Published online Aug 28, 2016. doi: 10.4329/wjr.v8.i8.735
Peer-review started: February 25, 2016
First decision: April 15, 2016
Revised: May 12, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: August 28, 2016
To analyse clinical and dosimetric results of helical tomotherapy (HT) and volumetric modulated arc therapy (VMAT) in complex adjuvant breast and nodes irradiation.
Seventy-three patients were included (31 HT and 42 VMAT). Dose were 63.8 Gy (HT) and 63.2 Gy (VMAT) in the tumour bed, 52.2 Gy in the breast, 50.4 Gy in supraclavicular nodes (SCN) and internal mammary chain (IMC) with HT and 52.2 Gy and 49.3 Gy in IMC and SCN with VMAT in 29 fractions. Margins to particle tracking velocimetry were greater in the VMAT cohort (7 mm vs 5 mm).
For the HT cohort, the coverage of clinical target volumes was as follows: Tumour bed: 99.4% ± 2.4%; breast: 98.4% ± 4.3%; SCN: 99.5% ± 1.2%; IMC: 96.5% ± 13.9%. For the VMAT cohort, the coverage was as follows: Tumour bed: 99.7% ± 0.5%, breast: 99.3% ± 0.7%; SCN: 99.6% ± 1.4%; IMC: 99.3% ± 3%. For ipsilateral lung, Dmean and V20 were 13.6 ± 1.2 Gy, 21.1% ± 5% (HT) and 13.6 ± 1.4 Gy, 20.1% ± 3.2% (VMAT). Dmean and V30 of the heart were 7.4 ± 1.4 Gy, 1% ± 1% (HT) and 10.3 ± 4.2 Gy, 2.5% ± 3.9% (VMAT). For controlateral breast Dmean was 3.6 ± 0.2 Gy (HT) and 4.6 ± 0.9 Gy (VMAT). Acute skin toxicity grade 3 was 5% in the two cohorts.
HT and VMAT in complex adjuvant breast irradiation allow a good coverage of target volumes with an acceptable acute tolerance. A longer follow-up is needed to assess the impact of low doses to healthy tissues.
Core tip: Using conventional techniques in breast and nodes irradiation, there could be suboptimal target coverage or great dose exposure to the normal structures. Our study suggests that helical tomotherapy (HT) and volumetric modulated arc therapy (VMAT) plans provide excellent target volume coverage and reduces high doses to organs at risk with an acceptable acute toxicity. At the same time, HT and VMAT deliver lower doses to larger volumes of normal tissues, suggesting in some cases an increased risk of second cancer. Nevertheless, the risk to benefit ratio seems to be in favour of HT and VMAT as opposed to three-dimensional conformal radiation therapy in complex target volumes, such as funnel chest, tumor in the inner quadrant when internal mammary chain and tumor bed boost are indicated, large breast size or unfavourable cardiac anatomy.
- Citation: Lauche O, Kirova YM, Fenoglietto P, Costa E, Lemanski C, Bourgier C, Riou O, Tiberi D, Campana F, Fourquet A, Azria D. Helical tomotherapy and volumetric modulated arc therapy: New therapeutic arms in the breast cancer radiotherapy. World J Radiol 2016; 8(8): 735-742
- URL: https://www.wjgnet.com/1949-8470/full/v8/i8/735.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i8.735
Adjuvant breast radiation therapy is standard of care after breast conserving surgery in early breast cancer, improving disease free survival and overall survival (OS)[1]. Benefit of lymph node irradiation [internal mammary chain (IMC) and supra and infra clavicular nodes (SN)] in patients with axillary lymph node involvement or at high risk of relapse has been shown by a meta-analysis of three randomised trials (MA.20, European Organisation for Research and Treatment of Cancer 22922/10925 and the Lyon breast cancer trial)[2]. Lymph nodes irradiation resulted in a significant improvement of OS [hazard ratio (HR) 0.88 (95%CI: 0.75-0.97)], disease free survival [HR 0.85 (95%CI: 0.77-0.94)] and distant metastasis free survival [HR 0.82 (95%CI: 0.73-0.92)]. In these trials, separated fields for breast and lymph nodes irradiation were used in two-dimensional (2D) or three-dimensional conformal radiation therapy (3D-CRT) treatment delivery. In recent years, intensity modulated radiation therapy (IMRT) has been developed to lessen organs at risk (OAR) exposure to high doses. Static breast cancer IMRT significantly reduced acute and late skin toxicity compared to standard techniques in 3 phase III randomised trials[3-5]. Then static IMRT techniques moved to helical tomotherapy (HT) or volumetric modulated arc therapy (VMAT) for pelvic and head and neck cancers. These two techniques (VMAT and HT) have been recently performed and assessed in breast cancers. Dosimetrics studies showed that HT or VMAT improved target volume coverage, allowed better dose homogeneity and decreased high doses to OAR compared to 3D-CRT[6-11]. At the other hand these two techniques increased low doses to OAR suggesting the possibility of greater risk for secondary malignancies[12].
Using conventional techniques in breast and nodes irradiation, field junction may result in a cold or hot spot and in complex cases (unfavourable cardiac anatomy, tumour in the inner quadrants, funnel chest for example) there could be suboptimal target coverage or great dose exposure to the normal structures. Some dosimetrics studies suggested a benefit of HT or VMAT in complex breast irradiations[13-17] but no large studies evaluated clinical results.
The purpose of this study is to report the clinical and dosimetrics results for patients treated with HT or VMAT using simultaneous integrated boost (SIB) in the setting of complex adjuvant breast and nodes irradiation.
A bi-centric retrospective study of breast cancer patients with complex anatomy and treated by HT or VMAT was conducted. Patients included in our study were treated from September 2010 to November 2013 in the HT cohort (n = 31) and from February 2011 to October 2013 (n = 42) in the VMAT cohort.
Inclusion criteria were: Stage I/III invasive breast cancers patients, breast conserving surgery, indication of lymph node irradiation (IMC, supraclavicular nodes ± axillary nodes). Patients with unacceptable dosimetry according to International Commission on Radiation Units and Measurements and Units 62 using 3D-CRT were selected for treatment using HT or VMAT by the treating radiation oncologist. The indication to proceed with HT or VMAT was validated at a quality control meeting comprised of staff radiation oncologists specializing in breast cancer. Patients with distant metastases, indication of bilateral breast irradiation and treated with total mastectomy with immediate breast reconstruction were excluded.
HT and VMAT indications were funnel chest (HT series and VMAT series respectively 11% and 5%), large breast size (5% and 17%), tumour in the inner quadrant (38% and 16%), interbreast reduced space (11% and 0%), axillary irradiation (0% and 19%) and suboptimal dosimetry (35% and 43%).
Patients were treated in the supine position, both arms above the head in both institutes.
Compared to 3D-CRT, when patients were treated with these two highly conformal techniques, contentions were added to limit set-up errors: A cervical thermoplastic immobilization was used in the HT group and a back Moldcare® was used in the VMAT group.
The CT data were transferred to a commercial treatment planning system in the two series (Eclipse 3D version 8.1; Varian Medical Systems Inc., Palo Alto, United States).
Clinical target volumes (CTVs) were the same when using 3D-CRT: The breast CTV was delineated using radiopaque markers defined at the clinical examination before the planning CT, nodal delineation was performed using established guidelines[16,17] and tumour bed boost was delineated using surgical clips, initial mammogram and postoperative scar[18]. There was an expansion of all CTVs, in all directions, of 5 mm (HT) and 7 mm (VMAT), except for the skin (Table 1). The planning target volume provided a margin around the CTV to compensate for the variability of treatment setup and motion of the breast with breathing[19]. Margins from CTV to PTV are different between the two groups, so we decided to present here the results of CTV coverage.
| HT | VMAT | |
| Breast PTV | [Breast CTV + 5 mm - (PTV tumor bed + 2 mm)] - 3 mm cutaneous | [Breast CTV + 7 mm - (PTV tumor bed + 2 mm)] - 5 mm cutaneous |
| Tumor bed PTV | (CTV tumor bed + 5 mm) - 3 mm cutaneous | (CTV tumor bed + 7 mm) - 5 mm cutaneous |
| Supra infra clavicular ± axillary PTV | (CTV SN + 5 mm) - 3 mm cutaneous | (CTV SN ± axillary + 7 mm) - 5 mm cutaneous |
| IMC PTV | (CTV IMC + 5 mm) - PTV breast | CTV IMC + 7 mm |
The heart was delineated from the apex to the roots of the major vessels and included pericardial fat. Lungs, spinal cord, thyroid and oesophagus were delineated entirely. Contralateral breast was defined using radiopaque markers defined at the clinical exam. Unspecified normal tissue corresponded to the volume enclosed by the whole patient skin contours.
All patients were treated with SIB. With SIB, the planning and delivery of whole breast and boost radiotherapy are integrated into a single plan that is used for the whole treatment course, with patients receiving a differential dose to the whole breast and to the tumor bed for every fraction. The reduction in overall treatment time and the increased dose per fraction to the tumor bed can also theoretically lead to improve local control[20]. In breast cancer treated with SIB, HT avoided unnecessary breast overdosage compared to 3D-CRT[21]. Treatments were in 29 fractions (f) in the both series. The dose to breast PTV was similar in both groups (52.2 Gy). 63.8 Gy (2.2 Gy/f) and 63.2 Gy (2.18 Gy/f) were delivered to the tumor bed respectively for HT and VMAT, 50.4 Gy (1.74 Gy/f) and 49.3 Gy (1.7 Gy/f) to the SN ± axillary nodes and 50,4Gy (1.74 Gy/f) and 52.2 Gy (1.8 Gy/f) to the IMC.
For a tumor α/β of 4[20], HT and VMAT fractionation schedule are respectively radiobiologically equivalent in 2 Gy fractions to 50.5 Gy in the whole breast, 65.9 Gy and 65.3 Gy to the tumor bed, 48.2 Gy and 46.8 Gy to the SN ± axillary nodes and 48.2 Gy and 50.5 Gy in the IMC.
For HT planning, the CT data and the structure sets were transferred to the TomoTherapy planning station (TomoTherapy HI-ART version 3.1.2.3; TomoTherapy Inc., Madison, United States). All plans used a jaw width of 2.5 cm, a pitch of 0.286 and a modulation factor of 2.5.
VMAT optimization was performed using the treatment planning system Eclipse version 8.9 (Helios, Varian, Palo Alto, CA). The plans were delivered in a Varian 21EX linear accelerator (Varian, Palo Alto, CA).
Acute oesophageal, lung and skin toxicity were assessed retrospectively using Common Terminology Criteria for Adverse Events v.3.0. A clinical exam was weekly performed during radiotherapy and one and three months following the completion of radiotherapy.
Patient’s characteristics are summarized in Table 2. Clinical characteristics, biological and prognostic factors were equally balanced in the two series except for inner quadrants tumours location.
| HT (n = 31) | VMAT (n = 42) | |
| Age | 50 | 52 |
| Laterality | ||
| Right | 56.8% | 50% |
| Left | 43.2% | 50% |
| Quadrant | ||
| IQ | 70.2% | 40% |
| Outer quadrants | 29.8% | 60% |
| Size (mm) | 25.4 | 25 |
| N stage | ||
| N0 | 37.8% | 21% |
| N1 | 48.6% | 42% |
| N2 | 13.5% | 23% |
| N3 | 0% | 14% |
| Grade | ||
| 1 | 2.7% | 7% |
| 2 | 45.9% | 31% |
| 3 | 51.4% | 62% |
| LVI | ||
| - | 59.5% | 77% |
| + | 40.5% | 23% |
| Hormone receptors | ||
| RH+ | 76% | 77% |
| RH- | 24% | 29% |
| Triple negative | 18.9% | 24% |
| HER2 | ||
| + | 16.2% | 14% |
| - | 83.8% | 86% |
| Tobacco | 16.2% | 20.9% |
| BMI (kg/m2) | 25.8 | 25.9 |
| Chemotherapy | ||
| Neoadjuvant | 30% | 29% |
| Adjuvant | 49% | 64% |
| Concurrent | 4% | 0% |
| Irradiation N | ||
| SN | 100% | 98% |
| IMC | 100% | 100% |
| Axillary | 16.2% | 19% |
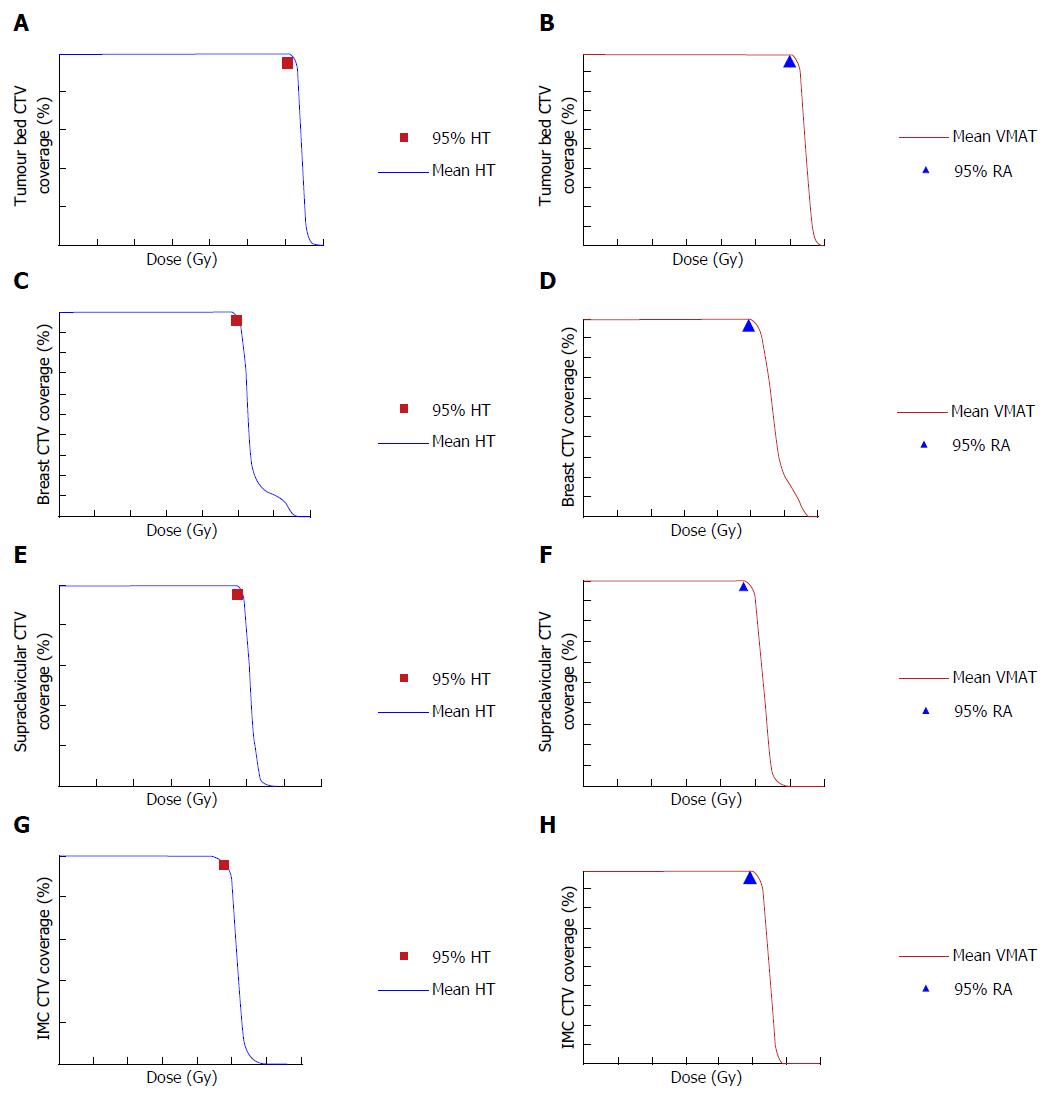
Tumour bed CTV: CTV V95 was 99.7% ± 0.1% with HT and 99.7% ± 0.5% with VMAT (Figure 1A and B).
Breast CTV: The breast CTV V95 was 98.4% ± 4.3% with HT and 99.3% ± 0.7% with VMAT (Figure 1C and D).
Supra and infra clavicular ± axillary nodes CTV: The supraclavicular ± axillary nodes CTV V95 were 99.6% ± 1.2% with HT and 99.3% ± 3% with VMAT (Figure 1E and F).
IMC CTV: The IMC CTV V95 was 96.5% ± 13.9% with HT and 99.6% ± 1.7% with VMAT (Figure 1G and H).
Doses to normal tissues are summarized in Table 3. There were little exposure of normal tissues to high doses; instead there were high volumes of normal tissues encompassed by small doses irradiation. Lung exposure and dosimetric constraints matched with Société Française Radiothérapie Oncologique (SFRO) recommendations[22] with V30 ipsilateral lung < 20% (8.8% ± 3.2% for VMAT and 10% ± 3% for HT) and V20 < 30% (20.1% ± 3.2% for VMAT and 20.9% ± 4.9% for HT).
| VMAT | HT | |
| Ipsilateral lung | ||
| V5 | 85.3% ± 9.6% | 78.5% ± 12.6% |
| V20 | 20.1% ± 3.2% | 21.1% ± 5% |
| V30 | 8.8% ± 3.2% | 10.1 ± 3.3 |
| Mean dose | 13.6 ± 1.4 Gy | 13.6 ± 1.2 Gy |
| Controlateral lung | ||
| V5 | 46% ± 14.1% | 35.4 ± 11.3 |
| V20 | 0.7% ± 0.5% | 0.1 ± 0.2 |
| Mean dose | 5.4 ± 1 Gy | 4.6 ± 0.8 Gy |
| Heart | ||
| Mean dose | 10.3 ± 4.2 Gy | 7.5 ± 1.4 Gy |
| V5 | 77.6% ± 21% | 59.8 % ± 14.6% |
| V30 | 2.5% ± 3.9% | 1% ± 1% |
| Controlateral breast | ||
| Mean dose | 4.6 ± 0.9 Gy | 3.6 ± 0.6 Gy |
| V5 | 32% ± 11.9% | 14.7% ± 7% |
| Spinal cord | ||
| V40 | 0 mm3 | 0 mm3 |
| V5 | 22.4 ± 8.8 mm3 | 25.2 ± 9 mm3 |
| Oesophagus | ||
| V45 | 0.4 ± 0.6 mm3 | 1 ± 1.2 mm3 |
| V10 | 8.8 ± 5.4 mm3 | 12.8 ± 5.7 mm3 |
| Thyroid | ||
| Mean dose | 28.3 ± 7 Gy | 26.7 ± 7.7 Gy |
| V30 | 44% ± 15.3% | 39.8% ± 17.6% |
| V5 | 97.1% ± 8.3% | 96% ± 9.4% |
| Unspecified tissues | ||
| V40 | 1977 ± 911 mm3 | 1880.9 ± 754 |
| V5 | 9770.3 ± 2551 mm3 | 8566.6 ± 1946.2 |
A maximum of 5% of grade 3 acute skin toxicity was observed regardless VMAT or HT use. Thirty-five percent (HT) and 40% (VMAT) grade ≤ 2 oesophagus toxicity was noticed. No lung toxicity was observed.
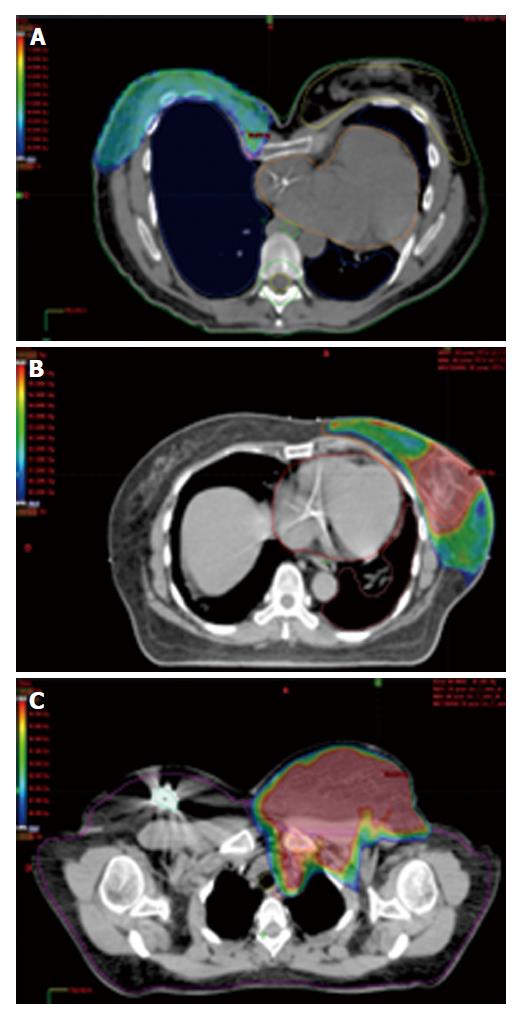
HT and VMAT could be an interesting option in case of complex anatomical cases of breast cancer patients by offering adequate and optimal target volumes coverage while lessening OAR exposure. Our results showed that 95% isodose covered at least 95% of PTV regardless IMRT techniques in case of funnel chest anatomy (Figure 2A), unfavourable cardiac anatomy (Figure 2B) or obese patients with superposition of breast and nodal volumes (Figure 2C).
A previous study, which assess the benefit of adjuvant breast hypo fractionated irradiation with HT, reported only 8% of grade ≥ 3 acute skin toxicity and 10% of grade ≥ 1 lung toxicity two months after treatment[23]. In this study only 13 patients received supraclavicular, infraclavicular and axillary irradiation and no patients received IMC irradiation. Our study is the largest to report clinical outcomes in the setting of complex adjuvant breast and nodes irradiation, including IMC, treated with VMAT or HT. Our study showed a lower incidence of grade 3-4 acute skin toxicities (5%), rather than tolerance reported after static IMRT (27%)[4], probably because inverse plan IMRT improve dose homogeneity compare to forward plan IMRT[11,24], and improve dose homogeneity translate into lower acute skin toxicity[4]. To reduce severe acute skin toxicity is a real challenge in breast cancer radiotherapy as it is related to a poor cosmetic outcome[25,26]. Static IMRT decreased its incidence when compared to 3D-CRT, but seems to be less effective when compared to HT and VMAT. Moreover, we have not yet observed clinical radiation pneumonitis in the two series while a meta-analysis mentioned a 14% incidence clinical radiation pneumonitis with 3D-CRT[27]. Given the negative selection bias towards patients with problematic anatomy, the favourable comparison of acute toxicity with historical trial data for a more standard cohort is encouraging.
To decrease the risk of late cardiac toxicity occurrence is one of the main challenges of breast cancer radiotherapy. Long-term breast cancer survivors are at high risk of cardiac events. Darby et al[28] showed an increased risk of ischemic heart disease (myocardial infarction, coronary revascularization, or death from ischemic heart disease) after breast cancer irradiation, which was related to the mean dose to the heart. No evident threshold has been observed but patients with pre-existing cardiac risk factors had a higher risk of developing such toxicities. This large cohort of patients was treated with standard 2D or 3D-conformal techniques of radiotherapy. The gain of the use of IMRT is to lessen heart exposure to high doses[6,8,9]. Our study reinforces these findings with a low value of V30Gy regardless HT or VMAT. However these techniques expose the heart to substantial low dose (V5Gy = 77.6% ± 21% in VMAT series and 59.8% ± 14.6% in HT series), which translates in a relative high mean heart dose [10.3 ± 4.2 Gy (VMAT) and 7.5 ± 1.4 Gy (HT)]. A longer follow-up is warranted to follow cardiac events occurrence after breast IMRT.
One limitation of the use of HT or VMAT in breast cancer is the lung exposure to low dose (i.e., lesser than 5 Gy). Our study showed a significant lung volume exposure to dose lower than 5 Gy, which is higher than lung exposure after 2D or 3D-CRT[6,8,9]. Similarly to heart exposure, late consequences of low doses to the lung are unknown. A carefully follow-up should be considered in patients treated with HT or VMAT.
Other unknown factors still remain as the contralateral breast exposure. Contralateral breast is rarely exposed after conventional techniques of radiotherapy or after static IMRT[29]. Here, the use of HT or VMAT exposed contralateral breast volume to low dose (lesser than 5 Gy; 4.6 ± 0.9 Gy in VMAT series and 3.6 ± 0.6 Gy in HT series). The main uncertainty of low dose exposure after HT or VMAT is the risk of radio-induced cancer[30,31]. The risk of radio-induced breast cancer has been widely reported after Hodgkin irradiation and young age and dose were the main risk factors[29,32]. Hence, the use of HT or VMAT should be carefully considered in young patients.
When examining normal tissues as a whole, there was less exposure to high doses using rotational techniques[8,9,33]. However, there were high volumes of normal tissue encompassed by small doses of irradiation (unspecified tissue V5: 9770.3 ± 2551 mm3 in VMAT cohort and 8566.6 ± 1946.2 mm3 in HT cohort) suggesting the possibility of a greater risk for secondary cancer, which could be a concern for young patients[12].
In conclusion, HT and VMAT are feasible techniques in cases of complex adjuvant breast and nodal irradiation and provide excellent target volume coverage with an acceptable acute toxicity. As low dose distribution with HT or VMAT is large, a careful follow-up regarding lung, heart, contralateral breast is warranted.
Since uncertainties still remain regarding the role of low dose, this technique should only be considered to a selected population of breast cancer such as funnel chest, high breast volume, tumour in the inner quadrants, unfavourable cardiac anatomy.
Benefit of lymph node irradiation in patients with axillary lymph nodes involvement has been proven by the MA.20 and European Organisation for Research and Treatment of Cancer 22922/10925 trials. The benefit of lymph node irradiation has been proven with two-dimensional or three-dimensional conformal radiation therapy techniques. In complex cases, there could be suboptimal target coverage or great dose exposure to the normal structures with standard techniques. Helical tomotherapy (HT) and volumetric modulated arc therapy (VMAT) are two techniques of rotational intensity modulated radiation therapy that provide excellent target volume coverage and reduce high doses to normal tissues. Some dosimetric studies suggested a benefit of HT or VMAT in complex breast irradiations but no large clinical studies evaluated clinical results.
This study is the first to report the feasibility of HT and VMAT in case of complex adjuvant breast and nodal irradiation.
The rationale of the study is based on the complexity of the irradiation of lymph nodes and breast with standard techniques, which could be responsible of poor target volume coverage, or great dose exposure of the normal structures, especially in complex anatomies. This study is the largest to report clinical outcomes in the setting of complex adjuvant breast and nodes irradiation, including internal mammary chain, treated with VMAT or HT. The data suggest that HT and VMAT are attractive techniques in the setting of complex adjuvant breast and nodes irradiation allowing good target volume coverage with an acceptable acute toxicity.
This study suggests that HT and VMAT are feasible techniques in complex adjuvant breast and nodes irradiation. It provides readers with the necessary information (patients selection, patient immobilization, dose prescription, target volume and organs at risk delineation, HT and VMAT planning) to carry out HT and VMAT in the setting of complex breast and nodes irradiation.
VMAT and HT are techniques of rotational intensity modulated radiation therapy. With VMAT, the beam radiation can be modulated by varying the gantry speed, move of the leafs and dose rate. HT is a 6-MV accelerator mounted on a ring gantry that rotates around the patient while the table advances slowly through the bore.
Very interesting and promising radiation management. This manuscript provides useful information to the medical students, clinicians, and researchers in this field.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sonoda K, Tsikouras PPT S- Editor: Gong XM L- Editor: A E- Editor: Zhang FF
| 1. | Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707-1716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2360] [Cited by in F6Publishing: 2514] [Article Influence: 193.4] [Reference Citation Analysis (0)] |
| 2. | Budach W, Kammers K, Boelke E, Matuschek C. Adjuvant radiotherapy of regional lymph nodes in breast cancer - a meta-analysis of randomized trials. Radiat Oncol. 2013;8:267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Mukesh MB, Barnett GC, Wilkinson JS, Moody AM, Wilson C, Dorling L, Chan Wah Hak C, Qian W, Twyman N, Burnet NG. Randomized controlled trial of intensity-modulated radiotherapy for early breast cancer: 5-year results confirm superior overall cosmesis. J Clin Oncol. 2013;31:4488-4495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, Vu TT, Truong P, Ackerman I, Paszat L. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085-2092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 523] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 5. | Donovan E, Bleakley N, Denholm E, Evans P, Gothard L, Hanson J, Peckitt C, Reise S, Ross G, Sharp G. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2007;82:254-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 6. | Popescu CC, Olivotto IA, Beckham WA, Ansbacher W, Zavgorodni S, Shaffer R, Wai ES, Otto K. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys. 2010;76:287-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Osman SO, Hol S, Poortmans PM, Essers M. Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation. Radiother Oncol. 2014;112:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Caudrelier JM, Morgan SC, Montgomery L, Lacelle M, Nyiri B, Macpherson M. Helical tomotherapy for locoregional irradiation including the internal mammary chain in left-sided breast cancer: dosimetric evaluation. Radiother Oncol. 2009;90:99-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Goddu SM, Chaudhari S, Mamalui-Hunter M, Pechenaya OL, Pratt D, Mutic S, Zoberi I, Jeswani S, Powell SN, Low DA. Helical tomotherapy planning for left-sided breast cancer patients with positive lymph nodes: comparison to conventional multiport breast technique. Int J Radiat Oncol Biol Phys. 2009;73:1243-1251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Chira C, Kirova YM, Liem X, Campana F, Peurien D, Amessis M, Fournier-Bidoz N, Pierga JY, Dendale R, Bey P. Helical tomotherapy for inoperable breast cancer: a new promising tool. Biomed Res Int. 2013;2013:264306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Lauche O, Kirova YM. Helical tomotherapy in breast cancer treatment. Breast Cancer Manag. 2014;3:441-449. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Abo-Madyan Y, Aziz MH, Aly MM, Schneider F, Sperk E, Clausen S, Giordano FA, Herskind C, Steil V, Wenz F. Second cancer risk after 3D-CRT, IMRT and VMAT for breast cancer. Radiother Oncol. 2014;110:471-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 13. | Coon AB, Dickler A, Kirk MC, Liao Y, Shah AP, Strauss JB, Chen S, Turian J, Griem KL. Tomotherapy and multifield intensity-modulated radiotherapy planning reduce cardiac doses in left-sided breast cancer patients with unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys. 2010;78:104-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Lamberth F, Guilbert P, Gaillot-Petit N, Champagne C, Looten-Vieren L, Nguyen TD. [Potential indications for helical tomotherapy in breast cancers]. Cancer Radiother. 2014;18:7-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Haertl PM, Pohl F, Weidner K, Groeger C, Koelbl O, Dobler B. Treatment of left sided breast cancer for a patient with funnel chest: volumetric-modulated arc therapy vs. 3D-CRT and intensity-modulated radiotherapy. Med Dosim. 2013;38:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kirova YM, Castro Pena P, Dendale R, Servois V, Bollet MA, Fournier-Bidoz N, Campana F, Fourquet A. Simplified rules for everyday delineation of lymph node areas for breast cancer radiotherapy. Br J Radiol. 2010;83:683-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Atean I, Pointreau Y, Barillot I, Kirova YM. [Organs at risk and target volumes: definition for conformal radiation therapy in breast cancer]. Cancer Radiother. 2012;16:485-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Kirova YM, Castro Pena P, Hijal T, Fournier-Bidoz N, Laki F, Sigal-Zafrani B, Dendale R, Bollet MA, Campana F, Fourquet A. Improving the definition of tumor bed boost with the use of surgical clips and image registration in breast cancer patients. Int J Radiat Oncol Biol Phys. 2010;78:1352-1355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Wiant DB, Wentworth S, Maurer JM, Vanderstraeten CL, Terrell JA, Sintay BJ. Surface imaging-based analysis of intrafraction motion for breast radiotherapy patients. J Appl Clin Med Phys. 2014;15:4957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Qi XS, White J, Li XA. Is α/β for breast cancer really low? Radiother Oncol. 2011;100:282-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Hijal T, Fournier-Bidoz N, Castro-Pena P, Kirova YM, Zefkili S, Bollet MA, Dendale R, Campana F, Fourquet A. Simultaneous integrated boost in breast conserving treatment of breast cancer: a dosimetric comparison of helical tomotherapy and three-dimensional conformal radiotherapy. Radiother Oncol. 2010;94:300-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Ortholan C, Estivalet S, Barillot I, Costa A, Gérard JP. [Guide for external beam radiotherapy. Procedures 2007]. Cancer Radiother. 2007;11:329-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Van Parijs H, Miedema G, Vinh-Hung V, Verbanck S, Adriaenssens N, Kerkhove D, Reynders T, Schuermans D, Leysen K, Hanon S. Short course radiotherapy with simultaneous integrated boost for stage I-II breast cancer, early toxicities of a randomized clinical trial. Radiat Oncol. 2012;7:80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Yin Y, Chen J, Sun T, Ma C, Lu J, Liu T, Wang R. Dosimetric research on intensity-modulated arc radiotherapy planning for left breast cancer after breast-preservation surgery. Med Dosim. 2012;37:287-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Ginot A, Doyen J, Hannoun-Lévi JM, Courdi A. [Normal tissue tolerance to external beam radiation therapy: skin]. Cancer Radiother. 2010;14:379-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol. 1990;57:751-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 219] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Gokula K, Earnest A, Wong LC. Meta-analysis of incidence of early lung toxicity in 3-dimensional conformal irradiation of breast carcinomas. Radiat Oncol. 2013;8:268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2429] [Cited by in F6Publishing: 2505] [Article Influence: 227.7] [Reference Citation Analysis (0)] |
| 29. | Boice JD, Harvey EB, Blettner M, Stovall M, Flannery JT. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med. 1992;326:781-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 336] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Kirova YM, Gambotti L, De Rycke Y, Vilcoq JR, Asselain B, Fourquet A. Risk of second malignancies after adjuvant radiotherapy for breast cancer: a large-scale, single-institution review. Int J Radiat Oncol Biol Phys. 2007;68:359-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-2106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3730] [Cited by in F6Publishing: 3517] [Article Influence: 185.1] [Reference Citation Analysis (0)] |
| 32. | Stovall M, Smith SA, Langholz BM, Boice JD, Shore RE, Andersson M, Buchholz TA, Capanu M, Bernstein L, Lynch CF. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys. 2008;72:1021-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Tsai PF, Lin SM, Lee SH, Yeh CY, Huang YT, Lee CC, Hong JH. The feasibility study of using multiple partial volumetric-modulated arcs therapy in early stage left-sided breast cancer patients. J Appl Clin Med Phys. 2012;13:3806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |