Published online May 28, 2013. doi: 10.4329/wjr.v5.i5.208
Revised: April 23, 2013
Accepted: May 9, 2013
Published online: May 28, 2013
AIM: To assess the value of enlarged perihepatic lymph nodes in determining hepatic histopathology for chronic hepatitis B (CHB) by magnetic resonance imaging (MRI).
METHODS: Sixty-seven patients who were clinically and histologically diagnosed with CHB and 18 healthy subjects without history of liver disease underwent abdominal MRI. Histological diagnosis and hepatic inflammation (grade 0-4) and fibrosis (stage 0-4) were assessed by a simplified system for scoring in chronic viral hepatitis. The major imaging protocol included an axial breath-hold fat suppressed fast spoiled gradient echo T2-weighted imaging (T2WI), axial breath-trigger fat suppressed fast recovery fast spin echo T2WI, and axial and coronal fast imaging employing steady-state acquisition. Perihepatic lymph nodes larger than 5 mm in shortest diameter were noted.
RESULTS: The numbers and size indexes of lymph nodes greater than 5 mm in shortest diameter in hepatic hilum suggested inflammatory activity for subjects with grade 2 or higher, with a high accuracy of diagnosis (the area under the curves > 0.9, P < 0.001). The numbers of lymph nodes were 2 or more with a sensitivity of 87.27%, a specificity of 90.00%, an accuracy of 88.24%, a positive predictive value of 94.12%, and a negative predictive value of 79.41% in patients with grade 2 or higher, and the size indexes were no less than 180 mm2 with a sensitivity of 83.64%, a specificity of 100%, an accuracy of 89.41%, a positive predictive value of 100%, and a negative predictive value of 76.92%. The numbers and size indexes of lymph nodes were not correlated with hepatic fibrosis. The signal intensity indexes of lymph nodes were no significant correlation with histological grading or staging of liver.
CONCLUSION: The numbers and size indexes of enlarged perihepatic lymph nodes for patients with CHB suggest inflammatory activity for subjects with grade 2 or higher.
Core tip: Chronic hepatitis B (CHB) is frequently associated with hyperplasia of lymph nodes in the hepatic hilum, and the enlarged lymph nodes can be a good indicator for inflammatory activity of the liver. Enlarged perihepatic lymph nodes for the patients with CHB can be sensitively demonstrated by magnetic resonance imaging, especially fat suppressed T2-weighted imaging. The numbers and size indexes of lymph nodes larger than 5 mm in shortest diameter suggest inflammatory activity for subjects with grade 2 or higher, with a high accuracy of diagnosis at a cutoff value of 2 for the numbers or 180 mm2 for the size indexes of lymph nodes.
- Citation: Shu J, Zhao JN, Han FG, Tang GC, Luo YD, Luo L, Chen X. Chronic hepatitis B: Enlarged perihepatic lymph nodes correlated with hepatic histopathology. World J Radiol 2013; 5(5): 208-214
- URL: https://www.wjgnet.com/1949-8470/full/v5/i5/208.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i5.208
Hepatitis B virus (HBV) is one of the most common causes of chronic hepatitis, and infected individuals are at an increased risk of developing cirrhosis, liver failure, and hepatocellular carcinoma (HCC)[1-3]. Several effective medications are available to inhibit HBV replication with liver fibrosis regression by reducing liver inflammation and cellular damage in most patients with chronic hepatitis B (CHB)[3-5]. These include injectable interferon and the oral nucleoside analogues: adefovir, lamivudine, and tenofovir[2,4,6]. So, the assessment of liver necroinflammatory activity (grading) and fibrosis (staging) for patients with CHB is helpful for determining prognosis and treatment strategy[4,7]. Liver biopsy is the gold standard for the assessment of liver histology for patients with CHB. However, it is more invasive, and often more expensive, than modern imaging methods, such as sonography, computed tomography (CT), or magnetic resonance imaging (MRI).
CHB is frequently associated with hyperplasia of lymph nodes in the hepatic hilum for patients with CHB[8-10], and enlarged lymph nodes can be a good indicator of inflammatory activity by the liver in CHB[9,10]. Sonography is frequently used to evaluate lymph nodes in the hepatic hilum for the patients with CHB and chronic hepatitis C (CHC)[8-12]. MRI can not only show the anatomy of the liver and pancreas clearly but can also depict enlarged perihepatic lymph nodes and their locations relative to adjacent bile ducts or vascular structures[13-15]. MRI can provide better contrast between the lymph nodes and adjacent tissue than does sonography or CT, and is superior to sonography for visualizing enlarged lymph nodes in the porta hepatis[13-16]. In patients with chronic hepatitis, MRI is usually performed to detect the presence of cirrhosis or HCC. In addition to the MRI features that may be present in acute hepatitis, focal inflammatory activity or fibrosis may develop in chronic hepatitis, resulting in diffuse or regional high signal intensity (SI) on T2-weighted images (T2WI) and early patchy enhancement or late linear enhancement on gadolinium-enhanced dynamic magnetic resonance images[17,18]. The magnetic resonance appearance of perihepatic lymph nodes in patients with CHC has been reported[14,15]. However, to our knowledge, there are no previous studies using MRI that reveal the significance of lymph nodes in the porta hepatis for patients with CHB.
The purposes of this study were to assess the value of enlarged perihepatic lymph nodes in determining the histopathology of CHB by magnetic resonance fat suppressed T2WI.
This study was conducted in accordance with the guidelines of the review board of our institution. Between January 2005 and July 2010, all consecutive inpatients at our medical center with chronic HBV infection who had undergone an abdominal magnetic resonance examination before antiviral treatment were selected retrospectively. In patients with CHB, MRI was usually performed to detect the presence of cirrhosis or HCC. The selection criteria for patients were a diagnosis of CHB with available pathology reports from the biopsy and clinical evaluation including positive serumal hepatitis B surface antigen for at least 6 mo. Patients with hepatic malignant neoplastic diseases such as HCC, or with other diseases of the liver and gallbladder such as cholecystitis, hepatic abscess and cholangitis, or with other hepatitis such as alcoholic hepatitis, viral hepatitis except hepatitis B and autoimmune hepatitis, or with systemic or abdominal diseases inducing hyperplasia of lymph nodes in the hepatic hilum such as lymphoma, abdominal tuberculosis and malignant neoplasm, were excluded following appropriate clinical, laboratory, and radiological investigations.
Finally, a total of 67 patients met the criteria for inclusion in this study. As controls, 18 subjects were recruited without liver biopsy, selected randomly from 45 healthy volunteers without abdominal disease on magnetic resonance images by a random digits table. In addition to the exclusion criteria mentioned above, the controls were normal for liver function tests and negative for hepatitis B surface antigen. All of the patients and controls were negative for anti-human immunodeficiency virus antibody.
Experienced hepatologists performed percutaneous liver biopsies in the right lobe of the liver with sonographic guidance using an 18-gauge spring-loaded biopsy device. All core biopsy samples with common 1.5 cm length were obtained within 3 d after the MRI examination and examined by the same pathologist, who was unaware of the clinical, biochemical and imaging data. Histological diagnosis, hepatic inflammation (grade 0-4) and fibrosis (stage 0-4) were assessed by a simplified system for scoring in chronic viral hepatitis according to Scheuer (1991)[19,20].
All magnetic resonance examinations were performed on a 1.5 T MRI scanner (Signa, GE Healthcare, United States) with 38 mT/m gradient subsystems and 120 T/m/s gradient switch rates using a phased-array torso coil. The imaging protocols mainly included an axial breath-hold fat suppressed fast spoiled gradient echo, T1-weighted imaging, axial breath-trigger fat suppressed fast recovery fast spin echo (FRFSE) T2WI, and axial and coronal fast imaging employing steady-state acquisition. Forty-one of the 67 patients underwent triple-phase dynamic MRI with liver acquisition in a volume acceleration sequence. Among the various sequences included in clinical examinations, only the FRFSE T2WI were reviewed for the purpose of this study with the following parameters: repetition time = 6000 ms, echo time = 89 ms, echo train length = 19, bandwidth = 62.5 kHz, matrix = 320 × 224, number of excitations = 2, section thickness = 8 mm, gap = 1 mm.
The original data were transferred to the workstation (Advanced Workstation 4.3, GE Healthcare) with 0.1 mm accuracy for distance and 0.01 for SI. All reviews and measurement of images were carried out on the workstation by two experienced radiologists blinded to clinical and pathologic findings with consensus opinions comparing each observation item and standard together.
Perihepatic lymph nodes greater than 5 mm in shortest diameter were counted[14,21], and the long and short axis diameters of each node were measured using electronic calipers on magnified fat suppressed FRFSE T2WI. The size of the lymph nodes were defined as an index, obtained as the product of the long and short axes. A size index of lymph nodes for each patient was recorded as the sum of the diameter products of all nodes (nodal numbers were less than or equal to 3) or the three largest nodes when there were more than three nodes[14,21]. The SI of these lymph nodes was measured for each patient and expressed as ratios relative to spleen on fat suppressed FRFSE T2WI[21]. A SI index of lymph nodes for each patient was recorded as the mean of all the ratios. The SI for each lymph node was measured in a circular region of interest with range in area from 10 to 30 mm2. The spleen region of interest, ranging in area from 200 to 500 mm2, was placed in the same or adjacent magnetic resonance section as the corresponding lymph node to avoid artifacts, spleen vessels, and heterogeneous areas. Each diameter or SI was measured three times, and the average of the three measurements was considered true measurements.
Quantitative data are presented as mean ± SD. The differences for multi-group quantitative data were analyzed for variance at a P value ≤ 0.05 level of significance, and for two groups by the independent-samples t test when the distribution of data was normal. When the distribution of data was not normal or there was homogeneity of variances, a nonparametric test was used, the differences for multi-group quantitative data were analyzed with the Kruskall-Wallis test, and the comparisons for two groups were assessed using the Mann-Whitney U test. In qualitative data, the comparisons among groups were assessed using χ2 test or Fischer’s exact test.
Because the size or SI indexes of the lymph nodes could simultaneously correlate with the grade and stage of liver histology, partial correlation was used to test the relationship between the nodal size indexes and the grade or stage, or between the nodal SI indexes and the grade or stage of liver histology. The accuracy of diagnostic criteria for the size indexes or numbers of lymph nodes in predicting inflammatory activity was determined by calculating the area under the curve from corresponding receiver operative characteristics (ROC) curves.
Values of P≤ 0.05 were considered statistically significant. All statistical analyses were performed with SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, United States).
Sixty-seven patients with CHB, comprising 52 men and 15 women (age range, 18-63 years; mean age, 40.8 ± 8.3 years), and 18 healthy volunteers, 13 men and 5 women (age range, 24-63 years; mean age, 42.4 ± 11.4 years), met the criteria for inclusion in this study. Between the patients and controls, there were no statistical differences between genders (P = 0.755, Fischer’s exact test) and ages (t = 0.550, P = 0.588). Liver histological findings for all subjects are shown in Table 1.
| Grading | Staging | Sample size (n) | Size indexes (mm2) | SI indexes |
| G0 (controls) | S0 | 18 | 43.52 ± 58.38 | 0.572 ± 0.599 |
| G1 | S0 | 6 | 63.33 ± 74.27 | 0.474 ± 0.519 |
| S1 | 3 | 92.48 ± 87.29 | 0.720 ± 0.627 | |
| S2 | 2 | 137.50 ± 50.20 | 1.130 ± 0.500 | |
| S3 | 1 | 0 | 0 | |
| G2 | S1 | 6 | 221.16 ± 68.83 | 1.218 ± 0.167 |
| S2 | 10 | 268.22 ± 164.69 | 1.213 ± 0.210 | |
| S3 | 1 | 162.06 | 1.878 | |
| S4 | 2 | 514.09 ± 141.30 | 1.301 ± 0.204 | |
| G3 | S1 | 3 | 404.33 ± 191.74 | 1.172 ± 0.153 |
| S2 | 6 | 647.02 ± 238.31 | 1.169 ± 0.171 | |
| S3 | 9 | 413.88 ± 417.78 | 1.094 ± 0.454 | |
| S4 | 2 | 511.21 ± 172.53 | 1.383 ± 0.152 | |
| G4 | S2 | 4 | 398.09 ± 219.81 | 1.209 ± 0.350 |
| S3 | 7 | 471.95 ± 154.13 | 1.065 ± 0.247 | |
| S4 | 5 | 315.45 ± 240.52 | 1.114 ± 0.073 |
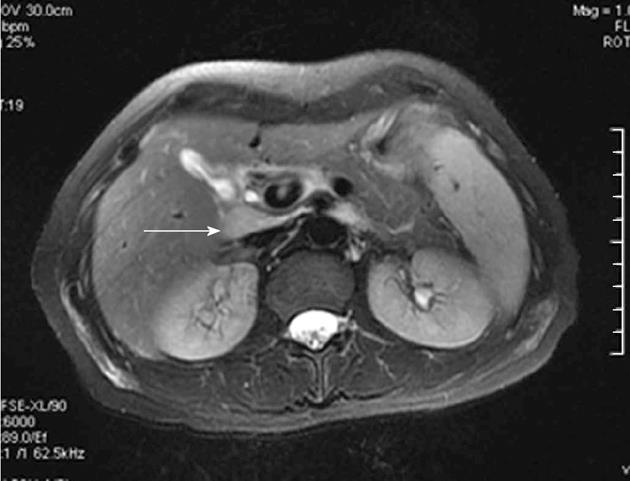
Enlarged perihepatic lymph nodes for the patients with CHB on magnetic resonance fat suppressed T2WI are shown in Figure 1. In the subjects without lymph nodes greater than 5 mm in shortest diameter in hepatic hilum, the size and SI index of lymph nodes was considered zero. The size and SI indexes of lymph nodes in hepatic hilum according to grade and stage groups are shown in Table 1. The average nodal size index was greater in individuals with grade 2 or higher than that in individuals with grades 0 and 1 [43.52 ± 58.38 mm2 for grade 0 (n = 18), 77.70 ± 74.12 mm2 for grade 1 (n = 12), 273.65 ± 155.04 mm2 for grade 2 (n = 19), 492.12 ± 324.97 mm2 for grade 3 (n = 20), and 404.58 ± 198.42 mm2 for grade 4 (n = 16)]. The average size index of lymph nodes for all subjects was 218.18 ± 262.65 mm2.
The partial correlation coefficient between the nodal size indexes and histological grading was 0.376 (P = 0.000), and 0.194 (P = 0.077) between the SI indexes and grading when controlling staging variables. There was no statistically significant correlation between the nodal size indexes and histological staging (r = 0.063, P = 0.572), or between the SI indexes and staging (r = 0.134, P = 0.226) when controlling grading variables.
The data for the nodal size indexes among partial groups of grading (grades 0 and 1) did not show normal distribution by tests of normality (P < 0.05, Shapiro-Wilk test) or by tests of homogeneity of variances for the nodal size indexes among groups of grading indicate heterogeneity of variance (F = 2.452, P = 0.006). The nonparametric Kruskall-Wallis test showed significant difference for the nodal size indexes among grading groups (χ2 = 49.557, P = 0.000). The nonparametric Mann-Whitney U test (exact probability) showed no significant difference for the nodal size indexes between grades 0 and 1 (P = 0.232), grade 2 and grade 4 (P = 0.061), and grade 3 and grade 4 (P = 0.498). However, there was a statistically significant difference between grades 1 and 2 (P = 0.000, Mann-Whitney U test with exact probability), which could suggest that the nodal size indexes in individuals with grade 2 or higher were larger than that in individuals with grades 0 and 1. All subjects were grouped into two new groups, group A comprising grades 0 and 1 (the average nodal size index, 57.19 ± 66.12 mm2) and group B comprising grade 2-4 (the average nodal size index, 391.18 ± 254.54 mm2). There was a statistically significant difference between groups A and B (U = 94.000, W = 559.000, Z = -6.741, P = 0.000, Mann-Whitney U test).
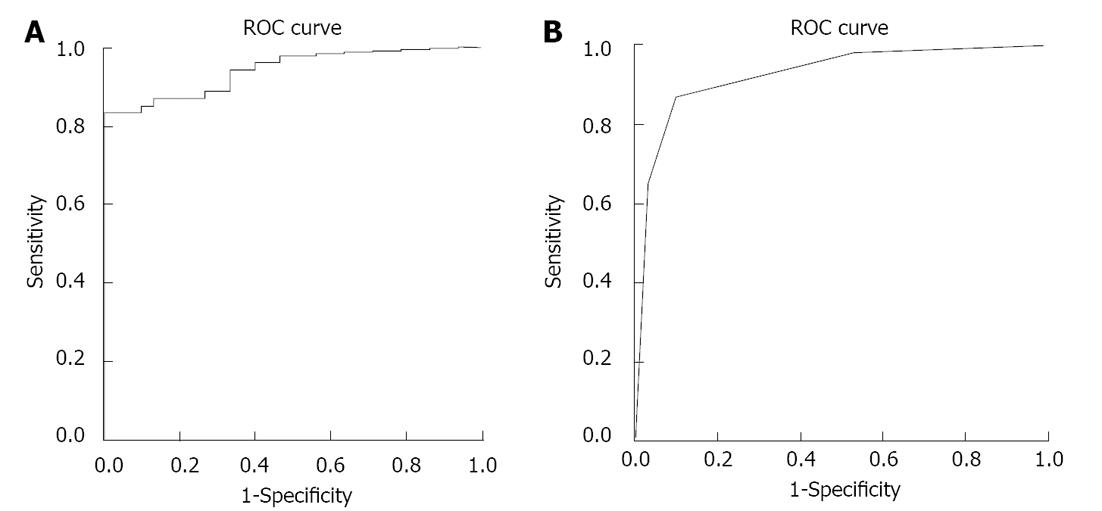
The ROC curve for the size indexes of lymph nodes predicting individuals with grade 2 or higher is shown in Figure 2A. The area under the curve was 0.943 (P = 0.000) with a cutoff value of 180.8 mm2. A cutoff value of 180 mm2 for the size indexes of lymph nodes had a sensitivity of 83.64%, a specificity of 100%, an accuracy of 89.41%, a positive predictive value of 100%, and a negative predictive value of 76.92% for a MR diagnosis of hepatic inflammation with grade 2 or higher.
The numbers of lymph nodes greater than 5 mm in shortest diameter in hepatic hilum among grade groups are shown in Table 2. There was statistically significant difference for the subjects with one lymph node or more among the grade groups (P = 0.000, Fischer’s exact test for R × C Table), for the subjects with two lymph nodes or more (χ2 = 49.556, P = 0.000), and for the subjects with three lymph nodes or more (χ2 = 33.727, P = 0.000), respectively. Presence rates of subjects with differing numbers of lymph nodes were larger in individuals with grade 2 or higher than that in individuals with grades 0 and 1 in Table 2. P value was 0.722 for subjects with one lymph node or more, 0.548 for subjects with two lymph nodes or more and 0.400 for subjects with three lymph nodes or more between grades 0 and 1, respectively (Fischer’s exact test). P value was 0.005 for subjects with one lymph node or more, 0.001 for subjects with two lymph nodes or more and 0.020 for subjects with three lymph nodes or more between grades 1 and 2, respectively (Fischer’s exact test). P value was 1.000 for subjects with one lymph node or more, 0.474 for subjects with two lymph nodes or more and 0.182 for subjects with three lymph nodes or more among grades 2-4, respectively (Fischer’s exact test). All subjects were grouped into two new groups, group A comprising grades 0 and 1 and group B comprising grades 2-4. There was a statistically significant difference between groups A and B (χ2 = 26.866, P = 0.000; χ2 = 48.295, P = 0.000; and χ2 = 30.475, P = 0.000, respectively) for the subjects with various numbers of lymph nodes.
| Grading | Sample size (n) | Subjects with various numbers of lymph nodes | |||
| 0 | ≥1 | ≥2 | ≥3 | ||
| G0 | 18 | 9 (50.00) | 9 (50.00) | 1 (5.56) | 0 |
| G1 | 12 | 5 (41.67) | 7 (58.33) | 2 (16.67) | 1 (8.33) |
| G2 | 19 | 0 | 19 (100) | 15 (78.95) | 10 (56.63) |
| G3 | 20 | 1 (5.00) | 19 (95.00) | 18 (90.00) | 16 (80.00) |
| G4 | 16 | 0 | 16 (100) | 15 (93.75) | 10 (62.50) |
| Total | 85 | 15 (17.65) | 70 (82.35) | 51 (60.00) | 37 (43.53) |
There were 15 subjects without lymph node greater than 5 mm in shortest diameter in hepatic hilum (9 subjects with grade 0, 5 subjects with grades 1 and 1 subjects with grade 3), 19 subjects with a single lymph node (8 subjects with grade 0, 5 subjects with grade 1, 4 subjects with grade 2, 1 subjects with grade 3 and 1 subjects with grade 4), 14 subjects with two lymph nodes (1 subjects with grade 0, 1 subjects with grade 1, 5 subjects with grade 2, 2 subjects with grade 3 and 5 subjects with grade 4), and 37 subjects with three or more lymph nodes (1 subjects with grade 1, 10 subjects with grade 2, 16 subjects with grade 3 and 10 subjects with grade 4). The ROC curve for the numbers of lymph nodes predicting individuals with grade 2 or higher is shown in Figure 2B. There was a high accuracy for numbers of lymph nodes predicting individuals with grade 2 or higher (the area under the curve = 0.926, P = 0.000, and cutoff value = 2). The sensitivity, specificity, accuracy, positive predictive value and negative predictive value for the diagnosis of hepatic inflammation with grade 2 or higher using 2 or more lymph nodes greater than 5 mm in shortest diameter in hepatic hilum were 87.27%, 90.00%, 88.24%, 94.12% and 79.41%, respectively.
There was a statistically significant difference for all subjects with one lymph node or more among stage groups (12 subjects with stage 0, 11 subjects with stage 1, 22 subjects with stage 2, 16 subjects with stage 3 and 9 subjects with stage 4, respectively), for subjects with two lymph nodes or more (1 subjects with stage 0, 9 subjects with stage 1, 19 subjects with stage 2, 14 subjects with stage 3 and 8 subjects with stage 4, respectively), and for subjects with three lymph nodes or more (5 subjects with stage 1, 14 subjects with stage 2, 11 subjects with stage 3 and 7 subjects with stage 4, respectively) (all three P value = 0.000, Fischer’s exact test for R × C table). However, there was no statistically significant difference between fibrosis groups (stages 1-4) (P = 0.305, 0.779 and 0.432, respectively, Fischer’s exact test for R × C table), which indicates that the presence of the lymph nodes could be not correlated with hepatic fibrosis and the difference between normal (stage 0) and fibrosis groups could come from differences in inflammatory activity of the liver.
In our study, we retrospectively reviewed the appearances of lymph nodes on axial fat suppressed FRFSE T2WI in patients with CHB, measured the presence, number, size, and SI of perihepatic lymph node, and assessed the relationship of these MR findings with liver histology for patients with CHB. We found that the number and size indexes of lymph nodes greater than 5 mm in shortest diameter in hepatic hilum suggested inflammatory activity for subjects with grade 2 or higher, with a high accuracy of diagnosis (the area under the curves > 0.9, P < 0.001). The number of lymph nodes was 2 or more with a sensitivity of 87.27%, a specificity of 90.00%, an accuracy of 88.24%, a positive predictive value of 94.12%, and a negative predictive value of 79.41% in patients with grade 2 or higher, and the size indexes were no less than 180 mm2 with a sensitivity of 83.64%, a specificity of 100%, an accuracy of 89.41%, a positive predictive value of 100%, and a negative predictive value of 76.92%. The number and size indexes of lymph nodes were not correlated with hepatic fibrosis. The SI indexes of lymph nodes were not significantly correlated with histological grading or staging of liver.
Lymph nodes are well known to exist in the hepatoduodenal ligament. They can consistently be detected in the dorsal part of the hepatoduodenal ligament adjacent to the cystic duct and common bile duct, and in the ventral hepatoduodenal ligament close to the orifice of the foramen epiploicum[22]. Enlarged lymph nodes in the hepatoduodenal ligament were prevalent in chronic viral hepatitis, especially CHC and CHB[8-12,23]. In ultrasound study, enlarged lymph nodes could be demonstrated in the hilus hepatis of almost all patients with CHB or CHC[8,9]. Lymph nodes in the hepatoduodenal ligament, especially those wider than 5 mm, suggested chronic HBV or HCV infection instead of only chronic hepatitis[8], and there was no significant difference in lymph node volume between patients with hepatitis B and those with hepatitis C[9]. Enlarged lymph nodes within the dorsal portion of the hepatoduodenal ligament can easily be identified on sonography, although it may be more difficult to detect lymph nodes in the ventral portion of the hepatoduodenal ligament because of surrounding fat deposition and connective tissue[22]. There was generally higher SI for enlarged perihepatic lymph nodes on magnetic resonance fat suppressed T2WI and better contrast between the lymph nodes and adjacent tissue than that on sonography or CT, which was superior to sonography for visualizing enlarged lymph nodes in the porta hepatica[14,21].
In patients with CHC, enlargement of perihepatic lymph nodes was associated with viremia and was predictive for the presence of severe inflammatory activity on sonography[9,22,24]. Total perihepatic lymph node volume changed according to the antiviral response: patients with CHC without response to antiviral therapy did not normalize the size of perihepatic lymph nodes, but successful antiviral therapy with histological improvement was reflected in a decline in perihepatic lymph node size[12,25].
In patients with CHB, the sonographically determined lymph node volume showed a significant correlation with serum aspartate transaminase, alanine transaminase, gamma-glutamyl-transpeptidase, histologic activity index, and necroinflammatory score, but not with fibrosis score and serum hepatitis B viremia[10].
Zhang et al[14] studied the magnetic resonance appearance of lymph nodes in relation to activity of CHC. They found that MRI could depict perihepatic lymph nodes in most patients with CHC, and that the number, size, and hyperintensity of lymph nodes were related to the activity of CHC while the results of liver function tests were not. Mitchell et al[21] found that the size index of lymph nodes was correlated with inflammatory activity of CHC but there was no correlation between Lymph node SI and any pathology using unenhanced MRI.
In our study, perihepatic lymph nodes in the patients with CHB were evaluated with MRI, especially fat suppressed FRFSE T2WI. Our results indicated that the number and size indexes of lymph nodes greater than 5 mm in shortest diameter in hepatic hilum correlated with inflammatory activity of CHB and did not correlate with fibrosis, in accordance with previous research for CHC or CHB[9,10,14,21]. The SI indexes of lymph nodes were not significantly correlated with histological grading or staging of liver, in accordance with research on CHC by Mitchell et al[21]. In our study, we also found that the number and size indexes of lymph nodes greater than 5 mm in shortest diameter suggested inflammatory activity for subjects with grade 2 or higher, with a high accuracy of diagnosis at a cutoff value of 2 for the numbers or 180 mm2 for the size indexes of lymph nodes. The findings suggest that MRI may reduce or displace liver biopsy for assessing liver inflammation in patients with CHB. Moreover, other liver diseases, such as CHC, may lead to perihepatic lymphadenectasis. The etiology diagnosis of this lymphadenectasis was difficult when depending only on MRI. In such cases, other tools such as serological examination, were available[26].
One limitation of our study is the relatively small sample size of groups. However, this should not significantly affect our results because appropriate statistical methods were applied. Additionally, in our study sequence one slice was obtained every 9 mm , with a slice-thickness of 8 mm and a gap of 1 mm. So, effect of partial volume cannot be ignored in the size index of lymph nodes for each subject. In addition, the size of the lymph nodes obtained as the product of the long and short axes could be slightly different from the true size. However, these would be only random errors without directionality in measurements, could not significantly affect our overall results.
In conclusion, enlarged perihepatic lymph nodes for the patients with CHB can be sensitively demonstrated by MRI, especially fat suppressed FRFSE T2WI. The number and size indexes of lymph nodes greater than 5 mm in shortest diameter suggest inflammatory activity for subjects with grade 2 or higher, with a high accuracy of diagnosis at a cutoff value of 2 for the numbers or 180 mm2 for the size indexes of lymph nodes.
Hepatitis B virus (HBV) is one of the most common causes of chronic hepatitis, and the infected individuals are at an increased risk of developing cirrhosis, liver failure, and hepatocellular carcinoma. Several effective medications are available to inhibit HBV replication with liver fibrosis regression by reducing liver inflammation and cellular damage in most patients with chronic hepatitis B (CHB). So, the assessment of liver necroinflammatory activity and fibrosis for patients with CHB is helpful for determining prognosis and treatment strategy.
CHB is frequently associated with hyperplasia of lymph nodes in the hepatic hilum, and these enlarged lymph nodes can be a good indicator for inflammatory activity of the liver in patients with CHB. Magnetic resonance imaging (MRI) can provide better contrast between the lymph nodes and adjacent tissue, especially T2-weighted imaging (T2WI). This has been reported for the magnetic resonance appearance of perihepatic lymph nodes in patients with chronic hepatitis C. However, there are no previous studies using MRI that reveal the significance of lymph nodes in the porta hepatis for patients with CHB.
Enlarged perihepatic lymph nodes in patients with CHB can be sensitively demonstrated by MRI, especially fat suppressed fast recovery fast spin echo T2WI. The number and size indexes of lymph nodes greater than 5 mm in shortest diameter suggest inflammatory activity for subjects with grade 2 or higher, with a high accuracy of diagnosis at a cutoff value of 2 for the numbers or 180 mm2 for the size indexes of lymph nodes.
The results support the suitability of MRI for assessment of liver inflammation in patients with CHB. Liver biopsy for assessment of inflammation can be reduced or displaced in patients with CHB. The study was performed directly on clinical subjects, and the findings can be readily applied to patient care
The authors attempt to validate the potential of using magnetic resonance fat suppressed T2WI in diagnosing inflammatory lymph nodes that lead to CHB. The study is a systematic and well thought out approach which, clearly shows the added advantages of magnetic resonance fat suppressed T2WI. Being a non-invasive tool for diagnosing the nodal inflammation, and the study was performed directly on clinical subjects; the findings can be readily applied to patient care.
P- Reviewers Darge K, Karmy-Jones R, Natarajan M S- Editor Huang XZ L- Editor Hughes D E- Editor Ma S
| 1. | Robotin MC. Hepatitis B prevention and control: Lessons from the East and the West. World J Hepatol. 2011;3:31-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Pradeep Kumar S, Medhi S, Asim M, Das BC, Gondal R, Kar P. Evaluation of adefovir & amp; lamivudine in chronic hepatitis B: correlation with HBV viral kinetic, hepatic-necro inflammation & amp; fibrosis. Indian J Med Res. 2011;133:50-56. [PubMed] [Cited in This Article: ] |
| 3. | Lin CL, Kao JH. Recent advances in the treatment of chronic hepatitis B. Expert Opin Pharmacother. 2011;12:2025-2040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Kim SU, Park JY, Kim do Y, Ahn SH, Choi EH, Seok JY, Lee JM, Park YN, Chon CY, Han KH. Non-invasive assessment of changes in liver fibrosis via liver stiffness measurement in patients with chronic hepatitis B: impact of antiviral treatment on fibrosis regression. Hepatol Int. 2010;4:673-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [PubMed] [Cited in This Article: ] |
| 6. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2125] [Cited by in F6Publishing: 2114] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 7. | Wright TL. Introduction to chronic hepatitis B infection. Am J Gastroenterol. 2006;101:S1-S6. [PubMed] [Cited in This Article: ] |
| 8. | Kuo HT, Lin CY, Chen JJ, Tsai SL. Enlarged lymph nodes in porta hepatis: sonographic sign of chronic hepatitis B and C infections. J Clin Ultrasound. 2006;34:211-216. [PubMed] [Cited in This Article: ] |
| 9. | Dietrich CF, Gottschalk R, Herrmann G, Caspary WF, Zeuzem S. Sonographic detection of lymph nodes in the hepatoduodenal ligament. Dtsch Med Wochenschr. 1997;122:1269-1274. [PubMed] [Cited in This Article: ] |
| 10. | Choi MS, Lee JH, Koh KC, Paik SW, Rhee PL, Kim JJ, Rhee JC, Choi KW, Kim SH. Clinical significance of enlarged perihepatic lymph nodes in chronic hepatitis B. J Clin Gastroenterol. 2001;32:329-332. [PubMed] [Cited in This Article: ] |
| 11. | Nakanishi S, Shiraki K, Sugimoto K, Tameda M, Yamamoto K, Masuda C, Iwata M, Koyama M. Clinical significance of ultrasonographic imaging of the common hepatic arterial lymph node (No. 8 LN) in chronic liver diseases. Mol Med Rep. 2010;3:679-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Dietrich CF, Stryjek-Kaminska D, Teuber G, Lee JH, Caspary WF, Zeuzem S. Perihepatic lymph nodes as a marker of antiviral response in patients with chronic hepatitis C infection. AJR Am J Roentgenol. 2000;174:699-704. [PubMed] [Cited in This Article: ] |
| 13. | Kim SY, Kim MJ, Chung JJ, Lee JT, Yoo HS. Abdominal tuberculous lymphadenopathy: MR imaging findings. Abdom Imaging. 2000;25:627-632. [PubMed] [Cited in This Article: ] |
| 14. | Zhang XM, Mitchell DG, Shi H, Holland GA, Parker L, Herrine SK, Pasqualin D, Rubin R. Chronic hepatitis C activity: correlation with lymphadenopathy on MR imaging. AJR Am J Roentgenol. 2002;179:417-422. [PubMed] [Cited in This Article: ] |
| 15. | Papakonstantinou O, Maris TG, Kostaridou S, Ladis V, Vasiliadou A, Gourtsoyiannis NC. Abdominal lymphadenopathy in beta-thalassemia: MRI features and correlation with liver iron overload and posttransfusion chronic hepatitis C. AJR Am J Roentgenol. 2005;185:219-224. [PubMed] [Cited in This Article: ] |
| 16. | Norton ID, Clain JE. The role of transabdominal ultrasonography, helical computed tomography, and magnetic resonance cholangiopancreatography in diagnosis and management of pancreatic disease. Curr Gastroenterol Rep. 2000;2:120-124. [PubMed] [Cited in This Article: ] |
| 17. | Mortele KJ, Ros PR. MR imaging in chronic hepatitis and cirrhosis. Semin Ultrasound CT MR. 2002;23:79-100. [PubMed] [Cited in This Article: ] |
| 18. | Semelka RC, Chung JJ, Hussain SM, Marcos HB, Woosley JT. Chronic hepatitis: correlation of early patchy and late linear enhancement patterns on gadolinium-enhanced MR images with histopathology initial experience. J Magn Reson Imaging. 2001;13:385-391. [PubMed] [Cited in This Article: ] |
| 19. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [PubMed] [Cited in This Article: ] |
| 20. | Hübscher SG. Histological grading and staging in chronic hepatitis: clinical applications and problems. J Hepatol. 1998;29:1015-1022. [PubMed] [Cited in This Article: ] |
| 21. | Mitchell DG, Navarro VJ, Herrine SK, Bergin D, Parker L, Frangos A, McCue P, Rubin R. Compensated hepatitis C: unenhanced MR imaging correlated with pathologic grading and staging. Abdom Imaging. 2008;33:58-64. [PubMed] [Cited in This Article: ] |
| 22. | Dietrich CF, Lee JH, Herrmann G, Teuber G, Roth WK, Caspary WF, Zeuzem S. Enlargement of perihepatic lymph nodes in relation to liver histology and viremia in patients with chronic hepatitis C. Hepatology. 1997;26:467-472. [PubMed] [Cited in This Article: ] |
| 23. | Soresi M, Bonfissuto G, Magliarisi C, Riili A, Terranova A, Di Giovanni G, Bascone F, Carroccio A, Tripi S, Montalto G. Ultrasound detection of abdominal lymph nodes in chronic liver diseases. A retrospective analysis. Clin Radiol. 2003;58:372-377. [PubMed] [Cited in This Article: ] |
| 24. | Cassani F, Valentini P, Cataleta M, Manotti P, Francesconi R, Giostra F, Ballardini G, Lenzi M, Zauli D, Bianchi FB. Ultrasound-detected abdominal lymphadenopathy in chronic hepatitis C: high frequency and relationship with viremia. J Hepatol. 1997;26:479-483. [PubMed] [Cited in This Article: ] |
| 25. | Dietrich CF, Zeuzem S. [Sonographic detection of perihepatic lymph nodes: technique and clinical value]. Z Gastroenterol. 1999;37:141-151. [PubMed] [Cited in This Article: ] |
| 26. | Akcam FZ, Tigli A, Kaya O, Ciris M, Vural H. Cytokine levels and histopathology in chronic hepatitis B and chronic hepatitis C. J Interferon Cytokine Res. 2012;32:570-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |