Published online May 28, 2012. doi: 10.4329/wjr.v4.i5.236
Revised: December 12, 2011
Accepted: December 19, 2011
Published online: May 28, 2012
Pulmonary tuberculosis is an opportunistic infection that can be reactivated in immunocompromised conditions, for example, in malignancy or after liver transplantation. Hepatocellular carcinoma (HCC) has a high mortality rate because it is frequently diagnosed at an advanced stage. Although surgical resection is the established curative measure for HCC, it is only feasible for early-stage HCC. Transcatheter arterial chemoembolization (TACE) is the most common treatment modality for patients with unresectable HCC. However, repeated TACE sessions and, occasionally, the tumor itself can further impair the reserve hepatic function and immunity. We report 3 recent cases of HCC with reactivation of pulmonary tuberculosis after TACE.
- Citation: Kim YJ, Goh PG, Moon HS, Lee ES, Kim SH, Lee BS, Lee HY. Reactivation of tuberculosis in hepatocellular carcinoma treated with transcatheter arterial chemoembolization: A report of 3 cases. World J Radiol 2012; 4(5): 236-240
- URL: https://www.wjgnet.com/1949-8470/full/v4/i5/236.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i5.236
Hepatocellular carcinoma (HCC) is a solid tumor associated with high mortality. HCC is usually diagnosed at an advanced stage; therefore, surgical resection is possible only in rare cases of early diagnosis[1-3]. Transcatheter arterial chemoembolization (TACE) is optimal for unresectable HCC