Published online May 28, 2017. doi: 10.4329/wjr.v9.i5.217
Peer-review started: November 2, 2016
First decision: February 15, 2017
Revised: February 23, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 28, 2017
Cholesteatoma is a collection of keratinous debris and stratified squamous epithelium. It is trapped in the middle ear and can lead to bony erosion. The disease is treated surgically often followed by a second-look procedure to check for residual tissue or recurrence. Cholesteatoma has specific signal-intensity characteristics on magnetic resonance imaging with very high signal intensity on diffusion weighted imaging (DWI). Various DWI techniques exist: Echo-planar imaging (EPI)-based and non-EPI-based techniques as well as new approaches like multi-shot EPI DWI. This article summarizes all techniques, discusses the significance in detecting cholesteatoma and mentions actual studies. Further recommendations for daily clinical practise are provided.
Core tip: Imaging cholesteatoma is either performed by computed tomography (CT) or by magnetic resonance imaging (MRI). CT is the method of choice for detection and for assessing exact location and extent. MRI with diffusion weighted imaging (DWI) is a powerful tool for the detection of local recurrence or residual cholesteatoma. Many DWI-techniques are available today; this review article gives an overview of the different sequences and the diagnostic procedure when using DWI with a clinical focus.
- Citation: Henninger B, Kremser C. Diffusion weighted imaging for the detection and evaluation of cholesteatoma. World J Radiol 2017; 9(5): 217-222
- URL: https://www.wjgnet.com/1949-8470/full/v9/i5/217.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i5.217
Cholesteatomas are defined as enlarging collections of keratinous debris within a sack of stratified squamous epithelium trapped in the middle ear[1]. It is a common inflammatory disease that grows progressively as the debris increases. It is seen as a kind of chronic otitis media with cell proliferation due to repeated inflammation or for congenital reason. Clinically the complications of cholesteatoma are related to bony erosion and destruction which is thought to be related to mechanical pressure. Evan small cholesteatoma can cause ossicular chain erosions with the threat of a conductive hearing loss. The diagnosis is usually made on clinical features.
Cholesteatomas of the middle ear are managed by surgery, generally with complete excision of the lesion with tympanoplasty or radical or modified radical mastoidectomy. This is often followed by a second-look procedure performed to check for residual or recurrent disease. This second-look is conducted 6-18 mo after the initial operation because most recur within the first 2 postoperative years, with 60% occurring during the first year after surgery[2,3]. The second-look surgery is mainly to assess residual or recurrent disease because both cannot adequately be diagnosed solely by clinical examination[4].
Computed tomography (CT) of the temporal bone is widely accepted to detect or confirm cholesteatoma and to assess the extension, the exact location and possible complications of the disease. Therefore it is mandatory for the initial preoperative description of the extent of cholesteatoma and for correct surgical planning. CT is further recommended for the evaluation of recurrent disease but it is not reliable when the postoperative, formed cavity is completely filled with a soft-tissue mass or partially filled with nonspecific imaging abnormalities[5,6]. This can be caused by recurrent cholesteatoma, granulation or fibrous tissue. This differential diagnosis is important since recurrent cholesteatoma needs middle ear surgery but there is no need for surgery if only granulation tissue is detected.
Magnetic resonance imaging (MRI) has several advantages over CT in detecting recurrent or residual disease: Beside delayed contrast-enhanced T1-weighted spin-echo (SE) imaging[7], diffusion weighted imaging (DWI) shows promising results in the data published so far[8-12]. Contrast-enhanced MRI can discriminate between the non-enhancing cholesteatoma and other contrast-enhancing findings, e.g., inflammation, scar or granulation tissue[7,13]. DWI is more practical with a shorter examination time than delayed contrast material-enhanced imaging; there is also no need for contrast injection. The technique relies on the principles of the Brownian motion of water molecules[14]. Cholesteatomas appear hyperintens on DWI obtained with b-factors of 800 or 1000 s/mm2 where the b-factor is a measure of the strength of the respective diffusion weighting. This visual characteristic is similar to a histologically identical lesion, the epidermoid cyst[15] - granulation tissue, fibrous tissue, cholesterol granuloma or serous fluid, on the other hand, have low signal intensity on DWI (at a b-factor of 800 s/mm2). Visual assessment of DWI images obtained with a b-factor of 800 s/mm2 without calculation of the apparent diffusion coefficient (ADC) is sufficient for the respective diagnostic analysis[9,12]. The reason for the high signal intensity is assumed to be due to a T2 shine-through effect or due to the restricted molecular diffusion of cholesteatoma. The T2 shine-through effect is observed in lesions with a prolonged relaxation time. Nevertheless, the real reason for the increased signal intensity on DWI is still unknown and under discussion in literature. DWI is a valuable tool to prevent unnecessary second-look surgeries in patients suspected for cholesteatomas and is therefore a reliable alternative to CT[16]. However, numerous artefacts can be generated during the acquisition of DWI, as, e.g., susceptibility artefacts, motion artefacts, ghosting artefacts and eddy current artefacts with the risk of false positive results[17].
So far a variety of different DWI-techniques has been used, which basically can be divided into echo-planar imaging (EPI)-based and non-EPI-based techniques. The choice of the actually used technique is thereby mainly influenced by the fact that imaging has to be performed near the scull base where problems due to different artefacts (e.g., motion, field inhomogeneities) can occur.
In addition DWI MRI is extremely useful for the assessment of possible complications such as erosion of the semicircular canal or invasion of the membranous labyrinth or the middle cranial fossa and to assess abscess formations[17].
Single-shot (SS) EPI-DWI can be seen as a widely available standard DWI technique. It is relatively insensitive to motion but prone to susceptibility artefacts, chemical shift and geometric distortion[14]. These artefacts can mask areas of restricted diffusion in a cholesteatoma[18]. A further limitation of EPI-DWI is its low spatial resolution and relatively thick sections. The size limit to detect a cholesteatoma with EPI-DWI is approximately 5 mm[9,11].
Turbo spin-echo (TSE)-based DWI is a spin-echo based SS or multi-shot (MS) technique with longer echo time and a higher signal-to-noise ratio than SS EPI-DWI. The sequence is known to lack significant image distortions and it does not show the susceptibility artefacts that are observed with standard EPI-DWI. Therefore a better spatial resolution in the middle ear is possible and it permits fast multiplanar imaging[19]. Furthermore thinner slices can be obtained than with EPI-sequences. This so called non-EPI sequence has therefore hardly any false-positive findings. False-negative findings are mostly a consequence of motion or empty retraction pockets[20]. The signal intensity of other postoperative findings has been reported much lower than that of residual and/or recurrent cholesteatoma[19,21].
In a study by Geoffray et al[22] non-EPI DWI was reliable to diagnose recurrent cholesteatoma also in children with a high sensitivity (87%) and specificity (71%). Nevertheless, they concluded that follow-up must be prolonged because small recurrence less than 5 mm may be missed.
The TSE-DWI can be combined with half-Fourier acquisition single-shot turbo spin-echo (HASTE), a single-shot technique with excellent motion insensitivity. HASTE is also less prone to susceptibility artefacts and geometric distortion than the EPI-Sequence[23,24]. The sensitivity and specificity of non-EPI DWI in depicting residual or recurrent cholesteatoma is very high, in literature it is between 90%-100% and also postulated higher than with EPI DWI[19-21,23-25]. The study by De Foer et al[25] prospectively evaluated a SS TSE-DWI sequence in detecting cholesteatoma with evaluation of the size of the middle ear cholesteatoma. They found 21 middle ear cholesteatomas at surgery with a size between 2 mm and 19 mm and 19/21 could be detected with DWI. Years later another study by De Foer et al[26] compared non-EPI DWI, delayed gadolinium-enhanced T1-weighted MRI and the combination of both techniques in the evaluation of patients with cholesteatoma. Sensitivity and specificity was 56.7%/67.6% with the delayed gadolinium-enhanced T1-weigthed images, 82.6%/87.2% with the non-EPI DWI images and 84.2%/88.2% for the combination of both kinds of images. They concluded that for the detection of cholesteatoma non-EPI DWI can be used alone.
Non-EPI sequences with periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER, GE Medical Systems, Milwaukee, Wisconsin/BLADE, Siemens Medical Solutions, Erlangen, Germany) have been reported as useful in avoiding geometric distortions. The k-space data are acquired in the form of rotating sections (blades). The resulting oversampling of the central k-space leads to an improved signal-to-noise ratio (SNR) and to the reduction of motion and susceptibility artefacts[23,27,28]. MS TSE-based DWI increases sensitivity, specificity and diagnostic accuracy compared to conventional single-shot EPI DWI[29].
In a systematic review of DWI in the assessment of postoperative cholesteatoma by Jindal et al[30] a combined sensitivity of 91.4% and positive predictive value of 97.3% was calculated for the non-EPI sequences. Non-EPI also showed a negative predictive value of 85% which means that it is very useful in avoiding second-look operations in healthy ears[30].
Recently it has been shown that an improved, MS EPI approach can provide high-resolution DWI with reduced geometric distortions, however, with longer imaging time[31-33]. Readout-segmented echo-planar (RESOLVE) DWI is a new approach for obtaining DWI images with high quality delivering sharp images at high spatial resolution and reduced slice thickness. Therefore it is possible to detect even small cholesteatomas. It uses the same diffusion preparation as SS EPI. By dividing the k-space trajectory into multiple segments in the phase encoding direction TE can be reduced to increase the quality of the acquired images. Further RESOLVE DWI is largely free of distortions, susceptibility and T2* blurring artefacts. As we mentioned before non-EPI seems to be superior to the EPI techniques in diagnosing recurrent or residual cholesteatoma, however, at the time of the systematic review by Jindal et al[30] the RESOLVE technique was not yet available. To date there are only few studies that evaluated this new approach, however, with promising results[31]. In our daily clinical routine RESOLVE has been proven as a robust and reliable approach for the detection of recurrent cholesteatoma.
DWI is a powerful tool that can replace CT and delayed gadolinium enhanced T1-weighted sequences. At our department and others contrast agent is not used anymore[26]. The best approach is to use non-EPI sequences or (if available) newer EPI-techniques as RESOLVE which provides high resolution and allows thinner slice-thickness. Single-shot EPI-DWI techniques are not recommended as they can provide false positive results due to artefacts. Further anatomical sequences (T1- and T2-weighted) should be added in coronal and/or axial orientation to better localize suspected lesions. Our department uses an axial T1 und T2 TSE sequence and a coronal T2 TSE sequence with fat-saturation. The fat saturation can help to detect fatty content of any detected lesion or structure. These sequences can also help to differentiate, e.g., the characteristic T1 hyperintensity of cholesterol granuloma. Dremmen et al[20] suggested to use conventional sequences to decrease the risk of misdiagnosis because transplanted fat within a postoperative cavity may show increased signal on DWI. Slice-thickness for the DWI (and its corresponding anatomical T1- or T2-weighted sequence) should not exceed 3 mm. If available, coloured image-fusion of DWI and anatomic sequences helps to better demonstrate the findings to patients or clinicians. On the basis of the findings by Steens et al[34] repeated follow-up DWI after surgery of cholesteatoma is recommended. Their study showed an evidence of cholesteatoma in 31% of the patients on repeated follow-up DWI.
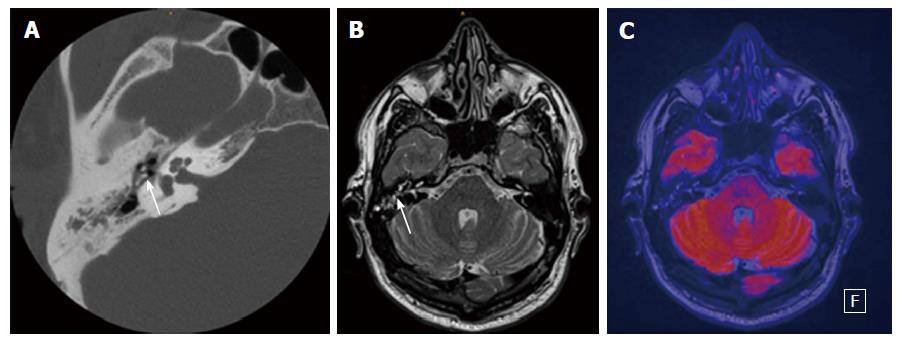
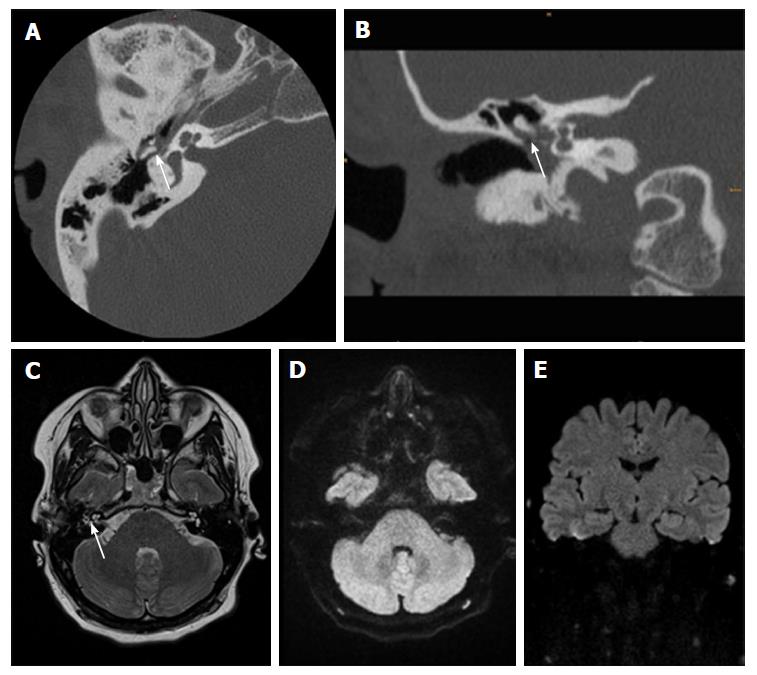
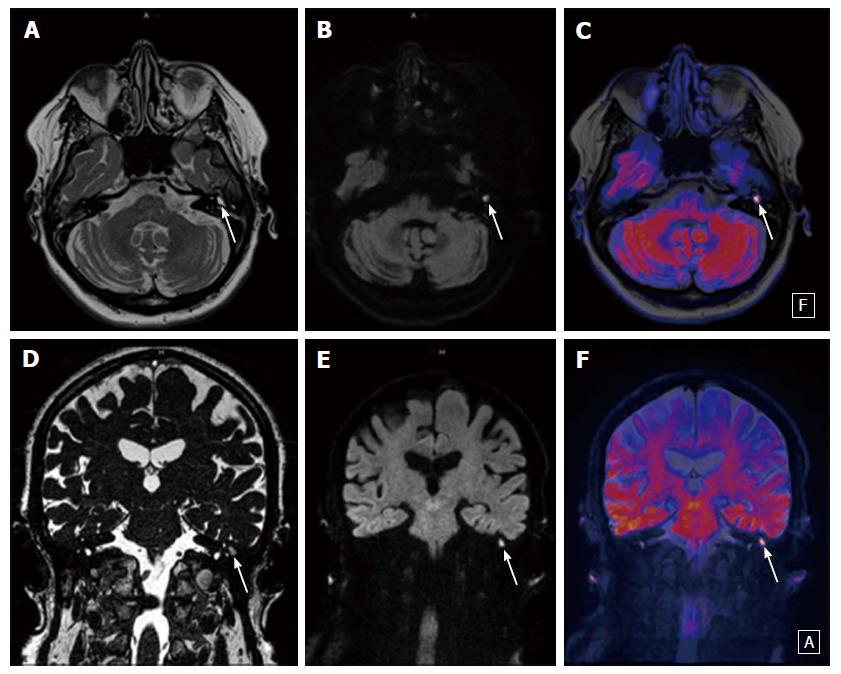
For the interpretation of DWI the reporting radiologist should look for hyperintense lesions on high b-values (800 or 1000 s/mm2), ADC-values should not be taken into the diagnostic decision as cholesteatomas can be hyperintense in ADC because of the T2 shine-through effect. If a lesion is detected the next step should be an anatomical correlation and signal interpretation on T1- and T2-contrast. This minimizes false positive results. Clinical examples are provided in Figures 1-3.
In conclusion, MRI with DWI can prevent unnecessary revision surgery in patients who are suspected of having recurrent or residual disease. Many techniques exist but non-EPI DWI and new MS EPI approaches (RESOLVE) are recommended to avoid false positive results due to different artefacts. The interpretation is simple but additional anatomical sequences are needed for exact localisation and differential diagnosis.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Austria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lim SM S- Editor: Song XX L- Editor: A E- Editor: Li D
| 1. | Swartz JD. Cholesteatomas of the middle ear. Diagnosis, etiology, and complications. Radiol Clin North Am. 1984;22:15-35. [PubMed] [Cited in This Article: ] |
| 2. | Shelton C, Sheehy JL. Tympanoplasty: review of 400 staged cases. Laryngoscope. 1990;100:679-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Gyo K, Sasaki Y, Hinohira Y, Yanagihara N. Residue of middle ear cholesteatoma after intact canal wall tympanoplasty: surgical findings at one year. Ann Otol Rhinol Laryngol. 1996;105:615-619. [PubMed] [Cited in This Article: ] |
| 4. | Yiğiter AC, Pınar E, İmre A, Erdoğan N. Value of Echo-Planar Diffusion-Weighted Magnetic Resonance Imaging for Detecting Tympanomastoid Cholesteatoma. J Int Adv Otol. 2015;11:53-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Williams MT, Ayache D. Imaging of the postoperative middle ear. Eur Radiol. 2004;14:482-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Blaney SP, Tierney P, Oyarazabal M, Bowdler DA. CT scanning in “second look” combined approach tympanoplasty. Rev Laryngol Otol Rhinol (Bord). 2000;121:79-81. [PubMed] [Cited in This Article: ] |
| 7. | Williams MT, Ayache D, Alberti C, Héran F, Lafitte F, Elmaleh-Bergès M, Piekarski JD. Detection of postoperative residual cholesteatoma with delayed contrast-enhanced MR imaging: initial findings. Eur Radiol. 2003;13:169-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Fitzek C, Mewes T, Fitzek S, Mentzel HJ, Hunsche S, Stoeter P. Diffusion-weighted MRI of cholesteatomas of the petrous bone. J Magn Reson Imaging. 2002;15:636-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Aikele P, Kittner T, Offergeld C, Kaftan H, Hüttenbrink KB, Laniado M. Diffusion-weighted MR imaging of cholesteatoma in pediatric and adult patients who have undergone middle ear surgery. AJR Am J Roentgenol. 2003;181:261-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Maheshwari S, Mukherji SK. Diffusion-weighted imaging for differentiating recurrent cholesteatoma from granulation tissue after mastoidectomy: case report. AJNR Am J Neuroradiol. 2002;23:847-849. [PubMed] [Cited in This Article: ] |
| 11. | Vercruysse JP, De Foer B, Pouillon M, Somers T, Casselman J, Offeciers E. The value of diffusion-weighted MR imaging in the diagnosis of primary acquired and residual cholesteatoma: a surgical verified study of 100 patients. Eur Radiol. 2006;16:1461-1467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Bergui M, Zhong J, Bradac GB, Sales S. Diffusion-weighted images of intracranial cyst-like lesions. Neuroradiology. 2001;43:824-829. [PubMed] [Cited in This Article: ] |
| 13. | Lemmerling MM, De Foer B, VandeVyver V, Vercruysse JP, Verstraete KL. Imaging of the opacified middle ear. Eur J Radiol. 2008;66:363-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45:169-184. [PubMed] [Cited in This Article: ] |
| 15. | Chen S, Ikawa F, Kurisu K, Arita K, Takaba J, Kanou Y. Quantitative MR evaluation of intracranial epidermoid tumors by fast fluid-attenuated inversion recovery imaging and echo-planar diffusion-weighted imaging. AJNR Am J Neuroradiol. 2001;22:1089-1096. [PubMed] [Cited in This Article: ] |
| 16. | Cimsit NC, Cimsit C, Baysal B, Ruhi IC, Ozbilgen S, Aksoy EA. Diffusion-weighted MR imaging in postoperative follow-up: reliability for detection of recurrent cholesteatoma. Eur J Radiol. 2010;74:121-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | De Foer B, Vercruysse JP, Pilet B, Michiels J, Vertriest R, Pouillon M, Somers T, Casselman JW, Offeciers E. Single-shot, turbo spin-echo, diffusion-weighted imaging versus spin-echo-planar, diffusion-weighted imaging in the detection of acquired middle ear cholesteatoma. AJNR Am J Neuroradiol. 2006;27:1480-1482. [PubMed] [Cited in This Article: ] |
| 18. | Attenberger UI, Runge VM, Stemmer A, Williams KD, Naul LG, Michaely HJ, Schoenberg SO, Reiser MF, Wintersperger BJ. Diffusion weighted imaging: a comprehensive evaluation of a fast spin echo DWI sequence with BLADE (PROPELLER) k-space sampling at 3 T, using a 32-channel head coil in acute brain ischemia. Invest Radiol. 2009;44:656-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Dubrulle F, Souillard R, Chechin D, Vaneecloo FM, Desaulty A, Vincent C. Diffusion-weighted MR imaging sequence in the detection of postoperative recurrent cholesteatoma. Radiology. 2006;238:604-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Dremmen MH, Hofman PA, Hof JR, Stokroos RJ, Postma AA. The diagnostic accuracy of non-echo-planar diffusion-weighted imaging in the detection of residual and/or recurrent cholesteatoma of the temporal bone. AJNR Am J Neuroradiol. 2012;33:439-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Dhepnorrarat RC, Wood B, Rajan GP. Postoperative non-echo-planar diffusion-weighted magnetic resonance imaging changes after cholesteatoma surgery: implications for cholesteatoma screening. Otol Neurotol. 2009;30:54-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Geoffray A, Guesmi M, Nebbia JF, Leloutre B, Bailleux S, Maschi C. MRI for the diagnosis of recurrent middle ear cholesteatoma in children--can we optimize the technique? Preliminary study. Pediatr Radiol. 2013;43:464-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Schwartz KM, Lane JI, Bolster BD, Neff BA. The utility of diffusion-weighted imaging for cholesteatoma evaluation. AJNR Am J Neuroradiol. 2011;32:430-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | De Foer B, Vercruysse JP, Bernaerts A, Deckers F, Pouillon M, Somers T, Casselman J, Offeciers E. Detection of postoperative residual cholesteatoma with non-echo-planar diffusion-weighted magnetic resonance imaging. Otol Neurotol. 2008;29:513-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | De Foer B, Vercruysse JP, Bernaerts A, Maes J, Deckers F, Michiels J, Somers T, Pouillon M, Offeciers E, Casselman JW. The value of single-shot turbo spin-echo diffusion-weighted MR imaging in the detection of middle ear cholesteatoma. Neuroradiology. 2007;49:841-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | De Foer B, Vercruysse JP, Bernaerts A, Meersschaert J, Kenis C, Pouillon M, De Beuckeleer L, Michiels J, Bogaerts K, Deckers F. Middle ear cholesteatoma: non-echo-planar diffusion-weighted MR imaging versus delayed gadolinium-enhanced T1-weighted MR imaging--value in detection. Radiology. 2010;255:866-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Forbes KP, Pipe JG, Karis JP, Heiserman JE. Improved image quality and detection of acute cerebral infarction with PROPELLER diffusion-weighted MR imaging. Radiology. 2002;225:551-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Más-Estellés F, Mateos-Fernández M, Carrascosa-Bisquert B, Facal de Castro F, Puchades-Román I, Morera-Pérez C. Contemporary non-echo-planar diffusion-weighted imaging of middle ear cholesteatomas. Radiographics. 2012;32:1197-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Elefante A, Cavaliere M, Russo C, Caliendo G, Marseglia M, Cicala D, Piccolo D, Di Lullo A, Brunetti L, Palma A. Diffusion weighted MR imaging of primary and recurrent middle ear cholesteatoma: an assessment by readers with different expertise. Biomed Res Int. 2015;2015:597896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Jindal M, Riskalla A, Jiang D, Connor S, O’Connor AF. A systematic review of diffusion-weighted magnetic resonance imaging in the assessment of postoperative cholesteatoma. Otol Neurotol. 2011;32:1243-1249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Yamashita K, Yoshiura T, Hiwatashi A, Kamano H, Dashjamts T, Shibata S, Tamae A, Honda H. Detection of middle ear cholesteatoma by diffusion-weighted MR imaging: multishot echo-planar imaging compared with single-shot echo-planar imaging. AJNR Am J Neuroradiol. 2011;32:1915-1918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Skare S, Newbould RD, Clayton DB, Albers GW, Nagle S, Bammer R. Clinical multishot DW-EPI through parallel imaging with considerations of susceptibility, motion, and noise. Magn Reson Med. 2007;57:881-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Flook E, Izzat S, Ismail A. Cholesteatoma imaging using modified echo-planar diffusion-weighted magnetic resonance imaging. J Laryngol Otol. 2011;125:10-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Steens S, Venderink W, Kunst D, Meijer A, Mylanus E. Repeated Postoperative Follow-up Diffusion-weighted Magnetic Resonance Imaging to Detect Residual or Recurrent Cholesteatoma. Otol Neurotol. 2016;37:356-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |