Published online Apr 28, 2016. doi: 10.4329/wjr.v8.i4.419
Peer-review started: October 6, 2015
First decision: November 24, 2015
Revised: December 3, 2015
Accepted: January 29, 2016
Article in press: January 31, 2016
Published online: April 28, 2016
Processing time: 206 Days and 20.2 Hours
AIM: To quantify cumulative effective dose of intensive care unit (ICU) patients attributable to diagnostic imaging.
METHODS: This was a prospective, interdisciplinary study conducted in the ICU of a large tertiary referral and level 1 trauma center. Demographic and clinical data including age, gender, date of ICU admission, primary reason for ICU admission, APACHE II score, length of stay, number of days intubated, date of death or discharge, and re-admission data was collected on all patients admitted over a 1-year period. The overall radiation exposure was quantified by the cumulative effective radiation dose (CED) in millisieverts (mSv) and calculated using reference effective doses published by the United Kingdom National Radiation Protection Board. Pediatric patients were selected for subgroup-analysis.
RESULTS: A total of 2737 studies were performed in 421 patients. The total CED was 1704 mSv with a median CED of 1.5 mSv (IQR 0.04-6.6 mSv). Total CED in pediatric patients was 74.6 mSv with a median CED of 0.07 mSv (IQR 0.01-4.7 mSv). Chest radiography was the most commonly performed examination accounting for 83% of all studies but only 2.7% of total CED. Computed tomography (CT) accounted for 16% of all studies performed and contributed 97% of total CED. Trauma patients received a statistically significant higher dose [median CED 7.7 mSv (IQR 3.5-13.8 mSv)] than medical [median CED 1.4 mSv (IQR 0.05-5.4 mSv)] and surgical [median CED 1.6 mSv (IQR 0.04-7.5 mSv)] patients. Length of stay in ICU [OR = 1.12 (95%CI: 1.079-1.157)] was identified as an independent predictor of receiving a CED greater than 15 mSv.
CONCLUSION: Trauma patients and patients with extended ICU admission times are at increased risk of higher CEDs. CED should be minimized where feasible, especially in young patients.
Core tip: We hypothesized that intensive care unit (ICU) patients, especially pediatric patients, are potentially vulnerable to increased cumulative effective doses (CED) from ionizing radiation. We found a relatively low CED in the majority of ICU patients, during their stay in the ICU. Nevertheless, physicians are beholden to keep radiation exposures from diagnostic imaging as low as reasonably practical and CED should be minimized where feasible, especially in young patients. Physicians should be aware that trauma patients and patients with extended ICU admission times are at an increased risk of high CEDs.
- Citation: Moloney F, Fama D, Twomey M, O’Leary R, Houlihane C, Murphy KP, O’Neill SB, O’Connor OJ, Breen D, Maher MM. Cumulative radiation exposure from diagnostic imaging in intensive care unit patients. World J Radiol 2016; 8(4): 419-427
- URL: https://www.wjgnet.com/1949-8470/full/v8/i4/419.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i4.419
There has been growing awareness in the medical literature of the potential for high lifetime cumulative radiation exposures incurred by patient subgroups with chronic disorders as a result of repeated use of diagnostic imaging. This is of particular concern in patients whose underlying illness begins at a young age and follows a chronic relapsing and remitting course; examples of this have been described with Crohn’s disease and cystic fibrosis[1]. The availability and improvements in diagnostic imaging have led to a sevenfold increase in the use of radiological imaging modalities[2]. This is especially true for computed tomography (CT), which imparts more than 50% of all radiation exposure from diagnostic imaging[3]. Studies report a 7.8% annual increase in the use of CT from 1996 to 2010 representing an overall doubling of the mean per capita effective dose of ionizing radiation[4].
The relationship of cumulative radiation exposure to a quantifiable risk of cancer induction is a controversial topic. However, protracted exposure to low-level ionizing radiation is widely believed to be associated with an increased risk of malignancy with several studies demonstrating a dose-dependent correlation between radiation exposure and cancer incidence[5-8].
Young patients appear to be 10 times more susceptible than adults to the risk of developing cancer from ionizing radiation exposure[9,10]. In terms of CT scans, children receive greater organ doses and several organs are more sensitive to radiation-induced cancer than adults[11]. Children are also inherently more at risk because they have many more subsequent years of life to develop a radiation-induced cancer.