patients, but many sessions of TACE result in an immunocompromised state due to deterioration of performance. Immunocompromised individuals who are infected with Mycobacterium tuberculosis (MTB) have an increased risk of tuberculosis (TB) reactivation. The possibility of HCC and coexisting TB is particularly high in countries with a high TB prevalence, such as South Korea[4,5]. We report 3 cases of HCC patients with TB reactivation after TACE.
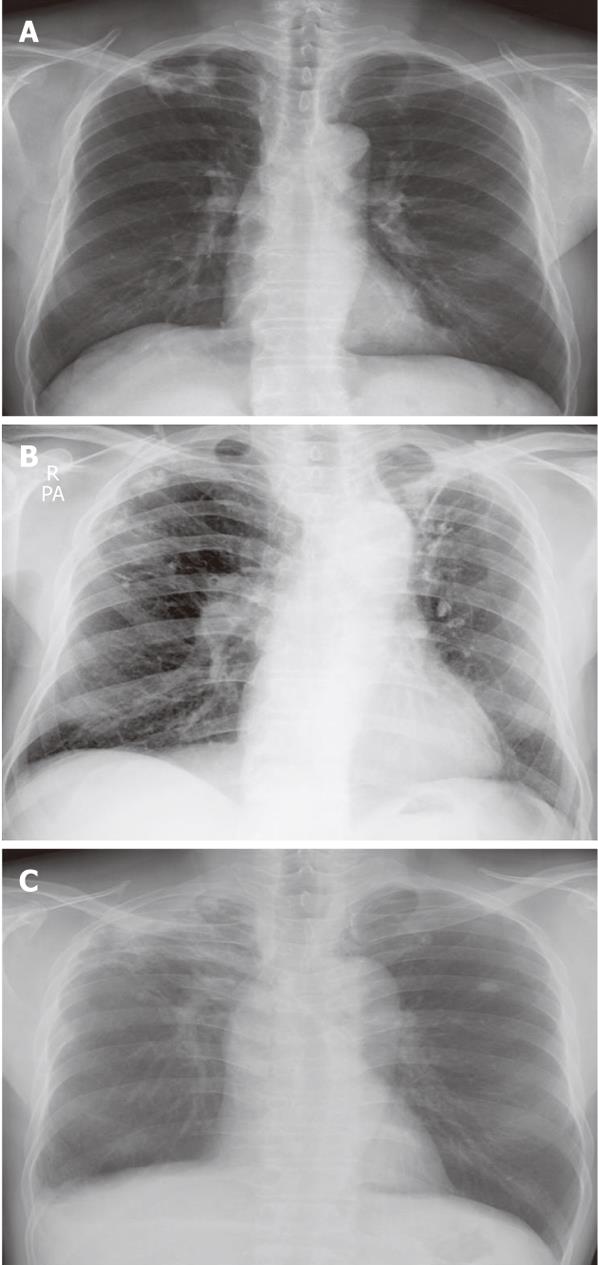
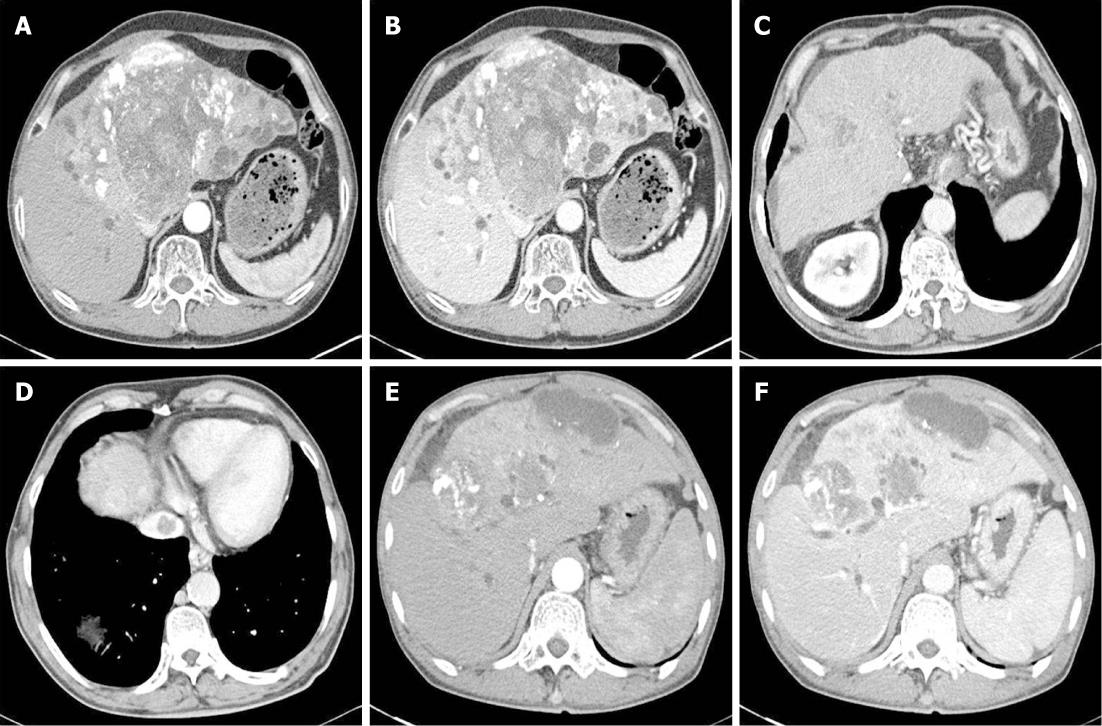
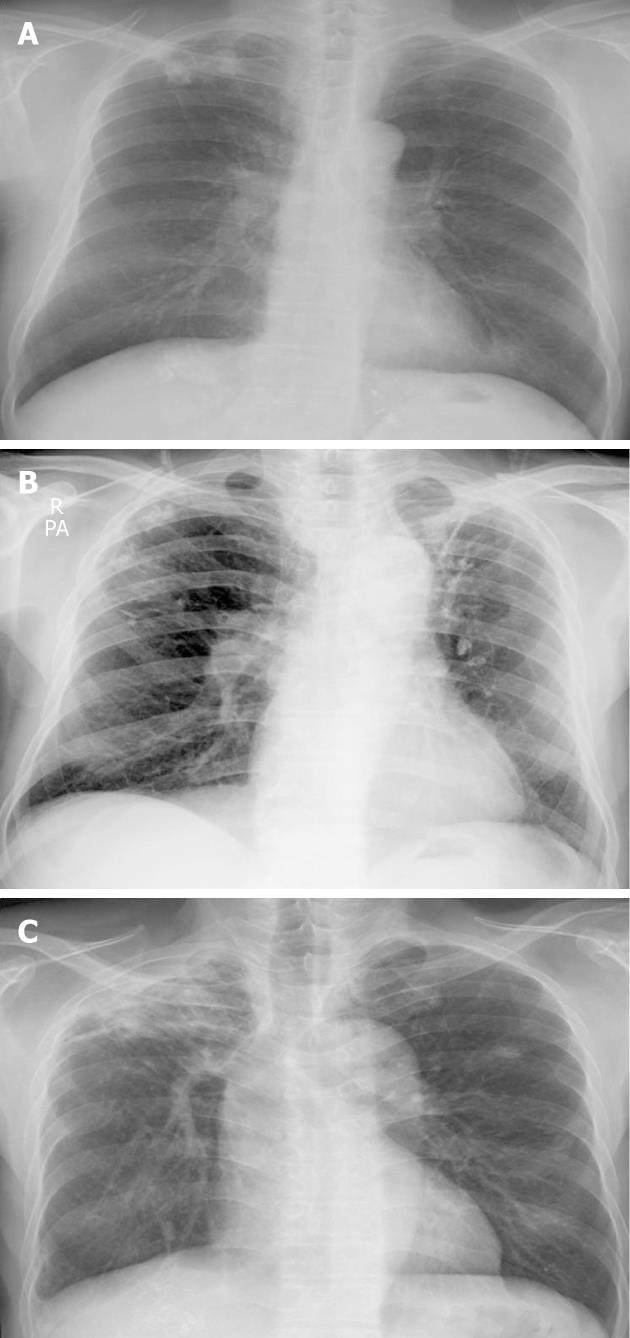
A 62-year-old man was referred to our hospital because of a hepatic mass detected in an abdominal computed tomography (CT) scan performed at a local clinic. The mass was large, measuring > 11 cm; it involved the S4, S5 and S8 hepatic segments and had invaded the bile duct. The α-fetoprotein (AFP) level was 16.7 (reference < 10 ng/mL). A diagnosis of HCC (stage IV) was concluded, and 5 cycles of chemotherapy were administered with TACE. We performed hepatic angiography and superselected a feeding artery of the HCC, and then performed embolization with 50 mg adriamycin and 9 mL lipiodol. Chest radiography on initial admission revealed a calcified granuloma suggestive of a former TB infection in the right upper lung field (Figure 1A). The patient was transferred to our Department of Hepatology because he complained of abdominal pain after the fifth TACE session. A general physical examination showed diffuse abdominal tenderness. Laboratory tests revealed the following results: leukocyte count, 7640 (3800 cells/mm3 < reference < 10 000 cells/mm3); hemoglobin level, 10.3 (13.5 g/dL < reference < 17.5 g/dL); platelet count, 226 000 (130 000 cells/mm3 < reference < 400 000 cells/mm3); aspartate aminotransferase (AST) level, 116 (reference < 37 IU/L); alanine transaminase (ALT) level, 101 (reference < 41 IU/L); alkaline phosphatase level, 444 (56 IU/L < reference < 119 IU/L); total bilirubin, 0.9 (reference < 1.2 mg/dL); albumin, 3.0 (4.0 g/dL < reference < 5.0 g/dL); and AFP, 32.4 ng/mL. He received supportive care for a few days. At the follow-up CT after the last TACE session, the interval of lipiodol uptake slightly increased in the large HCC located in the S2, S3, S4 and S5 segments; however, complete uptake was not noted (Figure 2A and B). The patient complained of cough with expectoration; therefore, chest radiography was performed. No interval changes were detected in the chest radiograph, but his sputum smear tested positive (1+) for acid-fast bacilli (AFB) (Figure 3A). Because his hepatic function was relatively stable, an anti-TB drug regimen comprising isoniazid (INH), ethambutol (EMB), and rifampicin (RFP) was prescribed. After 4 wk, this regimen was replaced with another regimen comprising EMB, cycloserine and moxifloxacin due to an elevation in his liver enzyme levels. However, after a few weeks his condition progressed to hepatic failure; therefore, the anti-TB treatment was discontinued. At present, the patient is receiving terminal care.
A 75-year-old man with liver cirrhosis due to regular alcohol consumption visited our Department of Gastroenterology; a hepatoma was detected in an abdominal CT scan. His AFP level was 0.1 (reference < 10 ng/mL). After the first TACE session, only 1 HCC (2.5 cm) in S5 exhibited complete lipiodol uptake, whereas no lipiodol uptake was detected in the HCC in S1. Finally, lobectomy of the caudate lobe and segmentectomy of S5 were performed; however, because the HCC recurred, 6 TACE sessions were conducted. At initial admission, a calcified granuloma suggestive of a former TB infection was detected in the right upper lung field on chest radiography (Figure 1B). After the sixth TACE session, he was readmitted to our Department of Gastroenterology with complaints of cough and mild fever. CT examination revealed a slight increase in the interval of lipiodol uptake, with no additional uptake in the small HCCs at S4 and S8 after a recent TACE session. However, a newly developed thrombosis was noted in the inferior vena cava (Figure 2C and D). A general physical examination showed normal findings. Laboratory tests revealed the following results: leukocyte count, 4670 (3800 cells/mm3 < reference < 10 000 cells/mm3); hemoglobin level, 10.7 (13.5 g/dL < reference < 17.5 g/dL); platelet count, 88 000 (130 000 cells/mm3 < reference < 400 000 cells/mm3); AST level, 85 (reference < 37 IU/L); ALT level, 54 (reference < 41 IU/L); alkaline phosphatase level, 93 (56 IU/L < reference < 119 IU/L); total bilirubin, 2.8 (reference < 1.2 mg/dL); albumin, 3.2 (4.0 g/dL < reference < 5.0 g/dL); and AFP, 402.3 ng/mL. No interval changes were noted in the subsequently obtained chest radiographs, and AFB staining of the sputum yielded negative results (Figure 3B). However, sputum culture and polymerase chain reaction (PCR) for MTB yielded positive results. He was therefore diagnosed with reactivated pulmonary tuberculosis and administered INH, EMB and RFP. However, the treatment regimen was changed after 4 mo due to progressive HCC and increased impairment of the residual hepatic function. The patient was then administered EMB only, but eventually, treatment with all anti-TB medications was discontinued because of progressive hepatic failure. The patient subsequently died while receiving supportive care.
An HCC was detected in a 55-year-old man during a regular follow-up for treatment of liver cirrhosis caused by chronic hepatitis B viral infection. He underwent TACE sessions at another hospital and was then transferred to our hospital for supportive care. CT examination revealed a large HCC with partial lipiodol uptake at S4, S5 and S8 along with dilatation of the intrahepatic duct (Figure 2E and F). A calcified granuloma suggestive of a former TB infection in the right upper lung field was detected on the initial chest radiograph (Figure 1C). The patient’s general condition was poor, and he complained of cough and fever. No interval changes were detected between the previous and present chest radiograph (Figure 3C). Laboratory tests revealed the following results: leukocyte count, 5670 (3800 cells/mm3 < reference < 10 000 cells/mm3); hemoglobin level, 9.6 (13.5 g/dL < reference < 17.5 g/dL); platelet count, 244 000 (130 000 cells/mm3 < reference < 400 000 cells/mm3); AST level, 260 (reference < 37 IU/L); ALT level, 49 (reference < 41 IU/L); alkaline phosphatase level, 650 (56 IU/L < reference < 119 IU/L); total bilirubin, 24.5 (reference < 1.2 mg/dL); and albumin 2.5 (4.0 g/dL < reference < 5.0 g/dL). AFB staining of the sputum yielded positive results (4+). Therefore, he was diagnosed with reactivated TB and was treated with a regimen comprising INH, EMB, and RFP. However, owing to progressive impairment of hepatic function, the treatment was replaced with EMB alone and eventually discontinued. He subsequently died of hepatic failure while receiving supportive care.
HCC is the most common form of primary liver cancer. This malignancy is associated with high mortality because it is often detected at an advanced stage. Therefore, it is the third most common cause of cancer-related deaths worldwide, and its incidence has been increasing in the United States[1]. Liver transplantation, surgical resection, and percutaneous ablation are the potential curative measures for HCC, but < 20% of HCC patients can opt for these measures due to factors such as old age, poor hepatic function, tumor extension and tumor localization[2-4]. Therefore, in clinical practice, TACE has been adopted as a palliative treatment for patients with unresectable HCC (advanced HCC with vascular invasion or extrahepatic spread), despite its debatable efficacy[5]. TACE-associated complications include signs or symptoms of fever, abdominal pain, elevated AST/ALT and serum bilirubin levels, cholecystitis, encephalopathy, pneumonia, and subphrenic abscess. Ruptured HCC following TACE is rarely reported[6]. The causes of death in patients undergoing TACE include hepatic failure, gastrointestinal bleeding, and tumor progression. Two of our patients died due to hepatic failure after multiple TACE sessions. However, no cases of TB reactivation following TACE have been reported.
TB infection caused by MTB is the most common opportunistic disease. TB is diagnosed by testing for MTB in culture or by AFB staining of the smears[7]. An immunocompromised state increases the risk of reactivation of TB. The tumor itself, chemotherapy for malignancy, or organ transplantation may reduce immunity. A higher incidence of mycobacterial infections has been reported in patients with malignancies as compared with the normal population. Screening for reactivation of TB is important because reactivated TB increases mortality associated with malignancy. A high incidence of TB has been reported in patients with neoplasms such as head and neck cancer, gastrointestinal cancer, lung cancer, and hematologic cancer[8,9]. However, TB reactivation in liver malignancy has not yet been reported. In addition, reactivation of TB after organ transplantation is an important clinical problem. This condition can often be life threatening for recipients of liver and kidney transplants[10,11]. However, diagnosis of reactivated TB may be delayed due to nonspecific symptoms. Cough and fever are common complaints among patients, as in the case of our 3 patients. The recommended treatment for TB comprises a 3- or 4-drug regimen, particularly in cases of increased drug resistance.
Several TACE sessions or the presence of a large HCC may result in an immunocompromised condition due to impaired reserve hepatic function and deteriorating general health. Therefore, TB infection may occur after TACE administration, particularly in HCC patients with a history of TB infection, as in the 3 cases reported above. However, the standard regimens could not be adopted in our 3 patients, and all anti-TB treatments were eventually discontinued in all 3 patients because of their preexisting liver disease. Therefore, we recommend that the possibility of TB coexisting with HCC be considered in patients with liver malignancies who are receiving chemotherapy or chemoembolization, particularly in countries with a high prevalence of TB, such as Korea. Treatment for TB can affect the survival of HCC patients with impaired hepatic function. Reactivation of TB should be considered in HCC patients who complain of cough and fever after TACE, even in the absence of interval changes on chest radiography. AFB staining, sputum culture, or PCR for MTB should be performed in these cases.
Peer reviewer: Charikleia Triantopoulou, MD, PhD, Head of Radiology Department, Konstantopouleion Hospital, 3-5, Agias Olgas street, Athens 14233 N. Ionia, Greece
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
| 1. | Cabrera R, Nelson DR. Review article: the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;31:461-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J Surg Oncol. 2010;101:476-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 3. | Biselli M, Andreone P, Gramenzi A, Trevisani F, Cursaro C, Rossi C, Ricca Rosellini S, Cammà C, Lorenzini S, Stefanini GF. Transcatheter arterial chemoembolization therapy for patients with hepatocellular carcinoma: a case-controlled study. Clin Gastroenterol Hepatol. 2005;3:918-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Belghiti J, Carr BI, Greig PD, Lencioni R, Poon RT. Treatment before liver transplantation for HCC. Ann Surg Oncol. 2008;15:993-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 383] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 6. | Battula N, Srinivasan P, Madanur M, Chava SP, Priest O, Rela M, Heaton N. Ruptured hepatocellular carcinoma following chemoembolization: a western experience. Hepatobiliary Pancreat Dis Int. 2007;6:49-51. [PubMed] [Cited in This Article: ] |
| 7. | API Consensus Expert Committee. API TB Consensus Guidelines 2006: Management of pulmonary tuberculosis, extra-pulmonary tuberculosis and tuberculosis in special situations. J Assoc Physicians India. 2006;54:219-234. [PubMed] [Cited in This Article: ] |
| 8. | Karnak D, Kayacan O, Beder S. Reactivation of pulmonary tuberculosis in malignancy. Tumori. 2002;88:251-254. [PubMed] [Cited in This Article: ] |
| 9. | Stefan DC, Kruis AL, Schaaf HS, Wessels G. Tuberculosis in oncology patients. Ann Trop Paediatr. 2008;28:111-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Chan AC, Lo CM, Ng KK, Chan SC, Fan ST. Implications for management of Mycobacterium tuberculosis infection in adult-to-adult live donor liver transplantation. Liver Int. 2007;27:81-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 11. | Chen CH, Lian JD, Cheng CH, Wu MJ, Lee WC, Shu KH. Mycobacterium tuberculosis infection following renal transplantation in Taiwan. Transpl Infect Dis. 2006;8:148-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |