screening for IBD may be a possibility. Biomarkers can also be used to predict response to therapy and objectively measure therapeutic response and disease severity. Due to the relapsing and remitting course of IBD, monitoring is necessary for assessing relapse, adverse outcomes and complications (e.g., strictures, fistulas and colorectal cancer). Most of these aspects necessitate endoscopic procedures and would benefit from biomarker substitutes. CD: Crohn’s disease; UC: Ulcerative colitis; IBD: Inflammatory bowel disease.
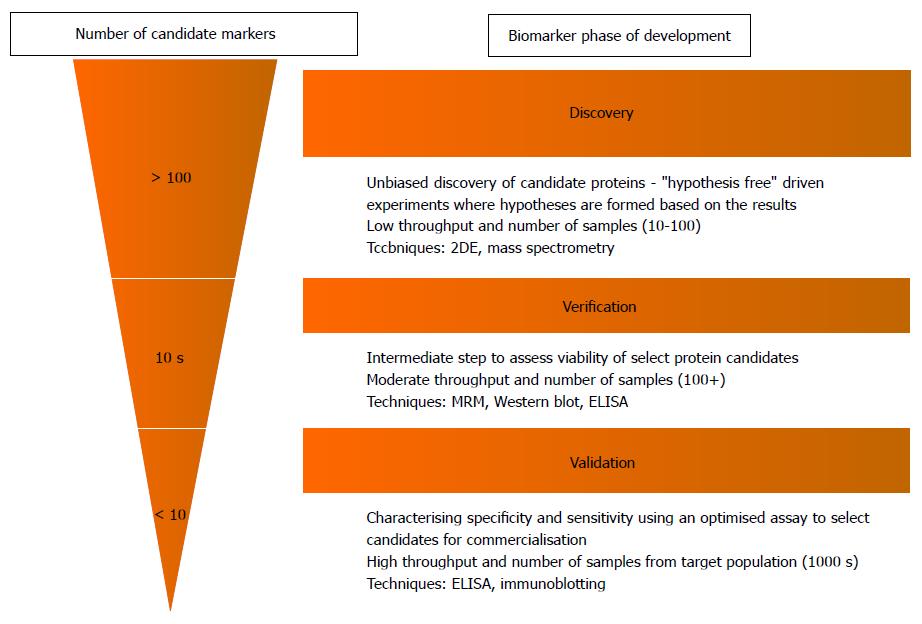
Figure 2 Biomarker “Pipeline” indicating the various stages from biomarker discovery to clinical application[20].
The number of candidate proteins (rough estimate of numbers indicated in figure) is narrowed down significantly in each step, selecting only the best candidates for further assessment and characterisation in a larger sample. The methodology also varies between the different phases. The early discovery phase uses low throughput methods such as 2-DE and mass spectrometry to screen large numbers of proteins in a low number of samples. Verification and validation require much more accurate quantitative methods as candidate proteins are narrowed down from the discovery phase and are assessed for their clinical utility in a large target population. This requires higher throughput methods such as MRM and immunoassays such as ELISA. CD: Crohn’s disease; UC: Ulcerative colitis; IBD: Inflammatory bowel disease; MRM: Multiple reaction monitoring.
- Citation: Chan PP, Wasinger VC, Leong RW. Current application of proteomics in biomarker discovery for inflammatory bowel disease. World J Gastrointest Pathophysiol 2016; 7(1): 27-37
- URL: https://www.wjgnet.com/2150-5330/full/v7/i1/27.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i1.27