High cumulative effective dose (CED) has been documented in a number of patient groups including Crohn’s disease[12], cystic fibrosis[13], end-stage kidney disease[14], and testicular cancer[1]. Studies have shown that intensive care unit (ICU) patients receive significantly higher cumulative doses of ionizing radiation than other hospitalized patients (17.9 mSv vs 11.3 mSv in one study)[15]. ICU patients are critically ill and often require several diagnostic radiology investigations that may need to be repeated within a short time frame.
Therefore, we hypothesize that ICU patients, especially pediatric patients, are potentially vulnerable to increased CEDs from ionizing radiation. Yet this particular subset of patients has received little attention, with only one study examining CED in pediatric patients published in the literature[16].
The aim of this study was to: (1) quantify the CED of ICU patients attributable to diagnostic imaging; (2) examine patterns of use of diagnostic imaging in ICU patients; (3) identify subgroups of patients that may be at risk of receiving higher CEDs; and (4) quantify CED in pediatric patients in the ICU.
This was a prospective, interdisciplinary study conducted at a large tertiary referral and level 1 trauma center over a 1-year period from March 2010 to February 2011. Patients were identified for possible inclusion on a daily basis from the general ICU database. All patients regardless of age admitted to the ICU for more than 4 h were included. Demographic and clinical data including medical record number, age, gender, date of ICU admission, primary reason for admission, acute physiology and chronic health evaluation (APACHE) II score, length of stay, number of days intubated, date of death or discharge, and re-admission data were collected and stored in an encrypted, anonymised database constructed using Microsoft Office Excel 2010 (Microsoft Corporation, CA, United States). Ethical approval for the study was granted by the local clinical research ethics committee.
Data on all diagnostic radiology investigations that utilized ionizing radiation including CT, conventional radiographs, and nuclear medicine studies was collected on all patients from the radiology department’s Picture Archiving and Communication System (IMPAX 6, Agfa Healthcare, NV, Belgium) and Radiology Information System (Keogh Radiology Information System version 2.7, KS Medical, Dublin, Ireland). The centralized nature of local radiology services and the use of one imaging center in our study assured the accuracy of the capture of all imaging studies performed in our study group.
CT examinations were performed with oral and intravenous contrast using a 64 slice multidetector row CT (LightSpeed VCT-XTe, General Electric Medical Systems, Waukesha, Wisconsin, United States) or a 4-slice multidetector row CT (Toshiba Aquilion, Toshiba Medical Systems, Zoetermeer, The Netherlands). Data on diagnostic investigations not utilizing ionizing radiation including ultrasound (US) and magnetic resonance imaging (MRI) was also recorded.
CED was calculated using the reference effective doses for diagnostic imaging studies published by the United Kingdom National Radiation Protection Board[17].
Data was exported from Microsoft Office Excel 2010 (Microsoft Corporation, CA, Statistical analysis) into GraphPad Prism version 6.0 (GraphPad Software Incorporated, San Diago, Statistical analysis) and Statistical Package for the Social Sciences version 22 (IBM, Chicago, IL, Statistical analysis) for further analysis. Data are described as mean and standard deviation for parametric distributions or as median and interquartile range (IQR) for non-parametric distributions. Distribution of variables was assessed using D’Agostino-Pearson omnibus normality test. Pearson and Spearman correlation tests were used to assess the relationship between continuous variables for parametric and non-parametric data respectively. Mann-Whitney U or Kruskal-Wallis tests were used to compare non-parametric distributions of two or more than two groups of continuous variables respectively. Dunn’s multiple comparisons test was used to assess dose differences between trauma, medical and surgical patients. Associations between patient characteristics and CED were analyzed by binominal logistic regression and expressed as odds ratios (OR). A CED greater than or equal to 15 mSv was used as the cut-off for low and high dose groups to facilitate logistic regression analysis. A subgroup analysis was performed in patients aged less than 17 years. P values less than 0.05 were considered to be statistically significant.
Four hundred and twenty-one patients with a median age of 58 (range 1-93) years were identified for inclusion in the study. The demographic and clinical characteristics of all patients in the study group are outlined in Table 1. Table 2 outlines the most common primary reasons for admission to the ICU.
| Total patients | 421 |
| Age (yr) | 58 (1-93) |
| Gender | 256 male (61%) |
| Length of stay (d) | 5 (range 1-100) |
| APACHE II score | 19 (range 0-49) |
| Number of days intubated (d) | 5.5 (1-226) |
| Number of deaths | 59 (14%) |
| Number of re-admissions | 46 (11%) |
| Total number of studies | 2737 |
| Total CED (mSv) | 1704 |
| Median CED (mSv) | 1.5 (IQR 0.04-6.6) |
| n (%) | |
| Pulmonary oedema | 42 (10) |
| Multiple trauma | 41 (10) |
| Overdose | 39 (9) |
| Sepsis | 39 (9) |
| Seizures | 34 (8) |
| Peripheral vascular surgery | 23 (5) |
| Cardiogenic shock | 23 (5) |
| Gastrointestinal disease | 21 (5) |
A total of 2737 studies that utilize ionizing radiation were performed with a mean 7.4 studies per patient (95%CI: 3.5-4.6). The total CED for the patient cohort was 1704 mSv with a median CED of 1.5 mSv (IQR, 0.04-6.6 mSv). Studies not using ionizing radiation accounted for 1% of all studies.
Seventy-nine diagnostic radiation studies were performed in 23 patients aged less than 17 years with a total CED of 74.6 mSv and a median CED of 0.07 mSv (IQR, 0.01-4.7 mSv).
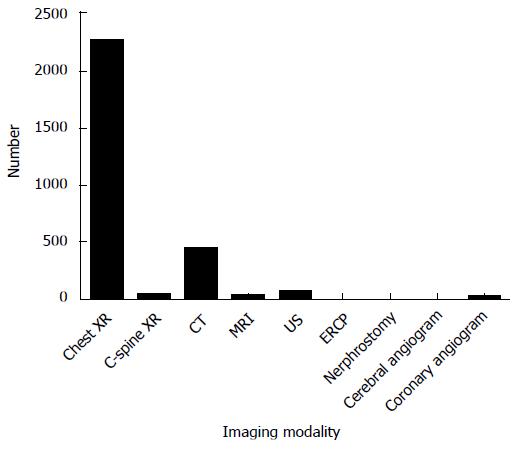
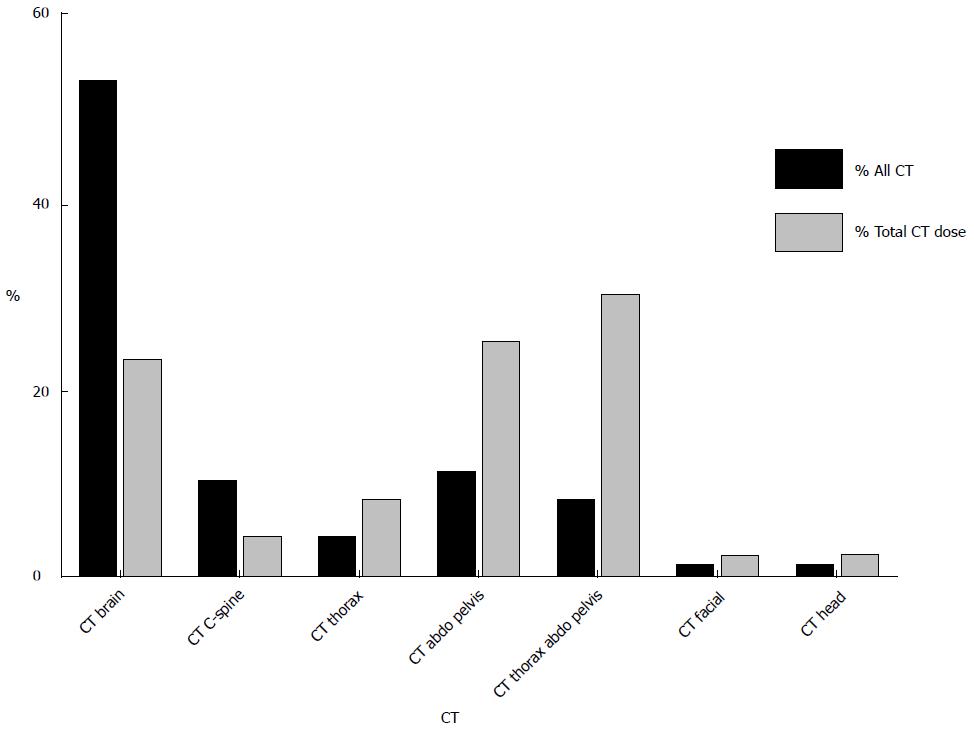
Chest radiography was the most commonly performed examination utilizing ionizing radiation accounting for 83% of all studies but only 2.7% of total CED (Figure 1). CT accounted for 16% all studies utilizing ionizing radiation and contributed 97% of the total CED for the study population. CT brain was the most frequently performed CT examination representing 53% of CT studies and contributing 23% of CED from CT alone. This was followed by CT abdomen and pelvis (11% of CT studies; contributing 25% of CED from CT) and CT thorax, abdomen and pelvis (8% of CT studies; contributing 30% of CED from CT) (Figure 2). MRI and US were performed in 4.5% and 12% of patients respectively.
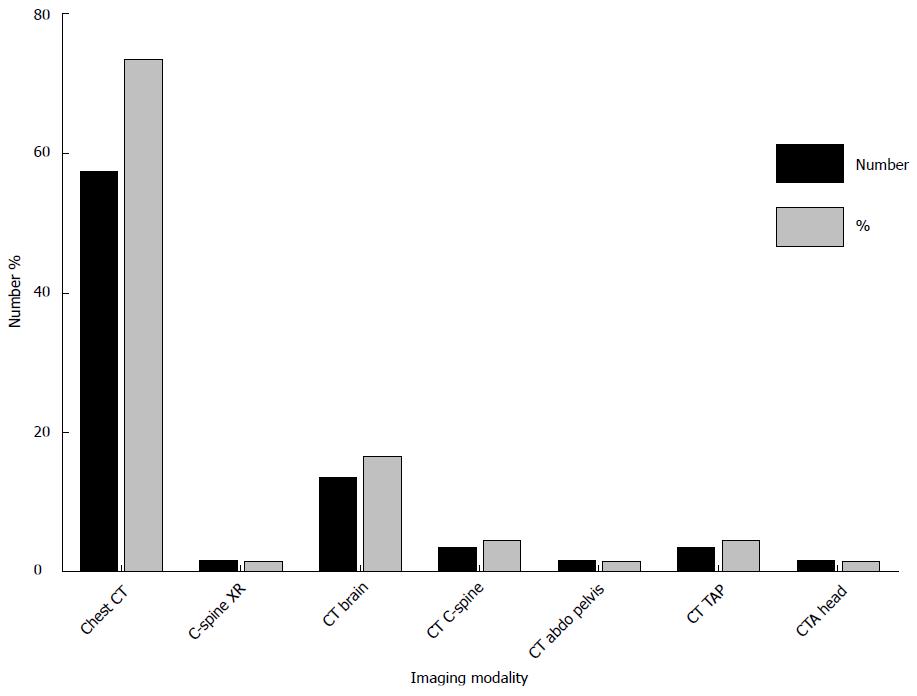
Amongst the 23 pediatric patients, chest radiography was the most commonly performed examination accounting for 72% of all studies and contributing to 1.5% of total CED (Figure 3). 21 CT examinations were performed in pediatric patients with a total CED from CT of 73.4 mSv accounting for 98% of total CED. CT brain was the most frequently performed CT examination in pediatric patients representing 62% of CT studies.
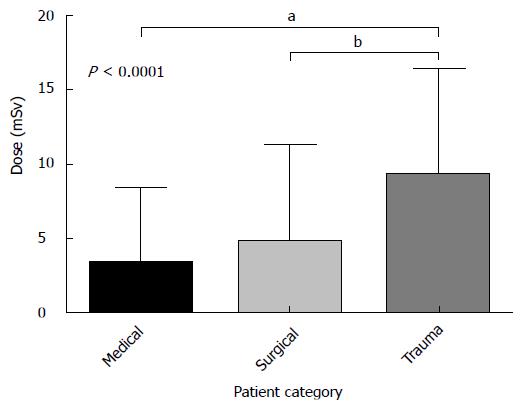
Trauma patients received a statistically significant higher dose [median CED: 7.7 mSv (IQR: 3.5-13.8 mSv)] than medical [median CED 1.4 mSv (IQR 0.05-5.4 mSv)] and surgical [median CED 1.6 mSv (IQR 0.04-7.5 mSv)] patients (Figure 4). Differences in CED between medical and surgical patients was not statistically significant. Length of stay had the strongest correlation of all variables with CED with a moderate strength of association (r = 0.49, P < 0.001).
A CED of greater than 15 mSv was observed in 12% (n = 49) of the study cohort. Binominal logistic regression was performed to assess the influence of a number of factors on the likelihood that a patient would receive a CED greater than 15 mSv. The model contained four independent variables (length of stay, APACHE II score, number of intubated days, and age at admission) and was statistically significant [χ2 (4, n = 421) = 65.459, P < 0.001], indicating the ability to distinguish between patients who received less or more than a 15 mSV CED. The model explained 28.1% (Nagelkerke R2) of the variance in CED and correctly classified 88.6% of cases. Length of stay in ICU [OR = 1.12 (95%CI: 1.079-1.157)] and decreasing age [OR = 0.967 (95%CI: 0.951-0.983)] were identified as independent predicators of receiving a CED greater than 15 mSv (Table 3).
Radiological examinations utilizing ionizing radiation are useful and necessary tools used for diagnostic and therapeutic purposes in ICU patients.
Quantifying cumulative radiation dose delivered by medical imaging is gaining increasing importance in the literature. The importance of monitoring CED is seen in the recent development by industry of radiation dose monitoring software systems, which are now commercially available and are gaining market traction. These systems are facilitating the process of monitoring radiation exposure in individual patients and also in continuous monitoring and audit of imaging departments’ performance, with respect to radiation exposures associated with individual imaging modalities and imaging protocols.
The international commission on radiological protection, as well as the National Academy of Sciences Committee on Biological Effects of Ionizing Radiation have estimated cancer risks associated with protracted exposures to low-dose ionizing radiation by extrapolating from published data of Japanese atomic bomb survivors who had, in large part, acute exposure to high doses of radiation[2]. As a result, a linear no-threshold model of risk associated with high-dose exposures is assumed to also be relevant for low-dose exposures. Epidemiological studies have provided evidence of increased cancer-related mortality following exposure to low levels of ionizing radiation from diagnostic imaging[18,19]. Other efforts to quantify the cancer risk from low-dose radiation exposure have focused on the cancer incidence among radiation workers in the nuclear industry. A retrospective cohort study examining the effects of low-dose protracted exposures to ionizing radiation in 15 countries involving almost 600000 workers demonstrated a dose-related increase in all cancer mortality[20]. However, use of data generated from atomic bomb survivors and nuclear industry workers to estimate cancer risk from diagnostic radiology examinations is not ideal and remains extremely controversial[21,22].
CED in ITU patients has been recorded in recent studies. Rohner et al[23] reported a median CED of 9.35 mSv (IQR: 0.18-27.4 mSv) in 74 patients in a surgical ICU and identified the number of CT studies and fluoroscopy minutes to be significantly associated with a dose greater than 50 mSv. Lutterman et al[15] examined CED in hospitalized patients and found dose estimates to be significantly higher for patients whose hospitalizations included time in the ICU (17.9 mSv vs 11.3 mSv). A study examining thoracic radiation exposure incurred by pediatric patients in the ITU reported a median total thoracic exposure of 3.71 mGy (range, 0.77-33.41 mGy) in 69 patients. Although our median CED estimates were lower for the majority of our study population than reported in other studies, a subgroup of patients within our study group did receive a significantly higher radiation exposure and we concluded that it was necessary to examine factors contributing to this.
Trauma patients received a significantly higher median CED (7.7 mSv) than medical (1.4 mSv) and surgical (1.6 mSv) patients. This primarily related to the use of whole-body CT and is in keeping with a previous study, which reported a mean CED of 22.7 mSv in trauma patients. We also identified length of stay in ICU to be predictive of receiving a higher CED (> 15 mSv). Although we only found a weak association [OR = 1.12 (95%CI: 1.079-1.157)] in our patient cohort, it is reasonable to assume that the longer a patient remains in ICU, the more likely he or she is to undergo additional and repeated imaging and incur a higher CED. Prior to requesting diagnostic radiology examinations for ICU patients with extended admission times, physicians should consider the justification for the examination in the context of the patient incurring a potentially high CED. A younger age was only weakly associated with the likelihood of receiving a higher CED. Number of intubated days and APACHE II score were not associated with a higher CED.
Similar to previous studies assessing CED[1,12], there was a disproportionately large contribution of CT (16% of all studies, 97% of CED) to CED, reflecting a possible overreliance on CT. Several studies report a notable increase in the use of CT in recent years with a parallel increase in radiation exposure from diagnostic imaging[12]. Therefore, strategies to reduce CED should initially be focused on reducing the use of and dose from CT.
Exposure to ionizing radiation is an important consideration when deciding on the most appropriate imaging modality to use in an ICU patient. This is especially true in young patients and patients with chronic conditions such as inflammatory bowel disease who have been shown in previous studies to be at risk of high cumulative radiation doses from diagnostic imaging[24]. If possible, imaging modalities that do not involve exposure to ionizing radiation such as MRI and US should be employed in the first instance. However, this may not be possible in many cases due to the lack of availability of MRI at some institutions, prolonged imaging times of MRI compared to CT, patient contra-indications to MRI, and the sub-optimal diagnostic capabilities of MRI compared to CT in certain clinical scenarios.
As previously discussed, CT is a valuable imaging technique when used judiciously in the ICU setting. It is vital that each radiology department takes due consideration of dose reduction strategies to keep patient doses as low as reasonably possible. There are several dose optimization strategies available that may be readily employed in patients in the ICU. These include omitting unnecessary images at the ends of acquired series[25], minimizing the number of phases acquired, and the use of automated exposure control as opposed to fixed tube current techniques[26]. Accurate patient centering is another important dose reduction strategy with poor centering resulting in significant dose increases[27]. In addition, new image reconstruction techniques that reduce radiation dose have been developed in recent years with promising results. These techniques use iterative reconstruction algorithms to attain diagnostic quality images with reduced image noise at lower radiation doses[28,29]. Recent studies have acquired diagnostic quality CT abdomen and pelvis examinations in the sub-mSv range equivalent to the dose of a conventional abdominal radiograph[30]. New filtration techniques used in combination with iterative reconstruction algorithms are also under development[31].
There are some limitations to our study. The study only measured ionizing radiation exposure while patients were in the ICU, but presumably many patients would have undergone additional imaging investigations prior to or following ICU admission, making their total exposure in hospital greater than recorded. This is especially true for trauma patients who may have had trauma series radiographs or whole-body CT on presentation to the emergency department. Patient CEDs were calculated using reference effective doses published by the United Kingdom National Radiation Protection Board[17]. However, due to the complexity of the cases in an ICU, it is possible that imaging studies may have involved higher doses than the quoted effective doses. We also only studied patients in a single center and our results may not be applicable to other centers. Finally, we did not measure lifetime CED in this study.
However, there are several strengths of our study. We had a relatively large sample size compared to previous studies assessing radiation dose in ICU patients. Requesting physicians were unaware of the study to avoid any confounding influence on physician behavior with regard to requesting radiology examinations. Our study population consisted of patients in a general ICU and therefore the results are more widely applicable. Additionally, we included pediatric patients in our study, which is arguably the most vulnerable subgroup.
In conclusion, we report a relatively low CED in the majority of ICU patients, during their stay in the ICU. Nevertheless, physicians are beholden to keep radiation exposures from diagnostic imaging as low as reasonably practical and CED should be minimized where feasible, especially in young patients. Physicians should be aware that trauma patients and patients with extended ICU admission times are at an increased risk of high CEDs. Reduction in CED may be achieved with the development of strategies to monitor CED, substituting CT with MRI and US where possible, and optimizing CT protocols based on clinical validation to keep patient doses as low possible.
There has been growing awareness in the medical literature of the potential for high lifetime cumulative radiation exposures incurred by patients as a result of repeated use of diagnostic imaging. This is especially true in certain patient groups such as those with chronic illness that require repeated imaging studies over their lifetimes. The relationship of cumulative radiation exposure to a quantifiable risk of cancer induction is a controversial topic. However, protracted exposure to low-level ionizing radiation is widely believed to be associated with an increased risk of malignancy with several studies demonstrating a dose-dependent correlation between radiation exposure and cancer incidence.
Quantifying cumulative radiation dose delivered by medical imaging is gaining increasing importance in the literature. The importance of monitoring cumulative effective dose (CED) is seen in the recent development by industry of radiation dose monitoring software systems, which are now commercially available and are gaining market traction. Several studies published recently in the literature have documented high CEDs in a number of patient groups including Crohn’s disease, cystic fibrosis, end-stage kidney disease, and testicular cancer.
Studies have shown that intensive care unit patients receive significantly higher cumulative doses of ionizing radiation than other hospitalized patients (17.9 mSv vs 11.3 mSv in one study). The authors found trauma patients and patients with extended intensive care unit (ICU) admission times to be at an increased risk of higher CEDs. CED should be minimized where feasible, especially in young patients.
ICU patients are critically ill and often require several diagnostic radiology investigations that may need to be repeated within a short time frame. Physicians should be aware that trauma patients and patients with extended ICU admission times are at an increased risk of high CEDs. Reduction in CED may be achieved with the development of strategies to monitor CED, substituting computed tomography (CT) with magnetic resonance imaging and ultrasound where possible, and optimizing CT protocols based on clinical validation to keep patient doses as low possible.
CED refers to the total radiation dose a patient has received as a result of undergoing diagnostic radiology examinations that involve the use of ionizing radiation.
The authors studied the CED from diagnostic imaging in ICU patients with good results.
P- Reviewer: Gao BL, Li YZ, Salemi VMC S- Editor: Qi Y L- Editor: A E- Editor: Jiao XK
| 1. | Sullivan CJ, Murphy KP, McLaughlin PD, Twomey M, O’Regan KN, Power DG, Maher MM, O’Connor OJ. Radiation exposure from diagnostic imaging in young patients with testicular cancer. Eur Radiol. 2015;25:1005-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: The National Academies Press 2006; . [Cited in This Article: ] |
| 3. | National Council on Radiation Protection and Measurements. Report No. 160 – Ionizing Radiation Exposure of the Population of the United States. Bethesda, Maryland: National Council on Radiation Protection and Measurements 2009; . [Cited in This Article: ] |
| 4. | Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, Greenlee RT, Kruger RL, Hornbrook MC, Roblin D. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307:2400-2409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 574] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 5. | Nakashima M, Kondo H, Miura S, Soda M, Hayashi T, Matsuo T, Yamashita S, Sekine I. Incidence of multiple primary cancers in Nagasaki atomic bomb survivors: association with radiation exposure. Cancer Sci. 2008;99:87-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res. 2000;154:178-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 7. | Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res. 2007;168:1-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1224] [Cited by in F6Publishing: 1181] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 8. | Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, Schubauer-Berigan M. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ. 2005;331:77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 373] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Valentin J. Low-dose extrapolation of radiation-related cancer risk. Ann ICRP. 2005;35:1-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2000 Report to the General Assembly, with Scientific Annexes. Volume II. Effects, Annex I. New York: United Nations, 2000. . [Cited in This Article: ] |
| 11. | Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2366] [Cited by in F6Publishing: 2122] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 12. | Desmond AN, O’Regan K, Curran C, McWilliams S, Fitzgerald T, Maher MM, Shanahan F. Crohn’s disease: factors associated with exposure to high levels of diagnostic radiation. Gut. 2008;57:1524-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 13. | O’Connell OJ, McWilliams S, McGarrigle A, O’Connor OJ, Shanahan F, Mullane D, Eustace J, Maher MM, Plant BJ. Radiologic imaging in cystic fibrosis: cumulative effective dose and changing trends over 2 decades. Chest. 2012;141:1575-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Coyle J, Kinsella S, McCarthy S, MacWilliams S, McLaughlin P, Eustace J, Maher MM. Cumulative ionizing radiation exposure in patients with end stage kidney disease: a 6-year retrospective analysis. Abdom Imaging. 2012;37:632-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Lutterman AC, Moreno CC, Mittal PK, Kang J, Applegate KE. Cumulative radiation exposure estimates of hospitalized patients from radiological imaging. J Am Coll Radiol. 2014;11:169-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Strawbridge H, Kurland G, Watson RS, Sheetz M, Wilkinson S, Weiner DJ. Radiation exposure from diagnostic imaging in the pediatric intensive care unit. Pediatr Crit Care Med. 2012;13:e245-e248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Tanner RJ, Wall BF. Radiation exposure of the UK population from medical and dental x-ray examinations. United Kingdom National Radiation Protection Board. [accessed 2015 Aug 31]. Available from: http: //cloud.medicalphysicist.co.uk /nrpb_w4.pdf. [Cited in This Article: ] |
| 18. | Andrieu N, Easton DF, Chang-Claude J, Rookus MA, Brohet R, Cardis E, Antoniou AC, Wagner T, Simard J, Evans G. Effect of chest X-rays on the risk of breast cancer among BRCA1/2 mutation carriers in the international BRCA1/2 carrier cohort study: a report from the EMBRACE, GENEPSO, GEO-HEBON, and IBCCS Collaborators’ Group. J Clin Oncol. 2006;24:3361-3366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Ronckers CM, Erdmann CA, Land CE. Radiation and breast cancer: a review of current evidence. Breast Cancer Res. 2005;7:21-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, Schubauer-Berigan M. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat Res. 2007;167:396-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 450] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 21. | Hendee WR, O’Connor MK. Radiation risks of medical imaging: separating fact from fantasy. Radiology. 2012;264:312-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 22. | Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251:13-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 331] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 23. | Rohner DJ, Bennett S, Samaratunga C, Jewell ES, Smith JP, Gaskill-Shipley M, Lisco SJ. Cumulative total effective whole-body radiation dose in critically ill patients. Chest. 2013;144:1481-1486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Desmond AN, McWilliams S, Maher MM, Shanahan F, Quigley EM. Radiation exposure from diagnostic imaging among patients with gastrointestinal disorders. Clin Gastroenterol Hepatol. 2012;10:259-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Kalra MK, Maher MM, Toth TL, Kamath RS, Halpern EF, Saini S. Radiation from “extra” images acquired with abdominal and/or pelvic CT: effect of automatic tube current modulation. Radiology. 2004;232:409-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Allen BC, Baker ME, Einstein DM, Remer EM, Herts BR, Achkar JP, Davros WJ, Novak E, Obuchowski NA. Effect of altering automatic exposure control settings and quality reference mAs on radiation dose, image quality, and diagnostic efficacy in MDCT enterography of active inflammatory Crohn’s disease. AJR Am J Roentgenol. 2010;195:89-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Toth T, Ge Z, Daly MP. The influence of patient centering on CT dose and image noise. Med Phys. 2007;34:3093-3101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 28. | Singh S, Kalra MK, Do S, Thibault JB, Pien H, O’Connor OJ, Blake MA. Comparison of hybrid and pure iterative reconstruction techniques with conventional filtered back projection: dose reduction potential in the abdomen. J Comput Assist Tomogr. 2012;36:347-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 29. | Yasaka K, Katsura M, Akahane M, Sato J, Matsuda I, Ohtomo K. Model-based iterative reconstruction for reduction of radiation dose in abdominopelvic CT: comparison to adaptive statistical iterative reconstruction. Springerplus. 2013;2:209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | O’Neill SB, Mc Laughlin PD, Crush L, O’Connor OJ, Mc Williams SR, Craig O, Mc Garrigle AM, O’Neill F, Bye J, Ryan MF. A prospective feasibility study of sub-millisievert abdominopelvic CT using iterative reconstruction in Crohn’s disease. Eur Radiol. 2013;23:2503-2512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Gordic S, Morsbach F, Schmidt B, Allmendinger T, Flohr T, Husarik D, Baumueller S, Raupach R, Stolzmann P, Leschka S. Ultralow-dose chest computed tomography for pulmonary nodule detection: first performance evaluation of single energy scanning with spectral shaping. Invest Radiol. 2014;49:465-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |