Published online Nov 28, 2018. doi: 10.4329/wjr.v10.i11.150
Peer-review started: April 30, 2018
First decision: June 14, 2018
Revised: August 30, 2018
Accepted: October 9, 2018
Article in press: October 9, 2018
Published online: November 28, 2018
To assess potential benefits of an additional unenhanced acquisition in computed tomography angiography (CTA) in patients with suspected acute aortic syndrome (AAS).
A total of 103 aortic CTA (non-electrocardiography-gated, 128 slices) performed due to suspected AAS were retrospectively evaluated for acute aortic dissection (AAD), intramural hematoma (IMH), or penetrating aortic ulcer (PAU). Spiral CTA protocol consisted of an unenhanced acquisition and an arterial phase. If AAS was detected, a venous phase (delay, 90 s) was added. Images were evaluated for the presence and extent of AAD, IMH, PAU, and related complications. The diagnostic benefit of the unenhanced acquisition was evaluated concerning detection of IMH.
Fifty-six (30% women; mean age, 67 years; median, 68 years) of the screened individuals had AAD or IMH. A triphasic CT scan was conducted in 76.8% (n = 43). 56% of the detected AAD were classified as Stanford type A, 44% as Stanford type B. 53.8% of the detected IMH were classified as Stanford type A, 46.2% as Stanford type B. There was no significant difference in the involvement of the ascending aorta between AAD and IMH (P = 1.0) or in the average age between AAD and IMH (P = 0.548), between Stanford type A and Stanford type B in general (P = 0.650) and between Stanford type A and Stanford type B within the entities of AAD and IMH (AAD: P = 0.785; IMH: P = 0.146). Only the unenhanced acquisitions showed a significant density difference between the adjacent lumen and the IMH (P = 0.035). Subadventitial hematoma involving the pulmonary trunk was present in 5 patients (16%) with Stanford A AAD. The difference between the median radiation exposure of a triphasic (2737 mGy*cm) compared to a biphasic CT scan (2135 mGy*cm) was not significant (P = 0.135).
IMH is a common and difficult to detect entity of AAS. An additional unenhanced acquisition within an aortic CTA protocol facilitates the detection of IMH.
Core tip: A computed tomography protocol in patients with suspected acute aortic syndrome should routinely include an unenhanced acquisition due to the added value in the detection of intramural hematoma.
- Citation: Panagiotopoulos N, Drüschler F, Simon M, Vogt FM, Wolfrum S, Desch S, Richardt D, Barkhausen J, Hunold P. Significance of an additional unenhanced scan in computed tomography angiography of patients with suspected acute aortic syndrome. World J Radiol 2018; 10(11): 150-161
- URL: https://www.wjgnet.com/1949-8470/full/v10/i11/150.htm
- DOI: https://dx.doi.org/10.4329/wjr.v10.i11.150
Acute aortic syndrome (AAS) is a life threatening condition that subsumes acute aortic dissection (AAD), intramural hematoma (IMH), and penetrating aortic ulcer (PAU)[1]. In-hospital mortality can be as high as 68% within the first 2 d after admission[2]. Recent advances in medical imaging and treatment have further emphasized the importance of rapid and accurate assessment of suspected AAS. In this emergency setting, computed tomography angiography (CTA) is today’s benchmark modality[3]. Although detection of an IMH might profoundly influence therapeutic decision-making compared to classic AAD, it is often missed in CT scans of patients with AAS. Thus changes to the routinely performed biphasic CTA protocol with an arterial and a venous acquisition seem necessary. The aim of this study was therefore to assess the potential benefit of a CTA protocol that includes an additional unenhanced acquisition added to contrast-enhanced scans in the diagnostic pathway of patients with AAS. The focus was on the impact on the detectability of IMH in particular. Moreover, we aimed at working out the morphological characteristics of the different entities of AAS (i.e., AAD, IMH, and PAU) as well as the prevalence of related complications (e.g., aortic root subadventitial hematoma and branch vessel involvement).
This retrospective cohort study was performed after approval by the institutional review board. CTA examinations of patients with clinically suspected AAS, who had been referred by the emergency department for aortic CTA, were screened. In the surveyed period of 33 mo, 103 aortic CTA scans had been conducted for the indication of AAS in our radiological department.
Scans were acquired at a 128-slice CT scanner (Somatom Definition AS+, Siemens Healthcare, Erlangen, Germany). No electrocardiography (ECG)-gating was used. Iodinated contrast agent (IMERON 300, Bracco Imaging, Milan, Italy) was applied by a contrast media injector (ohio tandem, Ulrich medical, Ulm, Germany) injecting 100 mL at a flow of 5 mL/s over an antecubital vein via a 18G cannula. We used a potentially triphasic CT protocol consisting of first an unenhanced spiral acquisition of the entire aorta. Secondly, an arterial CTA of the entire aorta was performed. Bolus timing was done using “care bolus” technique with the region of interest (ROI) within the ascending aorta with a threshold of 120 Hounsfield units (HU). If AAS was detected in the arterial phase, a venous phase scan was performed with a delay of 90 s after injection start. The scanning volume of all acquisitions reached from the aortic arch down to the groin.
CT images were digitally reviewed in a Picture archiving and communication system (PACS; IMPAX EE, Agfa HealthCare GmbH, Bonn). To visually delineate and locate AAD, IMH, PAU, and related complications predefined morphological criteria were applied. AAD was identified by findings like an intimal flap and a double lumen in the contrast-enhanced scans[4,5]. If present, the extent of dissection was determined and the Stanford classification system[6] was used according to involvement of the ascending aorta. Moreover, the true lumen of an AAD was distinguished from the false lumen by characteristic features as outer wall calcifications, differences in the caliber of the lumina, the beak sign or the cobweb sign. An essential part of the assessment of the false lumen was to identify a possible partial or total thrombosis by measuring the radiodensity of both lumina in predefined slices in the following regions of the aorta: (1) ascending aorta at the level of the main pulmonary artery; (2) aortic arch; (3) descending aorta at the level of the main pulmonary artery; (4) abdominal aorta at the level of the superior mesenteric artery origin; and (5) abdominal aorta above the bifurcation.
In AAD, radiodensity of both lumina in all acquired phases was measured by placing and adjusting a ROI into the true and false lumen respectively. The radiodensity was recorded as the mean value for each region and phase in HU. In a second step, the discrepancy in density between the true and false lumen was calculated for each region and phase, separately.
Likewise, morphological criteria were applied to identify an IMH. These lesions feature a crescentic, high-attenuating (60-70 HU) region of thickening of the aortic wall on non-contrast CT. The lesions apparently exhibit no enhancement in relation to the aortic lumen on contrast-enhanced scans. An intimal flap is not present. Analogously to AAD, the Stanford classification system was used to classify IMH. Its radiodensity was measured and the discrepancy in density between the wall hematoma and the aortic lumen was calculated and processed for each region and phase separately. The extent or amount of complications related to AAD or IMH like dissections and occlusions of branch vessels, ischemia of abdominal organs, aneurysmal dilatation, aortic rupture, and aortic root subadventitial hematoma were studied in this work as well. The ten evaluated branch vessels were: (1) brachiocephalic trunk; (2) left common carotid artery; (3) left subclavian artery; (4) celiac trunk; (5) superior mesenteric artery; (6) inferior mesenteric artery; (7) renal arteries, left and right; and (8) common iliac arteries, left and right.
For every scan the radiation exposure was measured by the CT dose-length product (DLP; in mGy*cm) which was found in the corresponding DICOM-Header. Gender and age of the study patients was stored and processed.
All data were first collected on standardized and anonymized data collection sheets and later transferred into an Excel-file (Microsoft Corporation, Redmond, United States) and SPSS (IBM, Armonk, United States) for statistical analysis. Prior to the comparison of continuous variables in different groups, chi-square test was used to confirm or rule out normal distribution. Since all of the tested groups revealed non-Gaussian distribution, the non-parametric Mann-Whitney U test was used to evaluate differences. P < 0.05 was considered to indicate statistical significance.
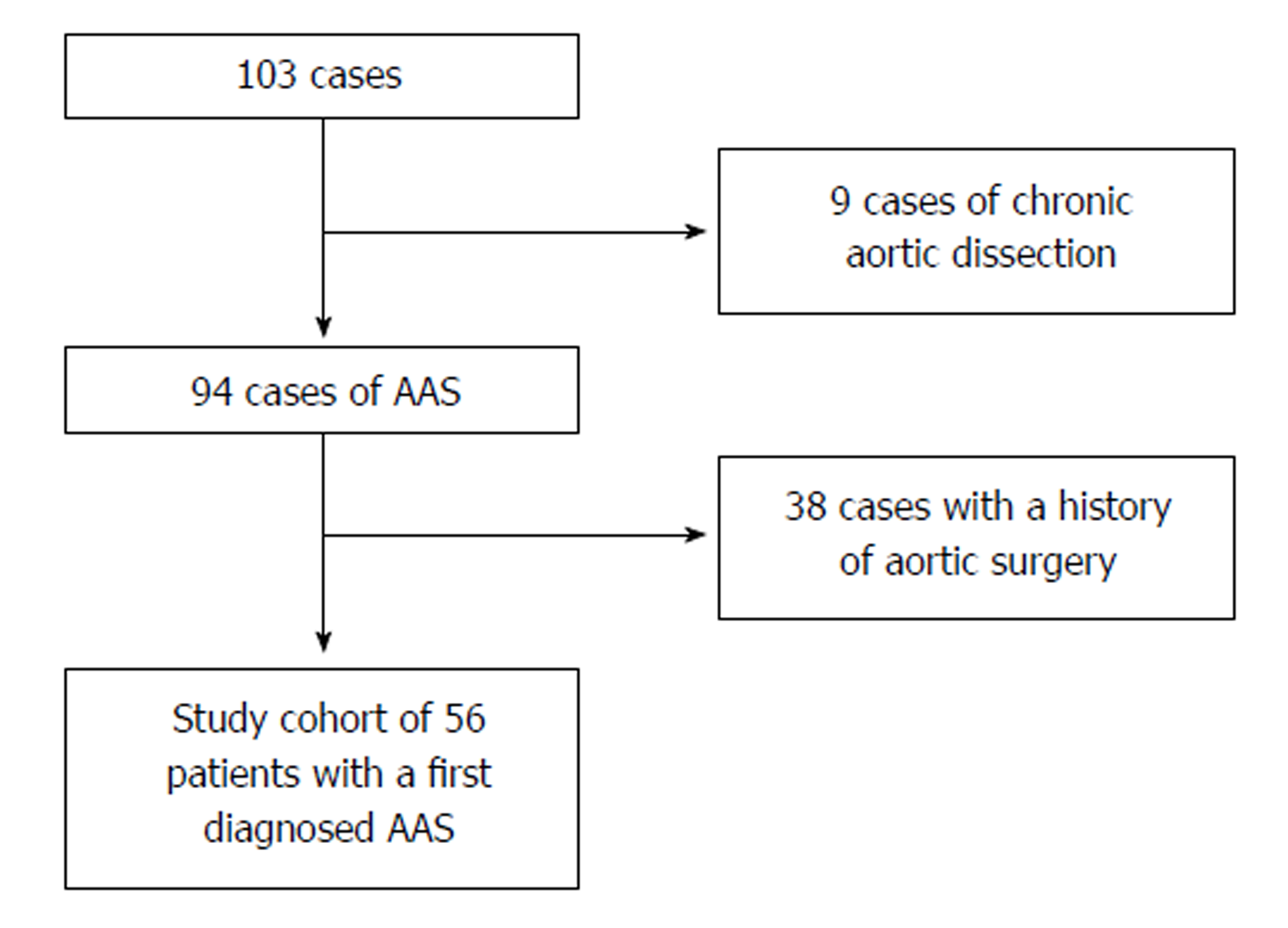
One-hundred-three patients had been diagnosed with aortic dissection or IMH. After the exclusion of those individuals who had chronic aortic dissection (n = 9) or a history of aortic surgery (n = 38) the resulting study cohort consisted of 56 individuals with first diagnosis of AAS (19 women; mean age 66.6 years, range 35-88 years, Figure 1 and Table 1). Among all patients diagnosed with AAS, 76.8% of patients were diagnosed with AAD compared to 23.2% with IMH. PAU was not found in our cohort. There was no significant percentage-difference of Stanford classification between AAD and IMH (P = 1). Age between AAD and IMH patients (P = 0.548) as well as between Stanford A and B (P = 0.650) patients did not differ significantly (Table 1).
| AAD | IMH | |||||
| n (%) | 43 (76.8) | 13 (23.2) | ||||
| mean age (yr) | 66 ± 12 | 69 ± 10 | ||||
| Stanford A | Stanford B | A vs B | Stanford A | Stanford B | A vs B | |
| 55.8% | 44.2% | 53.8% | 46.2% | |||
| mean age (yr) | 66 ± 11 | 67 ± 13 | P = 0.785 | 65 ± 10 | 75 ± 13 | P = 0.146 |
| Female | 41.7% | 10.5% | P = 0.039 | 28.6% | 50% | |
Radiation exposure as well as the share the unenhanced acquisition adds to the examination is depicted in Table 2. There was a higher mean radiation exposure of triphasic (2737.2 mGy*cm) compared to biphasic CT scans (2134.6 mGy*cm).
| DLP [mGy*cm] | |||
| Mean | Min | Max | |
| Triphasic CT scan (unenhanced, arterial and venous) | 2737.2 | 1583.0 | 4476.9 |
| Biphasic CT scan (arterial and venous) | 2134.6 | 766.2 | 3494.7 |
| Unenhanced acquisition (percentage of a triphasic CT scan) | 602.6 (22.0%) | ||
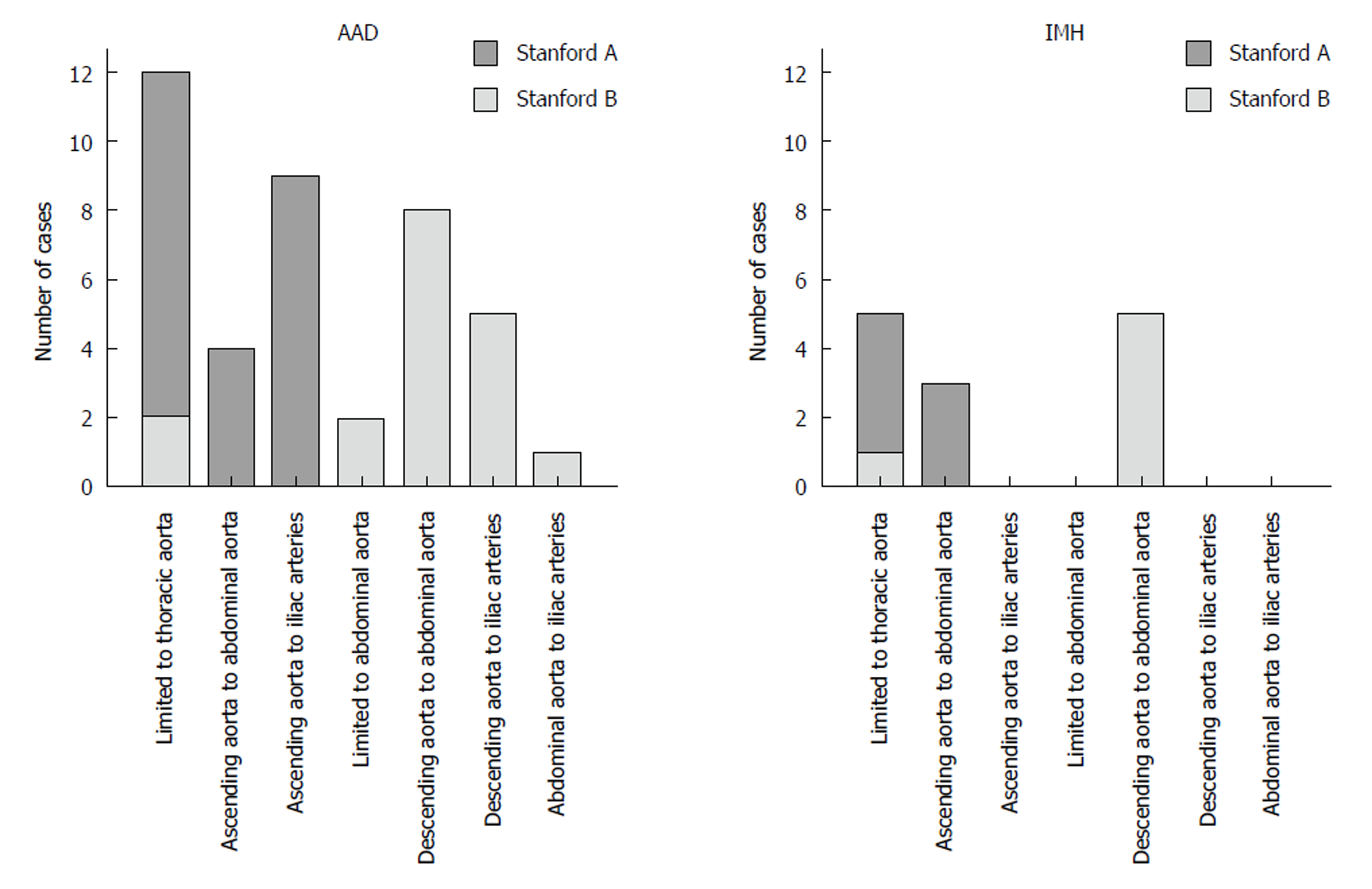
Extent and boundaries: Two data sets were excluded as the distal boundary of the AAD/IMH was not within the scan volume. In 31.5%, AAD and IMH were limited to the thoracic aorta. In contrast to IMH, which never involved the iliac arteries, AAD extended into the iliac arteries in 36.6% (Figure 2). Anatomic features such as kinking of the aorta, aortic bifurcation or aneurysm detained a distal progression of AAD and IMH in 41.5% and 53.8%, respectively (P = 0.528, Figure 3).
True and false lumen: In Stanford A AAD, in 95.8% the false lumen was located in the dextrolateral quadrant of the ascending aorta. Stanford A IMH was most frequently found in the sinistrolateral quadrant (57.1%). In the aortic arch, the false lumen and IMH mainly involved the anterior, caudal and posterior quadrant, less frequently they involved the ostia of the supraaortic branches. In Stanford A AAD, the false lumen of the descending thoracic and suprarenal abdominal aorta was predominantly present in the sinistrolateral and dorsal quadrant. In Stanford B AAD, there was only a slight predominance for the dorsal and dextrolateral quadrant. At the level of the renal arteries the false lumen was more often found on the right than the left side (right: Stanford A: 73.9%; Stanford B: 52.6%; left: A: 30.4%; B: 42.1%). In the infrarenal portion of the aorta, there was no significant difference in the position of the false lumen between Stanford A and B (dextroposterior). In the descending thoracic and abdominal aorta, IMH showed no predominance of location.
Involvement of branch vessels: Six data sets were excluded as an assessment of all ten predefined branch vessels was not possible. Branch vessel involvement was only 38.5% in IMH compared to 75.7% in AAD (P = 0.012). In 46% at least two branch vessels and in 20% at least four branch vessels were involved. In 34% there was no branch vessel and in one case (2%) seven branch vessels affected. In AAD there were a total of 225 branch vessels involved (A: 137; B: 88). 131 vessels originated from the true lumen [A: 69 (50.4%); B: 62 (70.5%)], 34 vessels from the false lumen [A: 27 (19.7%); B: 7 (8.0%)], and 60 vessels were supplied by both lumina [A: 41 (29.9%); B: 19 (21.6%)]. Branches that were supplied by the false lumen were patent in 23 cases [A: 19 (70.4%); B: 4 (57.1%)], partly thrombosed in 7 cases [A: 6 (22.2%); B: 1 (14.3%)] and occluded in 4 cases [A: 2 (7.4%); B: 2 (28.6%)]. Branches that were supplied by both the true and the false lumen were patent in 45 cases [A: 30 (73.2%); B: 15 (78.9%)], partly thrombosed in 6 cases [A: 5 (12.2%); B: 1 (5.3%)] and occluded in 9 cases [A: 6 (14.6%); B: 3 (15.8%)]. The brachiocephalic trunk was the vessel most often involved (57.2%). In Stanford A dissections involvement of the coeliac trunk and the superior mesenteric artery was more commonly seen than in Stanford B dissections (A: 38.5%, B: 26.7%; A: 41.7%, B: 13.3%; respectively). By tendency, renal artery involvement was more often present at the left side (A: 63.7%, B: 35.7%) compared to the right side (A: 36.4%, B: 28.6%), (P = 0.424).
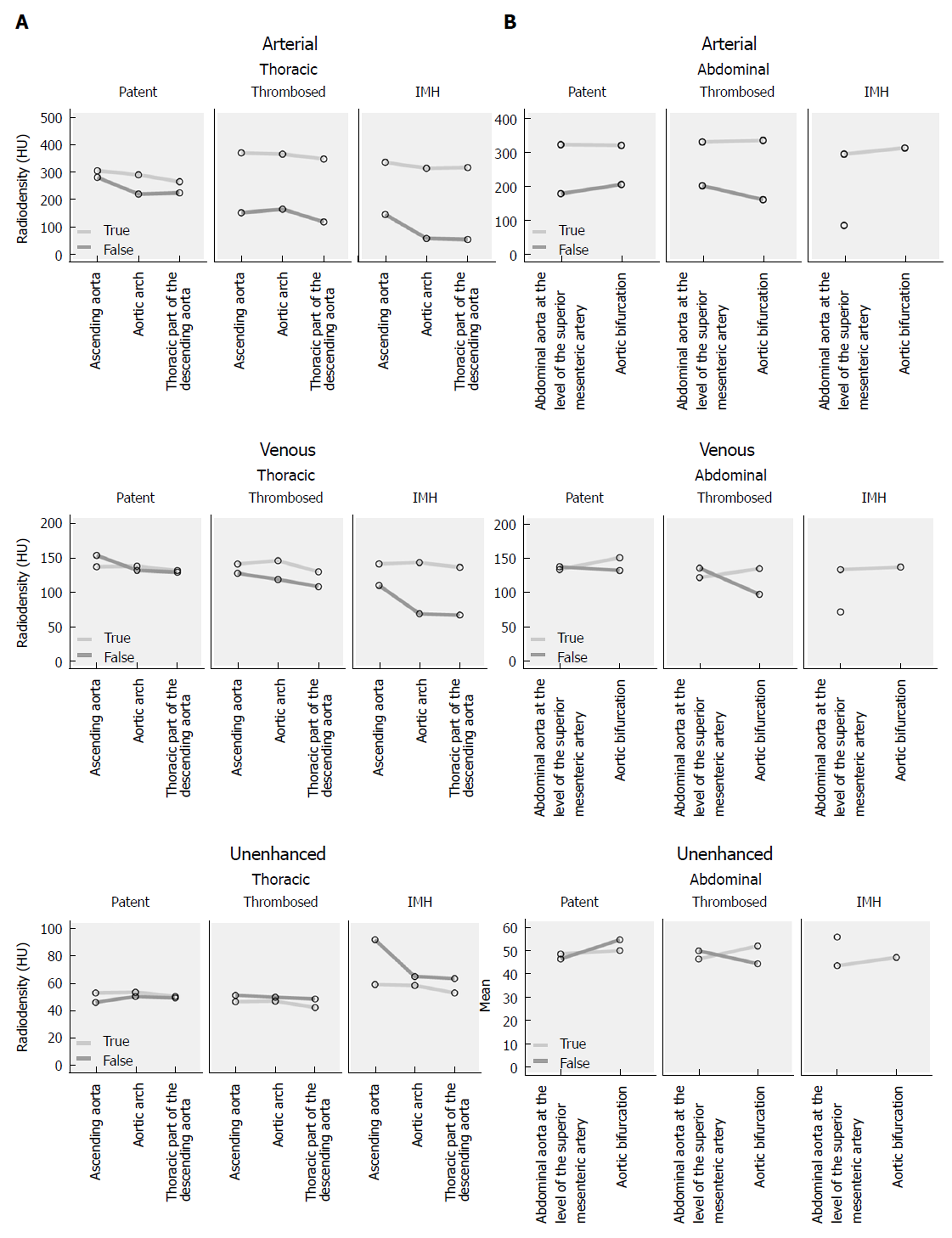
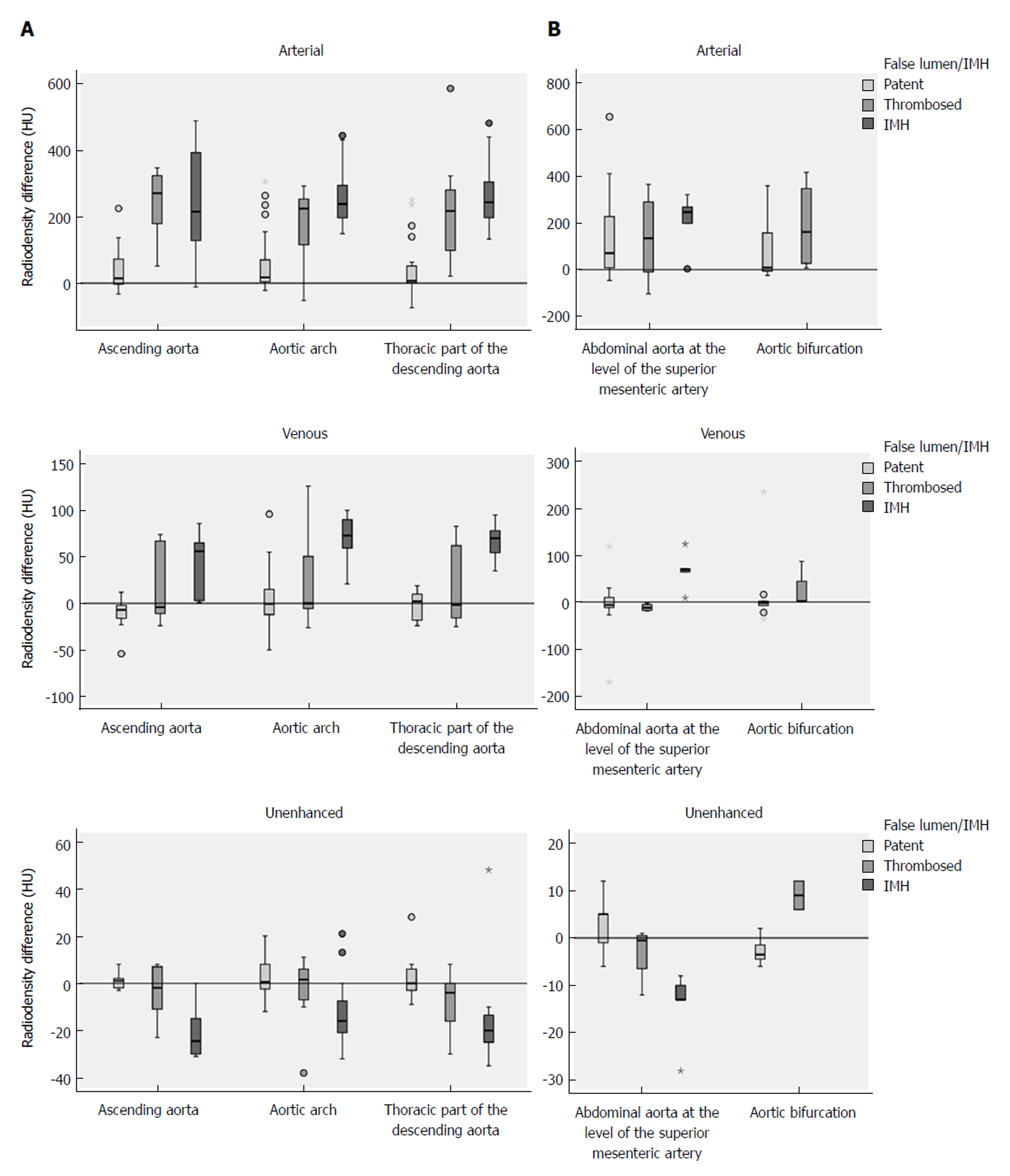
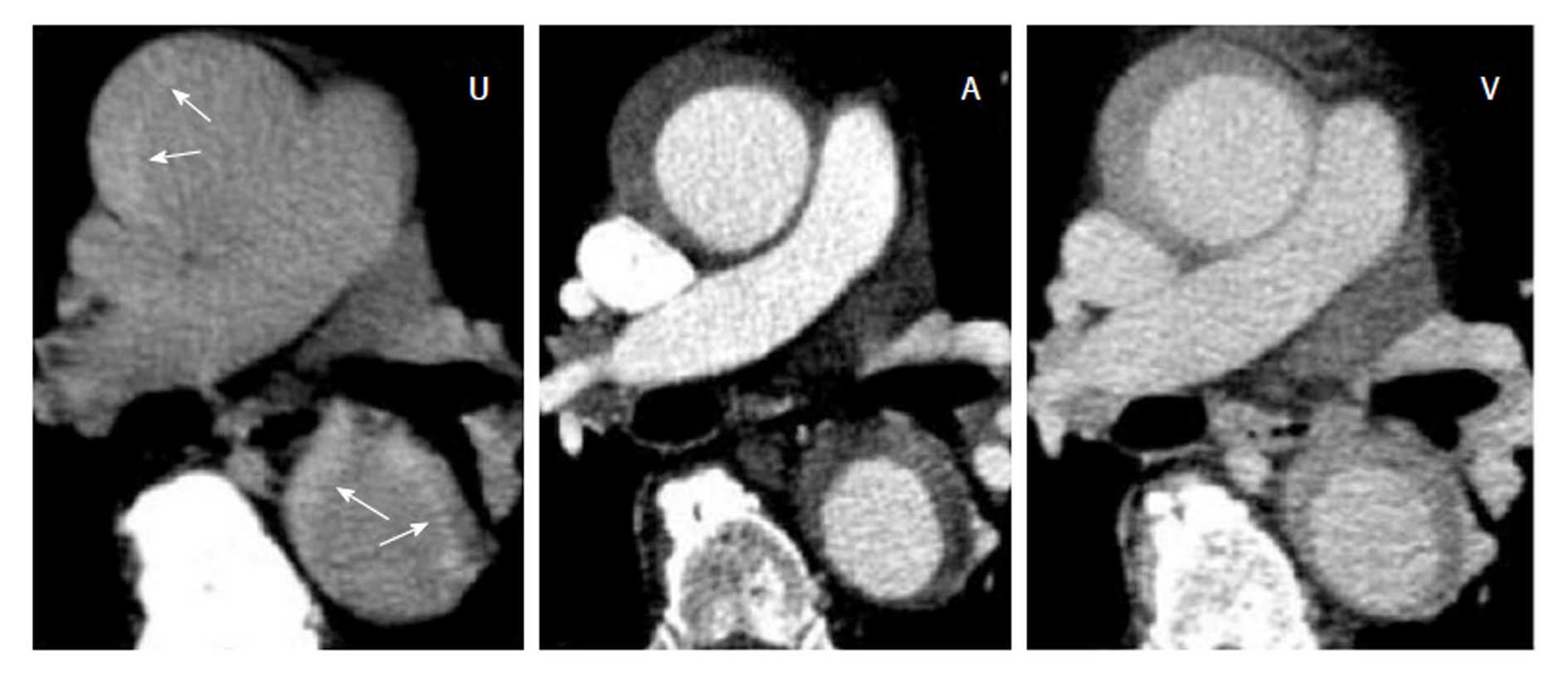
In the arterial phase the radiodensity of the patent false lumen of the thoracic aorta was comparable to that of the true lumen, while in the abdominal aorta the radiodensity in the patent false lumen was lower (on average more than 100 HU difference) than in the true lumen (Figure 4 and Figure 5). In the unenhanced and venous acquisitions 241 the radiodensity of the patent false lumen was comparable to that of the true lumen. A false lumen thrombosis resulted in higher radiodensity in the false lumen for the unenhanced acquisitions and lower densities in the arterial and venous phases. IMH had a lower radiodensity compared to the vessel lumen within the arterial and venous phases. In the unenhanced acquisition the radiodensity of the IMH was higher than the adjacent lumen in any of the regions of the aorta in which it occurred (Figure 6). Differences in radiodensity between true lumen and IMH as well as true lumen and thrombosed false lumen, respectively, were only significant in the unenhanced acquisition (P = 0.035).
Table 3 summarizes complications and secondary findings. Subadventitial hematoma involving the pulmonary trunk was present exclusively in patients with Stanford A lesions. It was found in 5 patients, i.e. in 16.1% of Stanford A lesions. In these patients pericardial effusion was present in 80%. In two cases there was pulmonary hemorrhage present. The subadventitial hematoma was hypodense in contrast enhanced scans compared to the lumen of the vessel (average HU differences arterial: 436 HU, venous 109 HU) while in non-contrast scans it was hyperdense (average HU difference -13 HU).
| Total | AAD | IMH | |||
| Stanford A | Stanford B | Stanford A | Stanford B | ||
| Subadventitial hematoma of pulmonary trunk | 5 (9.1) | 2 (8.3) | 0 | 3 (42.9) | 0 |
| Pericardial effusion | 20 (35.7) | 11 (45.8) | 4 (21.1) | 5 (71.4) | 0 |
| Pleural effusion | 14 (25.0) | 4 (16.7) | 6 (31.6) | 2 (28.6) | 2 (33.3) |
| Abdominal aortic aneurysm (AAA) | 17 (32.1) | 2 (8.7) | 12 (66.7) | 0 | 3 (50.0) |
| Bleeding/rupture | 4 (7.1) | 0 | 3 (15.8) | 0 | 1 (16.7) |
| Branch vessel involvement | 33 (66.0) | 17 (81.0) | 12 (70.6) | 3 (50.0) | 1 (16.7) |
| Ischemia | 10 (18.2) | 5 (20.8) | 4 (22.2) | 1 (14.3) | 0 |
| Kidney | 7 (12.5) | 4 (16.7) | 3 (15.8) | 0 | 0 |
| Liver | 2 (3.6) | 1 (4.2) | 1 (5.3) | 0 | 0 |
| Spleen | 3 (5.5) | 0 | 2 (11.1) | 1 (14.3) | 0 |
| Intestine | 1 (1.8) | 0 | 1 (5.3) | 0 | 0 |
Pericardial effusion was significantly more frequent in Stanford A lesions (P = 0.011). There was no significant difference in the occurrence of pleural effusion/hemothorax between Stanford A and B (P = 0.357). Abdominal aortic aneurysm was more frequent in Stanford B dissections (66.7%). Bleeding or rupture occurred in four Stanford B cases. Although less frequent in IMH, branch vessel involvement was the most frequent complication with 66% in total. Ischemia of abdominal organs was found in 18.2% with kidneys being the most often affected (12.5%).
When contemplating the assumption that a multiphasic CTA protocol adding a non-enhanced acquisition significantly improves the detectability of AAD and IMH there is the dimension of fast and accurate visualization and that of radiation protection that has to be evaluated. In this respect, this study shows that the radiodensity of the true lumen and the false lumen of AAD do not differ significantly in contrast-enhanced scans. On the contrary, unenhanced acquisitions featured a high-attenuating region of thickening of the aortic wall with a significant density difference between the IMH and the adjacent lumen in the range of 5 HU up to 21 HU for all examined regions of the aorta. A differentiation between IMH and a thrombosed false lumen was possible due to the significantly higher contrast between IMH and adjacent lumen compared to thrombotic material and adjacent lumen (P = 0.035). While the mere figures might seem rather small, the reason for the resulting critical improvement in the visualisation of the potentially life-threatening entity IMH can be found in the way windowing is possible if no contrast agent is used. By lowering the window level and narrowing the window width, the interpreting physician is able to zero in on those aspects that are of diagnostic value. While the arterial phase is essential for the delineation of pathognomonic findings like an intimal flap and a double lumen[7] and the venous acquisitions facilitate the assessment of the patency of the false lumen as well as a possible ischemia of the abdominal organs, adding an unenhanced acquisition of the thoracic aorta should be used whenever an AAS is suspected. In this study we were able to identify 13 IMH and 5 aortic root subadventitial hematomas and to distinguish both these entities from a false lumen thrombosis. This would not have been possible without the data of the unenhanced scan. The diagnosis of an IMH might profoundly influence the therapeutic decision-making compared to classic aortic dissection. First of all, IMH has a higher rate of rupture as compared to AAD[8]. Research groups around the world have argued about the right treatment of IMH. According to Tsai et al[9], similar to Stanford type A and B aortic dissection, surgery is advocated in patients with Stanford type A IMH and initial medical therapy in patients with type B IMH. On the contrary, a study conducted by Song et al[10] showed acceptable outcomes of a policy of urgent surgery for unstable Stanford type A IMH patients and initial medical treatment for stable patients with surgery for complications. In 2014 the European Society of Cardiology issued a guideline[11]. In accordance with this guideline, “emergency surgery of IMH is indicated in complicated cases with pericardial effusion (in this study present in type A: 71.4%, B: 0%), periaortic hematoma (in this study present in: type A: 42.9%, B: 0.0%), or large aneurysms [in this study present in (AAA): type A: 0.0%, B: 50.0%]. Surgery within 24 h after diagnosis is required in most of Stanford type A IMH. In elderly patients or those with significant comorbidities, initial medical treatment may be a reasonable option. Medical treatment is the initial approach to Stanford type B IMH. Endovascular therapy or surgery has the same indications as type B aortic dissection. While there might still be debate about the treatment that is clinically indicated if an IMH is detected, the importance of a rapid and accurate detection and assessment of potentially life-threatening complications is out of doubt. Nonetheless the ALARA principle (“as low as reasonably achievable”) dictates a limitation of the patients’ radiation exposure. Our data indicate that adding an unenhanced acquisition to a biphasic CT scan does, as expected, increase the radiation exposure, but to a moderate degree of only 22% to the total radiation dose. Therefore the risk of overlooking an IMH or aortic root subadventitial hematomata seems to be grossly disproportionate to the benefit of a reduction in radiation exposure. In our opinion the added radiation dose of a triphasic protocol is reasonable and can easily be justified. The data of this study substantiate the common perception that an IMH is a more circumscript thoracic pathology that rarely affects the abdominal aorta. This finding allowed us to further adjust our CTA protocol and operating procedures in a way that reduces the patients’ radiation exposure while maintaining most of the benefits the unenhanced acquisition offers. In cases in which there is no clinical suspicion of an abdominal involvement we now limit the unenhanced scan to the thoracic aorta, lowering the radiation exposure.
The 56 cases diagnosed with AAS would translate into an incidence of about 8.9/100000 a year if the population of Lübeck[12] is regarded as the hinterland of our department of radiology and nuclear medicine. This figure is considerably higher than the 2.9/100000/year incidence found in literature[2].
Studies based on the International Registry of Acute Aortic Dissections have found about 6.3% of AAS to be IMH[13]. In our cohort the prevalence was as high as 23.2%. This discrepancy partly originates from the study design and the selection process of the study cohort which excluded about 46% of patients with AAS because of a chronic aortic dissection or a history of aortic surgery. As many IMH evolve into an AAD there might be a selection bias due to the small study cohort as well. However, one might also suggest that IMH has often been overseen if the utilized CTA protocol misses a focus on detection of even small IMH. Therefore, adding an unenhanced scan to the CTA is suggested.
Although the screened cohort of 868 individuals was sizable, the resulting study cohort on which our data is based (n = 56) is not. The small size of this retrospective cohort study limits the generalizability of the results. A further limitation of this work can be found in the definition of branch vessel involvement. A branch vessel involvement was defined as an intimal flap or a hematoma that extended into the branch or alternatively the sole perfusion by false lumen. This definition leaves out any type of dynamic obstruction. Nonetheless, we believe that this study offers some motivational thoughts and descriptions of clinical relevance.
The life-threatening condition of an intramural hematoma (IMH) is often missed on routinely performed contrast enhanced computed tomography (CT) angiographies (CTA) in patients with suspected acute aortic syndrome (AAS).
To optimize the CT protocol for AAS.
To assess the potential benefit of a CTA protocol that includes an additional unenhanced acquisition added to contrast-enhanced scans in the diagnostic pathway of patients with AAS.
Aortic CTA of patients with suspected AAS were retrospectively evaluated for acute aortic dissection, IMH, or penetrating aortic ulcer. The spiral CTA protocol consisted of an unenhanced acquisition and an arterial phase. If AAS was detected, a venous phase (delay, 90 s) was added. Images were evaluated for the presence and extent of aortic pathologies, and related complications.
23% of patients with AAS had an IMH. There was no significant difference in the involvement of the ascending aorta or the average age between dissection and IMH. Only the unenhanced acquisitions showed a significant density difference between the adjacent lumen and the IMH. Subadventitial hematoma involving the pulmonary trunk was present in five patients.
IMH is a common and difficult to detect entity of AAS. An additional unenhanced acquisition within an aortic CTA protocol facilitates the detection of IMH.
The results underline the importance of a triphasic CTA as standard diagnostic procedure in patients with suspected AAS.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abdelghany M, Benson RA, Okutucu S S- Editor: Wang JL L- Editor: A E- Editor: Huang Y
| 1. | Hallinan JT, Anil G. Multi-detector computed tomography in the diagnosis and management of acute aortic syndromes. World J Radiol. 2014;6:355-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, Szép L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271-1278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 660] [Cited by in F6Publishing: 636] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 3. | Achenbach S, Barkhausen J, Beer M, Beerbaum P, Dill T, Eichhorn J, Fratz S, Gutberlet M, Hoffmann M, Huber A. [Consensus recommendations of the German Radiology Society (DRG), the German Cardiac Society (DGK) and the German Society for Pediatric Cardiology (DGPK) on the use of cardiac imaging with computed tomography and magnetic resonance imaging]. Rofo. 2012;184:345-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Chao CP, Walker TG, Kalva SP. Natural history and CT appearances of aortic intramural hematoma. Radiographics. 2009;29:791-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | McMahon MA, Squirrell CA. Multidetector CT of Aortic Dissection: A Pictorial Review. Radiographics. 2010;30:445-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 786] [Cited by in F6Publishing: 665] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Castañer E, Andreu M, Gallardo X, Mata JM, Cabezuelo MA, Pallardó Y. CT in nontraumatic acute thoracic aortic disease: typical and atypical features and complications. Radiographics. 2003;23 Spec No:S93-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Coady MA, Rizzo JA, Elefteriades JA. Pathologic variants of thoracic aortic dissections. Penetrating atherosclerotic ulcers and intramural hematomas. Cardiol Clin. 1999;17:637-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 211] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation. 2005;112:3802-3813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 330] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 10. | Song JK, Yim JH, Ahn JM, Kim DH, Kang JW, Lee TY, Song JM, Choo SJ, Kang DH, Chung CH. Outcomes of patients with acute type a aortic intramural hematoma. Circulation. 2009;120:2046-2052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2374] [Cited by in F6Publishing: 2764] [Article Influence: 276.4] [Reference Citation Analysis (0)] |
| 12. | Statistikamt Nord. Bevölkerung der Gemeinden in Schleswig-Holstein. Available from: http://www.statistik-nord.de/daten/bevoelkerung-und gebiet/bevoelkerungsstand-und entwicklung/dokumentenansicht/163/produkte-1. [Cited in This Article: ] |
| 13. | Harris KM, Braverman AC, Eagle KA, Woznicki EM, Pyeritz RE, Myrmel T, Peterson MD, Voehringer M, Fattori R, Januzzi JL. Acute aortic intramural hematoma: an analysis from the International Registry of Acute Aortic Dissection. Circulation. 2012;126:S91-S96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |