Published online Apr 28, 2014. doi: 10.4329/wjr.v6.i4.119
Revised: February 28, 2014
Accepted: April 9, 2014
Published online: April 28, 2014
Elderly people are prone to accidental falls and one of the main risk factor is considered muscle weakness. Several studies focused on muscle weakness and muscle morphology changes in the elderly that may be associated with vitamin D deficiency. The prevalence of vitamin D deficiency is higher than previously though representing an important issue for public health and prevention. There is an increased interest in vitamin D effects in skeletal muscle and imaging modalities are particularly involved in this field. In patients with vitamin D deficiency, ultrasound, computed tomography, densitometry and magnetic resonance imaging (MRI) can efficiently describe changes in muscle morphology and size. Moreover, new imaging modalities, such as MRI spectroscopy, may improve knowledge about the metabolic effects of vitamin D in skeletal muscle. In this narrative review we will discuss the role of skeletal muscle imaging in vitamin D-deficient individuals. The aim of this paper is to improve and encourage the role of radiologists in this field.
Core tip: Elderly people are prone to accidental falls and many fractures sometimes due to vitamin D deficiency. In this narrative review we will discuss the role of skeletal muscle imaging in vitamin D-deficient individuals. The aim of this paper is to improve and encourage the role of radiologists in this field.
- Citation: Bignotti B, Cadoni A, Martinoli C, Tagliafico A. Imaging of skeletal muscle in vitamin D deficiency. World J Radiol 2014; 6(4): 119-124
- URL: https://www.wjgnet.com/1949-8470/full/v6/i4/119.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i4.119
Elderly people are prone to accidental falls. Many factors are involved in falls risks such as visual impairment, neurological disorders, orthopaedic disabilities, and drug effects. More than 33% of people aged over 65 fall each year and this issue is closely related to muscle weakness[1]. It has been suggested that reduced muscle strength and weakness may be associated with vitamin D deficiency, which is common among elderly people[2]. Vitamin D deficiency in adults primarily affects bone, up to osteomalacia in severe cases, due to the combination of bone mineralization defects and resorption, the latter of which is a result of secondary hyperparathyroidism[3]. Vitamin D deficiency has also been associated with a decline in physical performance and loss of muscle strength and decreased muscle mass. Symptomatic myopathy can occur in osteomalacia, and a more subtle muscular impairment is often present in patients with moderate or mild vitamin D deficiency[4].
Beside the vitamin D skeletal and extraskeletal effects[5], there is an increased interest on vitamin D effects in skeletal muscle. Imaging modalities are particularly involved in this issue. Ultrasound (US), computed tomography (CT), densitometry and magnetic resonance imaging (MRI) can assess, with different physical principles the effects of vitamin D deficiency measuring changes in muscle morphology and size. Moreover, new imaging modalities, such as MRI spectroscopy, may improve knowledge about metabolic effects of vitamin D in skeletal muscle at a microscopical level. In this narrative review we will discuss recent developments in skeletal muscle imaging among elderly people with vitamin D deficiency.
The role of vitamin D in bone health and mineral homeostasis has been well established. There is growing evidence that vitamin D exerts several effects in skeletal muscle, including enhanced muscle strength, function and performance in general. Cell culture models suggested muscle vitamin D role in calcium handling, proliferation and differentiation, protection against insulin-resistance and arachidonic acid mobilization[6]. Vitamin D probably exerts its effects both indirectly, through calcium and phosphate pathways, and directly via binding of 1,25-dihydroxyvitamin D (1,25D) to the vitamin D receptor, that seems to be expressed in skeletal muscle cells, even if this topic is still under research[5,6].
It has been supposed that the vitamin D relationship with muscle strength may influence the increasing risk of accidental falls in the elderly[6]. Vitamin D deficiency is associated with proximal muscle weakness and reduction in performance speed, probably related to selective muscle atrophy of type II muscle fibers[5,6]. Moreover, proximal muscle weakness in severe vitamin D deficiency may be caused by secondary hyperparathyroidism and consequent hypophosphatemia[5]. It has been reported that proximal myopathy and muscle pain in subjects with severe vitamin D deficiency resolve in most cases with vitamin D supplementation[7]. In addition, a recent systematic review focused on healthy adults reported that higher 25-hydroxyvitamin D levels may have a positive effect for increasing muscle strength and reducing the incidence of injuries, but further researches are needed to strengthen these hypotheses[8]. It has also been suggested an association between low levels of vitamin D and fibromyalgia, in particular among women, but more researches in this field are still needed[7].
Therefore, vitamin D role in skeletal muscle and its association with muscle weakness and falls are an active area of research supported by clinical, biochemical and imaging parameters.
Muscle imaging is complex and presents unique anatomical and morphological challenges, which require a continuous integration of dynamic, physiologic, and functional capabilities of modern imaging techniques. The optimal use of US and MRI in various muscle disorders, including muscle strains and tears, delayed onset muscle soreness, myositis ossificans, muscle hernia, acute and chronic exertion compartment syndromes, inflammatory and infectious diseases, tumours and non-neoplastic masses is crucial to obtain the best information clinically useful. The value of CT, US, MRI in each muscle disorder has to weighted up to take advantage of strengths and weaknesses of the each technique and to understand the appropriate place of modern imaging in the clinical management of patients with muscle disease[9,10].
It is known that US, MRI, and CT are widely used with different purposes to visualize the normal anatomy of the musculo-skeletal system. However, in recent years, new and technologically advanced imaging techniques have been introduced for both clinical and research purposes to study the muscular anatomy from a physiological and microscopical point of view. For example, elastography has been introduced to assess the elastic properties of tendons and muscles. Using MRI, diffusion tensor imaging (DTI) has been introduced to study the architecture of the skeletal muscle. The DTI technique is based on the measurement of the apparent diffusion of water in a (biological) tissue. It is concluded that DTI fiber directions resemble fascicle directions visible in high-resolution images very well. Indeed, on DTI images it is possible to observe and study specific features of the skeletal muscle such as the pennate insertion on the aponeuroses and the pennation angle[9,10]. DTI has the potential to be introduced in biomechanical research on skeletal muscle function. The imaging evaluation of skeletal muscle is often performed on US. The use of small-sized probes working in general at high frequencies (frequency band 7-15 MHz) is suitable for very superficial muscles. On the other hand, if a large and deep muscle should be evaluated, for example, at the level of the lower limb it should be better to use low-frequency probes with high penetration of the US-beam (frequency band 3.5-10 MHz). It is important to remember that to depict the normal anatomy of a large wide muscle, such as the biceps femoris and the sartorius, it is possible to use an extended field-of-view which is an option frequently available on the majority of US machines currently available. The advantage of US over MRI is that the muscle may be studied “in vivo” in a relaxed status and during contraction.
Changes in muscle morphology in patients with severe vitamin D deficiency have been reported in literature. In the elderly, muscular atrophy with fatty infiltration is associated with vitamin D deficiency. Muscle trophism can be evaluated with clinical imaging modalities. In particular, to study the skeletal muscle with diagnostic imaging several modalities may be used: US, CT, dual-energy X-ray absorptiometry (DEXA) and MRI. The cross-sectional area (CSA) of a muscle is normally used to evaluate muscle size and volume. Moreover, CSA is directly related to muscle strength[11]. MRI and CT can both assess CSA and muscle composition with different physical principles. US has a limited field-of-view: this limitation hampers direct measurement of CSA of large muscles due to insufficient visualization of the whole muscle bulk[11]. US, MRI and CT can assess the presence of higher skeletal fat content than in normal muscles. Fatty replacement may present as a focal process or as a diffuse change involving one group of muscle of the entire skeletal muscle, as largely demonstrated for dystrophic muscle diseases[12]. Table 1 shows the prevalence of selective complete fatty degeneration and atrophy of the thigh muscles as recorded in our clinical practice and as demonstrated in literature[4]. This review has been focused on vitamin D deficiency and skeletal muscle, and it will not discuss the huge literature about skeletal muscle diseases.
| Rectus femoris | Adductors | Semitendinosus | Semimembranosus | Biceps femoris | Gracilis | Sartorius |
| 30% | 23% | 8% | 30% | 8% |
We will discuss the role of each modality in assessing skeletal muscle in vitamin D-deficient patient. Research studies in literature frequently refers to skeletal muscle and sarcopenia, a syndrome with loss of skeletal muscle mass and strength that occurs in the elderly[13]. In severe vitamin D deficient patients imaging of skeletal muscle may overlap with that of sarcopenia.
Using US it has been shown that quantitative muscle US is a potential alternative for assessment of disease severity and progression in several muscular diseases. The main findings in muscular diseases are increased echo intensity, reflecting increased infiltration of fat or fibrous tissue in muscles, and decreased muscle thickness, indicative of atrophy. Additional advantages of US are high discriminating ability, low cost, fast execution, and non-X-ray dependent nature. Structural abnormalities quantified with US may reflect muscle function or strength[14-16]. Moreover, US has been proposed as a reliable method for monitoring the extent of sarcopenia measuring muscle thickness, especially of musculus vastus medialis and musculus intermedius[11].
However, muscular US has been shown to have several disadvantages regarding the reliability of the results in terms of intra- and inter-observer agreement (Tagliafico et al[9], personal communication).
US elastography assesses tissue stiffness before and after compression[17]. Until now, there are limited data about the use of elastography to assess normal and pathologic skeletal muscle. However, it is likely that fatty infiltration of a muscle determines global reduce stiffness, as suggested in studies on inflammatory myositis[17].
There are no significant radiological studies about US assessment of skeletal muscle in individuals with vitamin D deficiency. We encourage research studies using US because this modality has the advantage of being available even at bedside, cheap and easily repeatable.
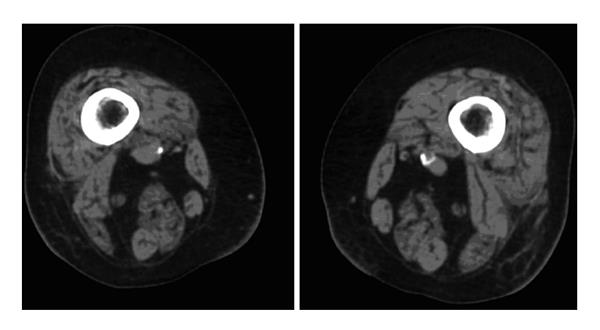
Regarding CT it has been shown that radiological measures of bone health measured with quantitative CT were not affected by persisting low vitamin D levels[18]. Non-enhanced CT scan of the pelvis demonstrated that the fat content varied among the hip muscles, with an antero-posterior gradient from the hip flexors to the hip extensors. This gradient increased after fifty years of age. Higher fat content was associated with poorer performance on physical tests, even after adjustment for the CSA of the muscle. Figure 1 shows cross-sectional CT of thigh muscles in a patient with vitamin D deficiency. Higher fat content was also associated with greater age, higher body-mass index, and lower physical activity[19]. This data has been supported by the data on muscle of the thigh and vitamin D deficiency[4]. Using very sophisticated and new techniques it has been found that the characteristic increase in osteoid-covered surfaces in vitamin D-deficient bone hampers remodelling of the remaining mineralized bone tissue. Using spatially resolved synchrotron bone mineral density distribution analyses and spectroscopic techniques, the bone tissue within the osteoid frame has a higher mineral content with mature collagen and mineral constituents, which are characteristic of aged tissue[20]. A recent cross-sectional study demonstrated a positive correlation between 1,25D levels and total skeletal muscle mass as measured on DEXA among subjects younger than 65 years[21]. This was supported by greater isometric knee extension moment in women with higher 1,25D levels. However, no association was found between 25D levels and muscle mass or strength or in those over 65 years of age. Among 26 subjects with chronic kidney disease, thigh muscle CSA on MRI correlated significantly with a model including 1,25D levels, calcium levels, and daily physical activity. Functional parameters assessing gait and proximal musculature also independently correlated with 1,25D[7,21].
By our group it has been shown that fatty degeneration of thigh muscles detected on MRI was associated with vitamin D deficiency and impaired balance and gait. Selective complete fatty degeneration of single muscles was observed[4]. These data are also supported by previous studies on muscle biopsy specimens[4].
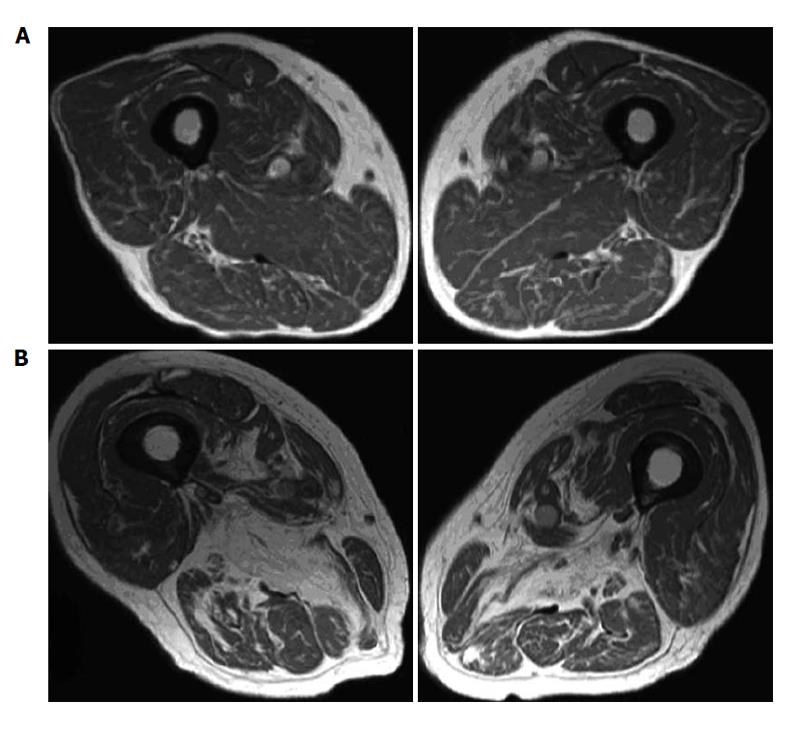
Fatty replacement and atrophy can be evaluated with MRI using a scoring system from 0 to 3 (grade 0 = normal appearance; grade 3 = severe changes)[4]. For illustration, Figure 2 shows MRI of two different grade of fatty atrophy involving thigh muscle in patients with vitamin D deficiency. The hypothesis that arose was that atrophy of skeletal muscle fibers and their replacement by fat tissue are the anatomic basis for the impairment in muscular performance described in older vitamin D-deficient people[4]. It may supposed that fatty substitution related to vitamin D deficiency may be the result of the lack of the known trophic effects of vitamin D on skeletal muscle cells[4]. If the thigh muscles are affected, the lack of enough muscular bulk may hamper balance and gait. Clinical scores were concordant with this observation. Concerning technical MRI protocols we suppose that a standard, patient-friendly protocol, including T1 and T2 weighted sequences may be sufficient for the follow-up of elderly people with potential vitamin D deficiency. In another study of 366 older patients receiving MRI of one shoulder for the investigation of potential rotator cuff injury, a correlation between higher fatty infiltration of rotator cuff muscles and lower serum levels of 25D was reported[22]. After multivariate linear regression analysis, this association remained statistically significant in two muscle groups (i.e., supraspinatus and infraspinatus muscles) but only among those whose MRI also demonstrated a full-thickness rotator cuff tear (228 patients).
Conventional MRI shows distribution pattern of fatty degeneration and it can be used for disease monitoring after treatment. Moreover, MRI allows standardize image protocols that can be useful to engage diagnostic or monitoring multi-centric trials.
New MRI sequences such as diffusion, perfusion and spectroscopy may improve the role of MRI in muscular evaluation of vitamin D role. In particular, spectroscopy magnetic resonance spectroscopy (MRS) may provide metabolic information on the musculoskeletal system. Phosphorus-31 MRS (31P-MRS) is a noninvasive technique that allows observation of mitochondrial function and oxidative phosphorylation in vivo. Based on the hypothesis that a suboptimal mitochondrial function may lead to myopathy in vitamin D-defiecient subjects, a recent study used 31P-MRS to observe that cholecalciferol therapy in vitamin D-deficient subjects improved both mitochondrial oxidative function and symptoms of myopathy and fatigue[23]; this result represented a direct sign of a link between vitamin D and mitochondria in skeletal muscle. Therefore, MRS lead to improve knowledge about vitamin D effects on skeletal muscle.
Many studies examined the specific effects of vitamin D on muscle function and physical performance. However, a very important review stated that comparing these studies is made very difficult by the variety of outcome measures used to assess muscle function[7]. We believe that including the role of diagnostic imaging to study the anatomy of skeletal muscle and not only its function may add new insights into vitamin D and muscle relationships. It has been suggested an association between vitamin D deficiency and structural muscle changes such as atrophy and fatty infiltration, both of which can be detected with cross-sectional imaging[12]. However, there are several confounders as, for example, disuse, drug use and denervation. Moreover, skeletal muscle is a highly metabolic tissue that respond not only to vitamin D effects, but to a variety of hormones and factors including, but not limited to, IL-6, brain-derived neurotrophic factor, insulin, glucocorticoids, thyroid hormones[7]. Each factor and hormone contributes and influences muscle differentiation, metabolism and function, through well known and emerging mechanisms[7]. In addition, one of the main topic is that imaging modalities can’t yet differ between extracellular fat and intracellular fat, of important pathophysiological significance[7].
On the other hand, imaging modalities permit to monitor muscle changes in size and tissue architecture. Moreover, new imaging modalities such as MRS may improve the knowledge about the direct effects of vitamin D on skeletal muscle.
In conclusion, we believe that the use of diagnostic imaging should be strongly encouraged to have radiological outcomes added to clinical and biochemical outcomes. Additional studies are needed to determine the exact role of imaging in the care of patients with vitamin D deficiency.
P- Reviewers: Arcangeli S, Shen J S- Editor: Gou SX L- Editor: A E- Editor: Liu SQ
| 1. | Venning G. Recent developments in vitamin D deficiency and muscle weakness among elderly people. BMJ. 2005;330:524-526. [PubMed] [Cited in This Article: ] |
| 2. | Semba RD, Garrett E, Johnson BA, Guralnik JM, Fried LP. Vitamin D deficiency among older women with and without disability. Am J Clin Nutr. 2000;72:1529-1534. [PubMed] [Cited in This Article: ] |
| 3. | Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477-501. [PubMed] [Cited in This Article: ] |
| 4. | Tagliafico AS, Ameri P, Bovio M, Puntoni M, Capaccio E, Murialdo G, Martinoli C. Relationship between fatty degeneration of thigh muscles and vitamin D status in the elderly: a preliminary MRI study. AJR Am J Roentgenol. 2010;194:728-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Wacker M, Holick MF. Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 6. | Dirks-Naylor AJ, Lennon-Edwards S. The effects of vitamin D on skeletal muscle function and cellular signaling. J Steroid Biochem Mol Biol. 2011;125:159-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34:33-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 335] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 8. | Redzic M, Lewis RM, Thomas DT. Relationship between 25-hydoxyvitamin D, muscle strength, and incidence of injury in healthy adults: a systematic review. Nutr Res. 2013;33:251-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Tagliafico A, Bignotti B, Airaldi S, Martinoli C. Correlation of Skeletal Muscle Anatomy to MRI and US Findings. Magnetic Resonance Imaging of the Skeletal Musculature. Berlin Heidelberg: Springer 2014; 27-39. [Cited in This Article: ] |
| 10. | Martinoli C, Airaldi A, Bignotti B, Tagliafico A. Imaging the Skeletal Muscle: When to Use MR imaging and When to Use Ultrasound. Magnetic Resonance Imaging of the Skeletal Musculature. Berlin Heidelberg: Springer 2014; 41-52. [Cited in This Article: ] |
| 11. | Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age (Dordr). 2013;35:2377-2388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 12. | Fischer D, Wattjes MP. MRI in Muscle Dystrophies and Primary Myopathies. Magnetic Resonance Imaging of the Skeletal Musculature. Berlin Heidelberg: Springer 2014; 241-254. [Cited in This Article: ] |
| 13. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6987] [Cited by in F6Publishing: 7588] [Article Influence: 542.0] [Reference Citation Analysis (0)] |
| 14. | Heckmatt JZ, Leeman S, Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. J Pediatr. 1982;101:656-660. [PubMed] [Cited in This Article: ] |
| 15. | Mayans D, Cartwright MS, Walker FO. Neuromuscular ultrasonography: quantifying muscle and nerve measurements. Phys Med Rehabil Clin N Am. 2012;23:133-48, xii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Pillen S, Tak RO, Zwarts MJ, Lammens MM, Verrijp KN, Arts IM, van der Laak JA, Hoogerbrugge PM, van Engelen BG, Verrips A. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol. 2009;35:443-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 372] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 17. | Drakonaki EE, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol. 2012;85:1435-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 18. | Dahlman I, Gerdhem P, Bergström I. Vitamin D status and bone health in immigrant versus Swedish women during pregnancy and the post-partum period. J Musculoskelet Neuronal Interact. 2013;13:464-469. [PubMed] [Cited in This Article: ] |
| 19. | Daguet E, Jolivet E, Bousson V, Boutron C, Dahmen N, Bergot C, Vicaut E, Laredo JD. Fat content of hip muscles: an anteroposterior gradient. J Bone Joint Surg Am. 2011;93:1897-1905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Busse B, Bale HA, Zimmermann EA, Panganiban B, Barth HD, Carriero A, Vettorazzi E, Zustin J, Hahn M, Ager JW. Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci Transl Med. 2013;5:193ra88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Marantes I, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ, Amin S. Is vitamin D a determinant of muscle mass and strength? J Bone Miner Res. 2011;26:2860-2871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Oh JH, Kim SH, Kim JH, Shin YH, Yoon JP, Oh CH. The level of vitamin D in the serum correlates with fatty degeneration of the muscles of the rotator cuff. J Bone Joint Surg Br. 2009;91:1587-1593. [PubMed] [Cited in This Article: ] |
| 23. | Sinha A, Hollingsworth KG, Ball S, Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J Clin Endocrinol Metab. 2013;98:E509-E513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |