INTRODUCTION
Allograft tissues obtained from human donor have wide range of clinical applications. Bone and soft tissue allografts are used for reconstruction of musculo-skeletal defects. Allogenic skin and amniotic membrane are used for treatment of burn injuries and non-healing ulcers. The use of autologous graft in clinical procedures has several disadvantages. The autograft tissues can be obtained in limited quantities, involves expense and trauma for acquisition of the grafts, and also results in donor site morbidity. Thus, the great need of the substitutes of autograft is satisfied by allografts. They offer several advantages like decreased morbidity, avoidance of the sacrifice of patient’s normal structure, reduction of prolonged hospital stays and cost, and availability of unlimited quantities of grafts bearing required functionality, size and shape. Allografts are banked throughout the world. Tissue allografts such as bone, cartilage, tendons, skin and amniotic membrane are processed and distributed by tissue banks. The general purpose of a tissue bank is to provide safe and effective allografts for transplantation. The use of allogeneic tissue grafts is beneficial; however, the possibility of disease transmission is a major concern with allografts.
TISSUE ALLOGRAFTS
Allografts have gained increasing popularity in reconstruction and their usage among surgeons has risen dramatically, resulting in impressive life-enhancing benefits. Allogenic tissues are obtained from living and cadaveric donors. Rigorous screening of all donors is carried out so that the donor material collected is free of pathogens that can transmit disease to the tissue recipients. Tissue allografts are simple and effective clinical tool for reconstructive surgery, while at the same time avoiding the pain, trauma, morbidity of a secondary surgical procedure necessary for acquiring autologous tissue.
Bone allografts have been successfully used in a variety of clinical situations for musculoskeletal reconstructive surgery. These include treatment of fractures and fracture defects, arthrodeses, filling of cavities in benign tumorous conditions and traumatic loss, management of large osseous defects in total knee arthroplasty (Figure 1). Major complications, such as cutaneous nerve damage, chronic donor site pain, vascular injury, infection and fracture are reported in autografted patients[1]. Due to complications associated with the harvesting of autogenic material[2,3], allografts have gained increasing popularity as treatment methods for musculoskeletal injuries. Allogenic grafts fulfil the demand of osteoconductivity. These grafts can either be cancellous or cortical in nature. Both variants allow the revascularization and the migration of bone forming and resorbing cells onto and into the tissues[4,5]. Therefore, the graft serves as a structure for new bone formation.
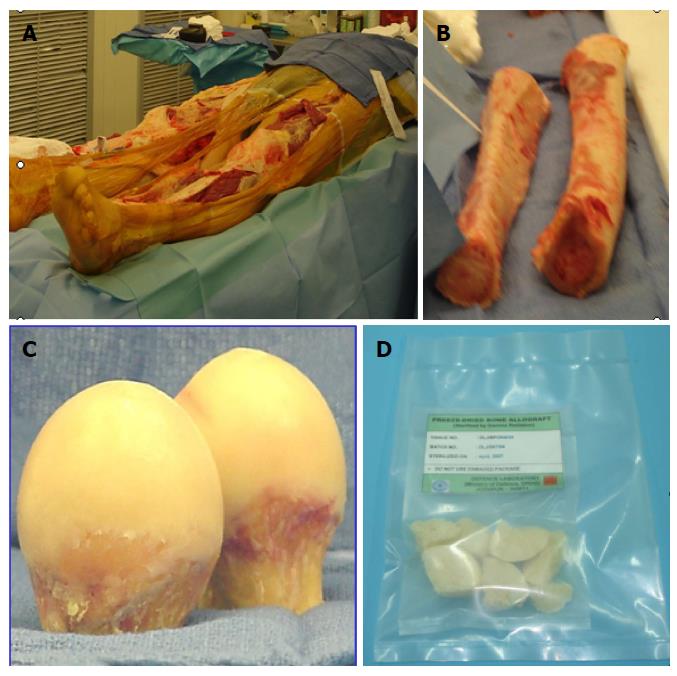
Figure 1 Bone allografts.
A: Bone collection from cadaveric donor; B: Cortical bone; C: Femoral heads excised during surgery; D: Processed bone allografts.
Human allogenic soft tissue has many indications in reconstructive surgery and have gained increasing popularity in anterior cruciate ligament reconstruction[6]. Soft tissue allografts (Figure 2) including the bone-patellar-bone graft, achilles tendon, and fascia lata are commonly used in reconstructive surgery[7]. Bone-patellar-bone grafts can be used to restore knee stability and achilles tendon can be used for ankle reconstruction or extra ocular eye surgery. Compared to autografts, the main advantage is the lack of any donor site morbidity and the faster return to normal activity.
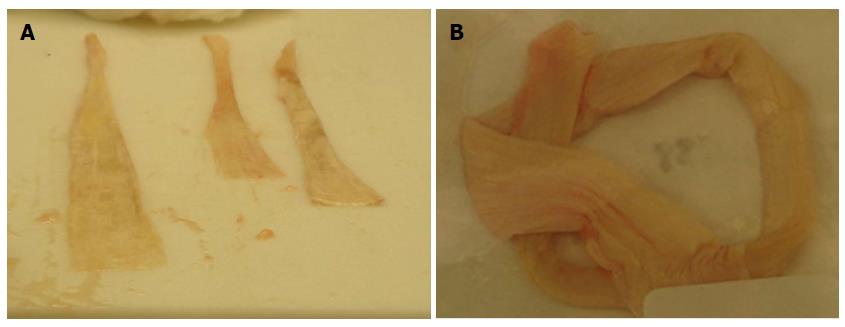
Figure 2 Soft tissue allografts.
A: Tendon allografts collected from cadaveric donor; B: Processed tendon allograft.
Human skin from cadaveric sources (Figure 3) has proved to be a very effective material to cover excised deep second or third degree burn wounds when insufficient amounts of autografts are available. Allograft skin has unique properties, which makes it indispensable in the treatment of serious burn injuries[8]. Allograft skin is used as temporary cover to provide conditions on the wound surface which favour re-epithelialization. Availability concerns do limit the use of this graft for wound therapy.
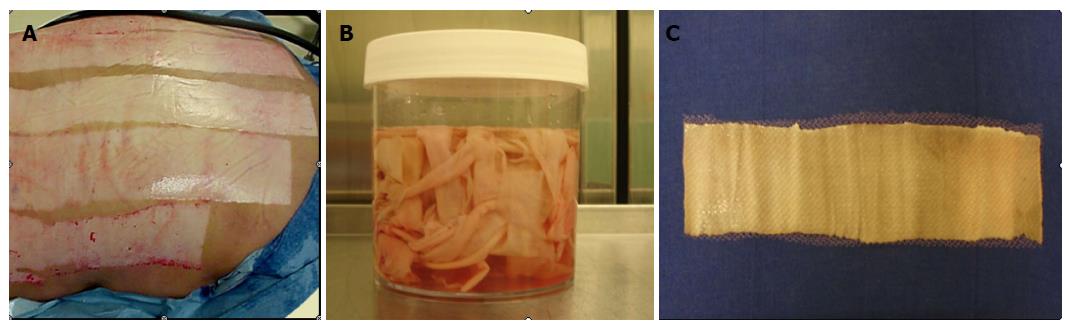
Figure 3 Allograft skin.
A: Cadaveric donor; B: Skin collected from cadaveric donor; C: Processed allograft skin.
Amniotic membrane is a collagen rich, thin, transparent, tough membrane, and lines the amniotic cavity. Amniotic membranes are obtained from the human placenta (Figure 4) after delivery and are available in bulk at major hospitals. Several properties contribute to the amnion as an ideal dressing. Amniotic membrane as a dressing adheres well to the wound, has bacteriostatic effect and acts as a barrier to external environment. Amniotic membranes have been used successfully as biological dressing for burn wounds and ulcers of various etiology[9,10]. Human amniotic membrane transplantation can promote tissue healing and reduce inflammation, tissue scarring and neovascularization.
Figure 4 Amniotic membrane.
A: Collection of amniotic tissue from placenta; B: Processed amniotic membrane dressing.
MICROBIAL CONTAMINATION OF TISSUE ALLOGRAFTS
Tissue safety is a major concern in transplantation. The transmission of infectious agents from donor to recipient with allografts is their major risk and disadvantage. The presence of microorganisms on processed tissues is unavoidable. Microbial contamination can occur from an infected donor, during collection of the tissue from donors or the environment, and during processing of the tissues. Hygienic practices during procurement and processing cannot eliminate the microbial contamination of tissues. A processed tissue in its final packaging prior to sterilization will inevitably have some microbial count, despite efforts to minimize it. Therefore, several steps should be undertaken by tissue bank operators to minimise the risk of infectious disease transmission with tissue allografts, such as careful donor selection, proper tissue processing and adequate sterilization of tissue allografts.
A number of fatal and nonfatal bacterial infections from allograft tissues obtained from cadavers have been reported[11]. Kowalski et al[12] carried out assessment of bioburden on tissue from 101 human donors and observed bioburden ranging up to > 28000 CFU. A number of studies have reported bacterial contamination of amniotic membrane[13,14]. The most prevalent organisms were Staphylococci species. Most of bacterial contaminations were related to donation process and the contamination pattern suggests procurement team as a source[13]. Other studies[15,16] have reported a range of microorganisms isolated from femoral head bone retrieved from living donors during surgery. The greatest number of isolates was Gram-positive cocci, predominantly coagulase-negative staphylococci. The second group most frequently isolated was Gram-positive bacilli, predominantly diphtheroids. Varettas et al[17] have reported coagulase-negative staphylococci as the predominant organism isolated from femoral head allografts of living donors. However, organisms such as Clostridium have become particularly important following report by Malinin et al[18] who showed a significant number of clostridial contamination in musculoskeletal allografts. Dennis et al[19] have reported Propionibacterium, coagulase-negative Staphylococcus, Pseudomonas aeruginosa, Klebsiella oxytoca, Lactobacillus species, Peptostreptococcus asaccharolyticus and Streptococcus sanguinis as the most frequently cultured organisms from the musculoskeletal allograft tissues. As in other studies[16] the organisms isolated from this study were predominantly skin flora. In living donors, contamination with regard to incidence and type of microorganisms is similar to that observed in surgical theatres during routine surgery[20].
Viral transmission may also come from infected donor. There are reports of transmission of hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), and human T-lymphotropic virus (HTLV) through tissue transplantation[21]. The incidence of viremia at the time of donation has been estimated to be 1 in 55000 for HBV, 1 in 34000 for HCV, 1 in 42000 for HIV, and 1 in 128000 for HTLV[22]. The first case of Acquired Immune Deficiency Syndrome surfaced in 1981 and the first case of HIV-1 transmission in bones was reported in 1984, followed by a second case in 1985[23]. Two cases of hepatitis C transmission were also reported in the 1990s, the second case occurring despite the existence of a first-generation screening test[23,24].
Safety issues regarding the transmission of microbial infections via allograft transplantation are of critical concern to both the surgeons and the recipients of allogenic tissue. Adequate donor screening coupled with aseptic processing reduce the risk of allograft associated disease transmission, but the possibility of infections is not completely eliminated. A sterilization process with high inactivation efficiency is therefore needed to assure the safety of allograft tissues for clinical use. Allograft tissues must be treated with sterilization methods to prevent the transmission of diseases from the donor to the recipient.
METHODS FOR STERILIZATION OF TISSUE ALLOGRAFTS
Sterilization of the tissue allografts is necessary to reduce the risk of transmission of infectious agents. Sterilization is a definitive method for eliminating microorganisms and can prevent life-threatening allograft associated infections[25]. Various sterilization techniques have been used to prevent infection through allografts. These include gamma irradiation[26], ethylene oxide gas[27], thermal treatment with moist heat[28], beta-propiolactone[29], chemical processing[30], and antibiotic soaks[31].
Ethylene oxide is widely used commercially for sterilization of health care products. It is a suitable method for sterilization of heat sensitive medical products and tissue allografts. Ethylene oxide is a chemical sterilization method which provides both bactericidal and virucidal effects at appropriate concentrations[32]. Ethylene oxide is applied in a gaseous state in mixture with inert diluents such as CO2[25]. Ethylene chlorohydrin is a toxic byproduct produced by ethylene oxide that influences the cell response and causes synovial inflammation. Ethylene oxide is thus not a suitable method of sterilization for tissue allografts[33].
Peracetic acid-ethanol sterilization procedure has been used for sterilization of bone grafts[34]. Several studies have demonstrated the antimicrobial efficacy of this method. Although the peracetic acid treatment is an established sterilization method of bone, dermis and amniotic membrane transplants with no evidence to impair the transplants properties, it has caused significantly reduced biomechanical strength and decreased remodeling activity in anterior cruciate ligament reconstruction tendon grafts[35]. Chemical processing and antibiotic soaks have certain limitations for sterilization of allograft tissues due to the lack of complete penetration for inactivation of deep-seated bioburden.
Thermodisinfection has been used for femoral heads excised during hip joint surgery. Fölsch et al[36] examined the influence of heat sterilization on pull out strength of cancellous bone and storage at different temperatures up to 2 years. Thermodisinfection of cancellous bone was found to preserve tensile strength necessary for clinical purposes.
Several investigators have also proposed the use of microwave for sterilization of medical appliances and materials[37]. Few studies are reported on the use of microwave for sterilization of bone allografts. Uchiyama et al[38] have used microwave as an alternative to bathtub for thermal treatment of bones. Dunsmuir et al[39] have reported sterilization of femoral head allograft by microwave. The process of microwave sterilization was found to be effective for sterilization of bone allografts processed from femoral heads contaminated with Gram-positive and Gram-negative bacteria[40].
Electron beam is a high energy electron treatment which is currently used for sterilization of medical devices and in radiation therapy. A number of tissue banks have used accelerated electron beam for sterilization of human tissues[25,41,42]. As compared to gamma radiation, accelerated electron beam has lower penetration into the material.
Most current sterilization procedures have inherent disadvantages affecting biological properties and mechanical function of the graft. Gamma radiation offers a better alternative for sterilizing tissues. The use of gamma radiations to sterilize non-viable tissue allografts is an extension of their utilization for the production of sterile single use disposable medical products[43].
RADIATION STERILIZATION OF TISSUE ALLOGRAFTS
Historical
Radiation sterilization is one of the most widespread and successful applications of radiation. It is based on the ability of ionizing radiation to kill microorganisms. The fact that ionizing radiation can kill microorganisms was recognized in 1896, shortly after the discovery of X-rays. In 1899, Pierre and Maria Curie observed the action of beta and gamma rays originating from natural isotopes on different materials and tissues. The phenomenon was investigated quite extensively in the 1920’s and 1930’s. Maria Curie considered the observations made by F. Holwek and A. Lacassagne and, in 1929, published a theoretical paper on the radiation inactivation of bacteria. However, more significant research and development on radiation sterilization commenced in the 1950’s when large sources of ionizing radiation became available. In 1956-1957, Ethicon Inc. (a subsidiary of Johnson and Johnson) in collaboration with High Voltage Engineering began commercial electron beam sterilization of sutures using a 6-Mev (4-kW) linear accelerator. A 0.5-MCi demonstration Cobalt-60 (60Co) gamma ray facility for the sterilization of plastic medical products was set up at the Wantage Research Laboratory of the United Kingdom Atomic Energy Authority in 1960. At the same time, the first commercial 60Co gamma radiation facility (2 MCi) was installed in Australia for the sterilization of goat hair. A commercial 0.15-MCi 60Co gamma sterilization facility was constructed for Ethicon in Edinburgh in 1964.
Sterilization plants and radiation sources
Radiation is an acceptable method for sterilization and is being used for more than five decades. When large radiation sources such as gamma radiation plants of either 60Co or Cesium-137 (137Cs) and electron accelerators became available, radiation sterilization was introduced to sterilize health care products on a commercial scale, and then to sterilize tissue allografts. With an increase in the use of disposable medical products, there has been a significant increase in the use of radiation sterilization and a large number of commercial 60Co irradiators have been established for sterilization with gamma radiation worldwide.
Sterilization is carried out both by 60Co gamma irradiation and, using a variety of electron accelerators, by electron-beam irradiation. The main disadvantage of electron beam sterilization is the relatively low penetrating power of electrons compared with 60Co gamma radiation. Nevertheless, the packages to be irradiated have relatively low densities (typically 0.15-0.2 g/cm-3), electron beams with energies of 5-10 MeV can be used to sterilize packages of many disposable medical products. Since electrons have relatively low penetration, the dose distribution through the irradiated product is less uniform than with more penetrating radiations such as gamma radiation. Gamma radiation is therefore most commonly used for sterilization of tissue allografts.
Radiation sterilization dose
Gamma radiation dose is measured in kilogray (kGy) units. One gray is the absorption of one joule of radiation energy by one kilogram of matter (one kGy = one joule/gram).
The choice of 25 kGy (2.5 Mrad) for sterilization of medical products was first suggested in 1959 by Artandli and Van Winkle. The dose was proposed based on minimum killing dose for about 150 microbial species. 25 kGy was selected as the dose for sterilization as it is 40% above the minimum dose required to kill the resistant microorganisms[44]. Accordingly, 25 kGy is the minimum irradiation dose established for sterilization. Radiation sterilization at a dose of 25 kGy provides such a high safety factor that test for sterility is generally considered superfluous.
For several decades, a dose of 25 kGy of gamma radiation has been recommended for terminal sterilization of medical products, including tissue allografts. Practically, the application of a given gamma dose varies from tissue bank to tissue bank. While many banks use 25 kGy, some have adopted a higher dose, while some choose lower doses. Radiation dose of 15 to 35 kGy have been used by different tissue banks. According to International Atomic Energy Agency (IAEA)[45], a radiation dose of 25 kGy is defined as the reference dose for the sterilization of the tissue grafts, but to keep intact the biomechanical and other properties of tissues, some tissue banks prefer lower radiation dose.
Bioburden and sterility assurance level
Several factors can affect the effectiveness of radiation sterilization process. One of the factors influencing the effect of irradiation is bioburden. Bioburden is the population of viable microorganisms present on or inside a product before sterilization. The process of radiation sterilization is more effective when the bioburden is low. The behaviour of the microbial population on exposure to ionizing radiation is of greatest relevance in radiation sterilization practice. The destruction of microorganisms by gamma radiation follows an exponential law. The probability of survival is a function of the number and types (species) of microorganisms present on the product (bioburden). The concept of sterility assurance level (SAL) is derived from kinetic studies on microbial inactivation, i.e., the probability of viable microorganisms being present on or inside a product unit after sterilization. The allografts must receive a sterilization dose high enough to ensure that the probability of an organism surviving the dosage is no greater than one in one million units tested (10-6). The sterilization process must be validated to verify that it effectively and reliably kills any microorganisms that may be present on the presterilized allograft.
Gamma irradiation of tissue allografts
Gamma irradiation is the process of exposure to gamma rays from radionuclide isotopes 60Co and 137Cs. Gamma irradiation has been proved to be successful in sterilization of medical products. It has an extensive history of use for sterilizing tissue allografts. Gamma radiation has bactericidal and virucidal properties. Gamma irradiation as a sterilization method was first approved in 1963 by British Pharmacopoeia and was later accepted by United States Pharmacopeia XVII and the European Agency for the Evaluation of Medicinal Products. It is currently the most common method for sterilization of tissue allografts.
Sterilization of tissue allografts should be carried out in plastic bags resistant to radiation dose to ensure the safety of allografts. The packaging polymer should also not react with the chemical components of tissues during the sterilization process[25]. The efficacy of gamma irradiation is significantly higher when treated in the liquid, hydrated state as compared to tissues in the frozen or freeze-dried state[32,46].
IAEA has actively supported radiation technology for sterilization purposes. The IAEA has developed and issued many guidelines and standards applicable to radiation sterilization. The IAEA’s promotion and financial support has resulted in the establishment of tissue banks and the application of ionizing radiation for the sterilization of tissue allografts in different countries of Asia and the Pacific region[47].
MECHANISM OF RADIATION STERILIZATION
The lethal effect of ionizing radiation is primarily due to the genetic damage and inhibition of cell division of the microorganisms. There are two mechanisms for the cell damage and inactivation of bacteria, fungi and viruses due to the direct effect and indirect effect of gamma radiation.
Direct effect of radiation
The damaging process may be caused directly by ionizing radiation. Ionizing radiation can incur damage directly by interaction with critical biological molecules leading to excitation, lesion and scission of polymeric structure. High energy photons of ionizing radiation or active radical produced by the ionization process can damage the DNA strands[48]. Single-strand breaks (SSBs) in the sugar phosphate backbone of the individual polynucleotide strands, double strand breaks due to adjacent or near adjacent breaks in the two polynucleotide strands, cross-linking within single strand, intermolecular cross-linking between two strands and base alterations may occur due to exposure to ionizing radiation. Ionizing radiation induces structural damage in DNA which inhibits DNA synthesis, causes errors in protein synthesis, and this leads to cell death.
Indirect effect of radiation
Another effect of radiation is called indirect effect which is due to the aqueous free radical formation as a result of radiolysis of water in microorganisms. Indirect action involves aqueous free radicals as intermediaries in the transfer of radiation energy to biological molecules. Radiation interacts with water leading to the production of free radicals and peroxy radicals that damage biological molecules like DNA and inactivate the process of reproduction causing death of microorganisms. The indirect effect of ionizing radiation is especially significant in the presence of oxygen. Hydroxyl radicals produce peroxide radicals and peroxides in the presence of oxygen and the damaging effect to DNA is therefore enhanced. DNA lesion commonly caused by indirect effects of ionizing radiation include single and double strand breaks of DNA, intra-strand cross-links and base or sugar modifications. Figure 5 illustrates the common types of DNA damage due to ionizing radiation.
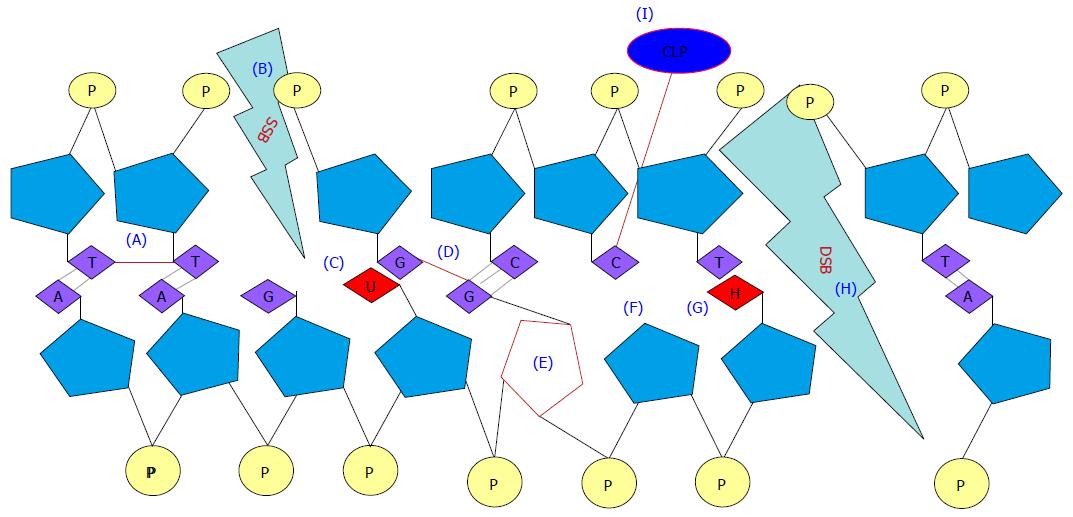
Figure 5 Types of DNA damage by ionizing radiation.
A: Intrastrand crosslink; B: SSB; C: Base deamination; D: Interstrand crosslink; E: Sugar residue alteration; F: Abasic site and hydrogen breakage; G: Base oxidation; H: DSB; I: CLP. SSB: Single strand break; DSB: Double strand break; CLP: Crosslinking protein.
DNA repair mechanisms
DNA repair system can be subdivided into several distinct mechanisms based on the type of DNA lesion. Base excision repair (BER) and SSB repair pathways are useful for the repair of damaged bases and SSBs, and these pathways overlap in certain processes; for example the ability of BER to also repair SSBs via the action of a DNA polymerase and ligase. Nucleotide excision repair is a highly versatile pathway that can recognize and repair bulky and helix-distorting lesions from DNA including intrastrand, interstrand and DNA-protein crosslinks. Repair of double-strand breaks comprise both homologous recombination repair and non-homologous end-joining. Both prokaryotes (bacteria) and eukaryotes (moulds and yeasts) are capable of repairing DNA. Radiosensitivity of a strain depends on the capability to repair DNA. Strains lacking the ability of DNA repair are more radiosensitive than the others.
Response of microorganisms to radiation
The radiation resistance of a microorganism is measured by the decimal reduction dose (D10 value), which is defined as the radiation dose (kGy) required to reduce the number of that microorganism by 10-fold (one log cycle) or required to kill 90% of the total number. Survival curve for a microorganism is obtained by exposing equal sized population to different doses of radiation and determining the survival fraction. Dose response or inactivation curve is plotted using the surviving fraction at different doses of treatment[49].
Microorganisms have higher resistance to radiation as compared to the higher forms of life. The sensitivity to radiation is inversely correlated to size with viruses being the most resistant to radiation. The radiation sensitivity of microorganisms is determined genetically and the Gram-negative bacteria are reported to be more radiation sensitive than the Gram-positive bacteria. The high radiation resistance of Micrococcus radiodurans and Streptococcus species. is due to the presence of efficient mechanisms for DNA repair. The effect of radiation on fungi is slightly complicated since fungi possess more complex morphology, cytology and life cycles[48]. Prions are extremely resistant to most chemical and physical sterilizing agents, including ionizing radiation. Enzymes, pyrogens, toxins and antigens of microbial origin have higher resistance to radiation as compared to the living cells.
Factors influencing response to radiation
The effect of radiation on a microorganism is dependent on the physical and physiological factors during irradiation. Most microorganisms show greater resistance to radiation in the stationary growth phase than in the logarithmic growth phase. It may be due to slow DNA degradation and a greater capacity for the repair of single strand DNA breaks in stationary phase. Environmental conditions before, during and after irradiation also have a significant effect on the response of microorganisms to radiation. Microorganisms are much more sensitive in liquid solution than when suspended in the frozen state. This is due to the immobilization of the free radicals and prevention of their diffusion when the medium is frozen, so that the indirect effect which they cause is nearly prevented. Microorganisms are more sensitive to radiation in the presence of oxygen than in its absence. Free radicals may react with molecules of oxygen and such reactions are of great radiobiological significance since they may lead to the production of peroxy radicals both of hydrogen and of important organic molecules, some of which have been shown to be biologically damaging. In low water activity or dry conditions, the yield of free water radicals produced by radiation is lower and thus the indirect damage. Microorganisms are thus more radiation resistant when dry than in the presence of water or high water activity. Protectors such as alcohol, glycerol, reducing agents, dimethyl sulphoxide, proteins and carbohydrates increase resistance.
ADVANTAGES OF RADIATION STERILIZATION
Radiation sterilization is a simple, safe and energy efficient process. Gamma radiation is used at commercial scale to sterilize healthcare products. The sterilization of tissue allograft can be achieved safely and effectively by gamma irradiation. Radiation process is a cold sterilization and is the preferred method for sterilization of biological tissues because of the several advantageous factors[50]. One of the principal advantages of radiation sterilization arises from its ability to destroy contaminating microorganisms with an insignificant rise in the temperature of the irradiated materials, thereby preserving the properties and characteristics of tissues. The high penetration of gamma radiation enables the bulk of the hard and soft tissues to be sterilized in their final packaged form. The effect is instantaneous and simultaneous for the whole target. Since materials can be effectively sterilized by radiation in their final packages, this method provides considerable flexibility in packaging for sterilization and allows the product to be retained in the sterile form until the package is opened or damaged. The sterilization of materials at the terminal phase in its final packaging material and its suitability to a variety of different kinds of packaging materials have brought additional value to radiosterilization. Radiation sterilization is efficient at room temperature and even at temperatures below zero. The process control is precise and can be applied accurately to achieve sterility. Irradiation time is the only parameter which needs to be controlled.
Gamma irradiation sterilization has been proven to eliminate viruses, bacteria, fungi and spores from tissue without affecting the structural or biomechanical attributes of tissue grafts. The efficacy of allograft sterilization is supported by the absence of bacterial or viral allograft-associated infections in tissues processed by this method[51]. Radiation sterilization offers many advantages over conventional methods based on heat or ethylene oxide. Radiosterilization does not exhibit any of the toxicological and ecological problems that ethylene oxide and formaldehyde sterilization do because of solvent residues that may stay on the material even after the quarantine process. However, radiation sterilization is more expensive than the other sterilization methods that require large facilities. The need for large facilities with proper radiation protections for personnel and the environment makes this procedure highly costly.
VALIDATION OF THE RADIATION STERILIZATION PROCESS
It is vital that the sterilization processes applied to tissue allografts are validated to ensure sterility. A number of standards have been used for validation of the sterilization of medical products. ANSI (American National Standards Institute), AAMI (Association for the Advancement of Medical Instrumentation), ISO (International Organization for Standardization), and ASTM International (American Society for Testing and Materials) have established standards for validation of the radiation sterilization process. International standards for sterilization have had a significant impact on radiation sterilization. The ISO standard 11137 and the European standard EN 552 have been available since 1994 and are widely accepted.
Tissue banks using gamma radiation for the sterilization of tissues followed ISO 11137[52] and ISO/TR 13409[53] to validate the process[47]. Twenty-five kGy as sterilization dose for tissue allograft is validated and substantiated according to ISO 13409. In 2006, ISO 11137:2006[54] was adopted in order to replace the ISO 11137:1995[52]. Methods 1 and 2 of the ISO 11137[54-56] as before allow selection of doses other than 25 kGy. The new VDmax approach included in the new ISO document, depending on bioburden of the product, offers the validation of 15 kGy (VDmax15 Method) as well as the substantiation of 25 kGy (VDmax25 Method). The revised document Sterilization of Health Care Products - Radiation is divided into three parts. Part 1 is Requirements for Development, Validation, and Routine Control of a Sterilization Process for Medical Devices specifies requirements for development, validation, process control, and routine monitoring in the radiation sterilization for health-care products. Part 1 applies to continuous and batch-type gamma irradiators using the radionuclides Co60 or Cs137, and to irradiators using a beam from an electron or X-ray generator. Part 2 on Establishing the Sterilization Dose describes methods that can be used to determine the minimum dose necessary to achieve the specified requirement for sterility, including methods to substantiate 15 or 25 kGy as the sterilization dose. Part 3 is Guidance on Dosimetric Aspects provides guidance on dosimetry for radiation sterilization of health-care products and dosimetric aspects of establishing the maximum dose (product qualification); establishing the sterilization dose; installation qualification; operational qualification; and performance qualification[54-56].
The ANSI/AAMI/ISO 11137:2006 standard (Sterilization of Health Care Products - Radiation) was published originally in 1995. AAMI Technical Information Report on Substantiation of a Selected Sterilization Dose - Method VDmax was published in 2005[57]. In 2007, the IAEA published a code of practice entitled “Radiation Sterilization of Tissues Allografts: Requirements for Validation and Routine Control” for guiding tissue bankers in the proper use of ionising radiation technique for sterilization of tissue allografts[58].
EFFECT OF GAMMA RADIATION ON PROPERTIES OF TISSUES
The biological properties of tissue allografts, their immunogenicity, their rate of resorption, their ability to induce regeneration processes, e.g., osteoinductive capacity of bone grafts, and, in some cases, their mechanical properties, are of great importance from the clinical point of view. The requirements are, however, different for various types of grafts, depending on the role which they should fulfill in the recipient. Cartilage grafts used in reconstructive surgery should be unresorbed as long as possible. The mechanical properties are very important in the case of tendons or structural, weight-bearing bone grafts, but they are not significant when morsellised bone is used to fill up bone defects after removal of benign tumours, or when preserved skin and amniotic membranes are used as a temporary dressing for the treatment of burns. Preserved, radiation sterilized connective tissue allografts serve as a kind of biological prosthesis and, in most cases undergo subsequent resorption and substitution by the host’s own tissues.
No deleterious effect of radiation sterilization with doses up to 25 kGy on physical and biological properties of tissue allografts has been confirmed in laboratory and clinical studies[25,59]. Direct effect due to free radicals induced by irradiation cause scission of collagen molecules[60,61] and at the same time creation of new immature collagen crosslinks by indirect effect[25]. The impact of these processes on the final effects may differ depending on irradiation conditions (dose, temperature), physical state of a sample[25] and the type of irradiation source used. High doses of ionizing radiation (above 50 kGy) can evoke numerous chemical and physical changes that may affect the biological quality of tissue allografts, such as the osteoinductive capacity of bone, the mechanical properties of bone and other connective tissue allografts as well as the rate of their resorption in vivo.
Effect of radiation sterilization on the structural and functional properties of allograft tissues have been studied using a number of techniques. Scanning electron microscopy (SEM) for collagen structure[62], infrared spectroscopy for chemical structure of amniotic membrane[63], bone graft models for osteoinduction and bone absorption[64], and compression or bending tests for mechanical properties have been used[26,65-67]. Voggenreiter et al[62] studied the bone surface structure of cortical bone grafts using SEM and observed no deleterious effects of cryopreservation and irradiation. Yamamoto et al[68] reported that the gamma irradiation of femurs to a dose of 25 kGy increased the crystallinity, whereas, there was no change in the Raman spectrum. The authors concluded that increased crystallinity may be due to the adverse effects of gamma radiation on bone allografts. However, Jinno et al[69] observed that incorporation of syngeneic and allogeneic grafts was not affected significantly. The study thus showed that syngeneic and allogeneic graft incorporation was not influenced by the crystallinity of bone.
The mechanical and biological properties of bone allografts terminally sterilized by gamma radiation from cobalt-60 sources are affected on irradiation and the changes have been observed to be dose-dependent[70]. Mechanical properties are reported to significantly decrease on gamma irradiation at doses above 25 kGy for cortical bone and above 60 kGy for cancellous bone[70]. Biocompatibility, osteogenic capacity, biomechanical strength and architecture are all important factors in the successful incorporation of graft bone and can determine the speed of recovery. Sterilization by gamma irradiation has been demonstrated to decrease osteogenic potential by reducing biocompatibility through the production of peroxidized lipids[71], as well as diminishing the biomechanical stability of the bone[72,73].
Effect of gamma radiation on biomechanical properties of tissues
Sterilization of tissue allografts is an important prerequisite to prevent disease transmission. However, mechanical tissue properties are compromised by most current sterilization procedures. Numerous experiments have been done to study the effect of irradiation on mechanical properties of bone allografts. Most of them used gamma rays as an irradiation source[66,74-76]. High doses of irradiation up to 50 kGy do not have significant effect on the biomechanical properties of bone[25,65]. However, in most of the reports, the decrease of maximum load of cortical bone was observed after gamma irradiation with doses over 30 kGy[63,66,74,75].
Gamma irradiation has adverse effect on the mechanical and biological properties of bone allografts due to degradation of collagen in the bone matrix. Burstein et al[77] described that the plasticity of bone depends on the structure of collagen fibres. Irradiation can cause damage to collagen fibres and changes in inter- and intramolecular crosslinks of collagen which may result in the loss of mechanical properties. This finding was also described by other authors[78-81]. Hamer et al[67] reported that the plastic properties of bone grafts was altered by irradiation depending on dose. Irradiation at low temperatures was observed to prevent the damage of collagen. Free radicals are generated due to radiolysis of water molecules on irradiation that react with collagen molecules and induce cross linking reactions. Mechanical properties of bone allograft are decreased with increasing doses of gamma radiation. Effect on mechanical properties of cortical bone is observed above 25 kGy and for cancellous bone above 60 kGy[70].
Early research showing dose-dependent reductions in musculoskeletal tissue biomechanics at high gamma doses (≥ 30 kGy)[82,83] has prompted tissue banks to employ lower doses that remain extremely efficient in deactivating microorganisms while minimizing tissue damage. Studies have shown that low dose gamma irradiation at 15-20 kGy does not alter the biomechanical properties of bone-patellar tendon-bone (BTB), tibialis, and semitendinosus tendon allografts[84,85].
Several studies have shown that the compression strength of bone allografts are not altered by radiation doses less than 30 kGy[86,87]. Komender[76] showed that 90% of torsion strength is maintained upto 30 kGy. In contrast, when the irradiation dosage was increased to 60 kGy, the specimens showed a reduction in bending, compression and torsion strength. The torsion strength was decreased to 65% by irradiation at a dose of 60 kGy and to 70% by a combination of irradiation at 30 kGy and freeze-drying. Hamer et al[67] showed that the bending strength of bone was reduced to 64% of control values after irradiation with 28 kGy and that the reduction in strength was also dose dependent. Zhang et al[88] showed that there was no statistical significant difference between irradiated and non-irradiated groups for both deep-frozen allograft and freeze-dried tricortical iliac crest allografts at a radiation dosage of 20-25 kGy. Kaminski et al[89] studied the effect of 25 kGy and 35 kGy gamma radiation on mechanical properties of non-defatted or defatted compact bone grafts. Irradiation of bone grafts was carried out on dry ice or at ambient temperature. Significant decrease in the ultimate strain and toughness was found on irradiation with both the doses[89]. Significant increase in the elastic limit and resilience was observed on irradiation at 25 kGy. Maximum load, elastic limit, resilience, and ultimate stress were found to decrease on irradiation at ambient temperature[89]. The results of study suggest that the damage of collagen structure by gamma radiation may effect the mechanical properties of bone grafts[89]. No noticeable effect of gamma irradiation on mechanical properties of defatted trabecular bone allografts was observed[90]. Cornu et al[74] observed that ultimate strength, stiffness and work to failure of frozen bone was not reduced significantly on irradiation. However, decrease in the properties was observed on freeze-drying of bones before or after irradiation.
Sterilization of soft tissue allografts with high dose 60Co gamma radiation has been shown to have adverse effects on allograft biomechanical properties. Studies have shown that gamma irradiation significantly alters the initial biomechanical characteristic of soft tissue allografts in a dose-dependent manner. A number of studies are reported on the effect of gamma irradiation on biomechanical properties of human BTB allografts. Maintaining tissue mechanical integrity is particularly relevant towards accelerated rehabilitation of the injured knee, where the cyclic function of patellar tendon allografts is critical. Fideler et al[82] have reported that the initial biomechanical strength of fresh-frozen allografts was reduced up to 15% when compared with fresh-frozen controls after 2.0 Mrad of irradiation. Maximum force, strain energy, modulus, and maximum stress demonstrated a statistically significant reduction after 2.0 Mrad of irradiation (P < 0.01). Stiffness, elongation, and strain were reduced but not with statistical significance. A 10% to 24% and 19% to 46% reduction in all biomechanical properties were found after 3.0 (P < 0.005) and 4.0 (P < 0.0005) Mrad of irradiation, respectively. Curran et al[91] studied the cyclic and failure mechanical properties of paired BTB allografts, with and without low-dose irradiation of 20 kGy. Failure load averaged 1965 ± 512 N for irradiated grafts and 2457 ± 647 N for nonirradiated grafts. The authors concluded that the diminished strength of irradiated grafts may contribute to overt anterior cruciate ligament graft failure, and the increase in cyclic elongation may also be detrimental to graft function. Baldini et al[92] found that the stiffness and strength of anterior or posterior tibialis tendons at 20-28 kGy did not affected allograft strength as compared to grafts treated with supercritical CO2. McGilvray et al[93] studied the effects of 60Co gamma radiation dose on initial structural biomechanical properties of ovine BTB allografts. They observed that low dose radiation (15 kGy) does not compromise the mechanical integrity of the allograft tissue, yet high dose radiation (25 kGy) significantly alters the biomechanical integrity of the soft tissue constituent[93].
Radio-protective treatment for preserving tissue properties on gamma irradiation
Protection of tissue properties against ionizing radiation using radio-protective treatment based on crosslinking and free radical scavenging have been suggested for musculoskeletal allografts[94]. Combination of radio-protectants and optimized, high-dose gamma irradiation is a viable method for producing safer cancellous bone grafts that have the mechanical strength of existing grafts. Cancellous bone dowels treated with a radio-protectant solution and 50 kGy of optimized irradiation had an ultimate compressive strength and modulus of elasticity equal to conventionally irradiated (18 kGy) and non-irradiated control bone grafts[95]. Radio-protective effect of free radical scavenger N-acetyl-L-cysteine was observed on the mechanical properties of bovine femur cortical bone sterilized by gamma radiation[96]. Grieb et al[97] have also reported that high dose of gamma irradiation following pretreatment with a radio-protectant solution can reduce infectious risks associated with soft tissue allografts while maintaining the preimplantation biomechanical performance of the tissues. Studies by Seto et al[98] suggested that radio protective treatment improves the strength and the stability of tendon allografts. Radio-protection via combined crosslinking and free radical scavenging maintained initial mechanical properties of tendon allografts after irradiation at 50 kGy.
CLINICAL EFFICACY OF RADIATION STERILIZED TISSUE ALLOGRAFTS
Radiation sterilized bone allografts have been successfully employed in orthopaedics for a variety of purposes. The incorporation of a bone graft is the result of creeping and substitutional events that reduce the grafted bone and replace it by newly formed bone from the host bone. Bone grafts are often described by the terms osteogenicity, osteoinductivity and osteoconductivity. Osteogenicity is the presence of bone-forming cells within the bone graft[99]. Osteoinductivity is the ability of a graft to stimulate or promote bone formation. Osteoconductivity is the ability of the graft to function as a scaffold for ingrowth of new bone and sprouting capillaries. Bone allografts have an organic structure, the mineralized bone matrix, which when implanted stimulates the response of the adjacent tissue, muscle or bone, so that the capillary vessels get into the allograft and endothelial cells become osteoblasts and form new bone. This process is called osteoconduction and it is a form of indirect osteogenesis[100]. Osteoconductive grafts such as the cortical and cancellous chips and particles are used to fill defects as in the treatment of benign intraosseus tumours, in the revision of the acetabular or femoral components in hip arthoplasty[101] and in cases where the surgeon wants to take advantage of the indirect osteogenetic effect like fracture nonunions and posterior spine arthrodesis[102]. Structural osteoconductive allografts are used in major metaepiphyseal defects requiring resistance to compression as in the acetabular walls and columns reconstruction in hip revision arthoplasty[101].
Bone allografts are generally required to have no immunogenicity, possess good osteogenesis potential, maintain sufficient strength until incorporation, and not transmit a disease. Fresh allografts are less frequently used than processed allografts. The freeze-dried, irradiated bone acts as a scaffold for deposition of new bone by the host bed. New bone is formed by osteoconduction, a process in which mesenchymal cells migrating from the recipient site together with new capillaries grow into the grafted bone. This leads to a slow process of creeping substitution of the graft. The ideal allograft incorporation involves the envelopment of the necrotic graft by the new host bone containing a remodeling unit consisting of haematopoietic cells and osteoblasts. Allograft integration takes place through ingrowth (creeping substitution) or apposition of new host bone[103]. This requires optimal osteoclast-mediated bone resorption as well as bone formation.
Radiation-sterilized bone allografts have been demonstrated to be safe and effective in reconstructive oral surgery[104]. Allograft bones sterilized by irradiation have also been used for fractures of tibial plateau. Feng et al[105] evaluated irradiated bone allografts for treatment of tibial plateau fractures on 21 patients. Bone allografts were frozen for 4 wk at -70 °C and irradiated at 25 kGy. Rasmussen score and X-rays were used for clinical assessment of the patients for 1-2 years. Gamma-irradiated bone allograft was found to integrate with the surrounding host bone and is thus a viable treatment option for tibial plateau fractures. A number of clinical studies on radiation sterilized bone allografts are reported and have been proved clinically to be viable alternative to autografts[106-109].
Clinical studies have demonstrated the functional and clinical efficacy of radiation sterilized amniotic membranes for healing of burn wounds[9,10], non-healing ulcers[110], and split skin graft donor site[111]. Sterilization by gamma radiation has been found not to affect the clinical function of the amniotic membrane. This is further supported by the work of Branski et al[112] who have reported that sterilization with gamma radiation does not significantly affect the growth factor content in human amniotic membrane.
SAFETY OF RADIATION STERILIZED TISSUE ALLOGRAFTS
The increased use of allograft tissues has brought more focus to the safety of allogeneic tissue and the efficacy of various sterilization techniques. Gamma radiation is established as a procedure for inactivating bacteria, fungal spores and viruses[95,113], and thus has become a popular sterilization technique for tissue allografts. Gamma irradiation can eliminate the danger of disease transmission through allografts terminal sterilization. The sterile product, which is free from any potential source of infection, will therefore be safe for clinical use. However, radiation sterilization treatment is by no mean a substitute for stringent donor screening and validated tissue-processing procedures in tissue banking[49]. Radiation treatment provides an additional safety measure against infection, since tissues are generally procured and processed in clean but non-sterile conditions. Tissue sterilization by radiation have high SAL of 10-6 set for medical products that come in contact with human tissues[45]. The main objective of radiation sterilization of tissue allografts is to eliminate any risk to recipient with the use of contaminated graft.
Tissue allografts should be assuredly free of viral contamination besides the microbial sterility. The risk of viral transmission is greatly reduced following strict donor screening, aseptic practices and disinfection steps during the collection and processing of tissues. Terminal sterilization of tissues by gamma radiation further assures the elimination of viruses and the safety of tissue allografts. Inactivation of viruses has been reported at low dosages of gamma irradiation[82,97,114,115]. A number of studies have also demonstrated the efficacy of gamma irradiation for inactivation of HIV[114-116]. A dose of 30 kGy of gamma radiation has been shown to inactivate HIV when present at high density[117,118]. However, doses lower than 30 kGy would be sufficient for inactivation of lower density levels of HIV present in screened and NAT tested tissues. Conway et al[114] have reported D10 value of approximately 4 kGy for HIV. Based on D10 value of 4 kGy, it is assumed that 3 or 4 log reduction of HIV can be achieved at doses of 12 or 16 kGy. The process of terminal sterilization of allograft tissues using gamma irradiation has been shown to inactivate both enveloped and non-enveloped DNA or RNA viruses[119]. The final step of sterilization in the processing of allografts tissues from screened human donors provides an additional assurance of safety from viral transmission for clinical use.
CONCLUSION
Gamma irradiation is a simple, safe and highly effective sterilization method for biological tissues. Several studies have validated the efficacy of this method on viruses, bacteria, fungi, and spores and compared with other sterilization methods. Radiation sterilization of allograft tissues offers a clear advantage in terms of safety compared with other sterilization techniques. Fortunately, most tissues, including bone, soft tissues, skin and amniotic membrane can be treated with gamma radiation to kill microorganisms, without affecting their functionality. At the same time, sterilization by gamma irradiation significantly reduces the risk of infectious disease transmission with tissue allografts.
ACKNOWLEDGMENTS
The authors are extremely grateful to Dr. S.R. Vadera, Director; Sh. G.L. Baheti, Head, NRMA Division and Sh. S.G. Vaijapurkar, Head, RDP Group, Defence Laboratory, Jodhpur for the encouragement and support.