Published online Apr 26, 2017. doi: 10.4330/wjc.v9.i4.339
Peer-review started: October 17, 2016
First decision: December 15, 2016
Revised: December 29, 2016
Accepted: January 16, 2017
Article in press: January 18, 2017
Published online: April 26, 2017
To investigate the survival benefit of bilateral internal mammary artery (BIMA) grafts in patients with left ventricular dysfunction.
Between 1996 and 2009, we performed elective, isolated, primary, multiple cardiac arterial bypass grafting in 430 consecutive patients with left ventricular ejection fraction ≤ 40%. The early and long-term results were compared between 167 patients undergoing BIMA grafting and 263 patients using left internal mammary artery (LIMA)-saphenous venous grafting (SVG).
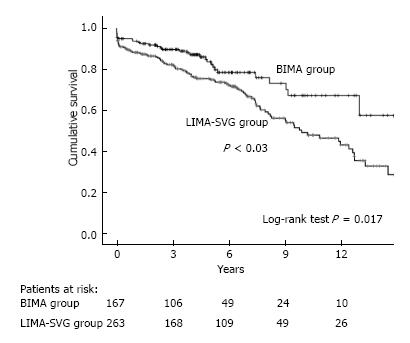
The mean age of the overall population was 60.1 ± 15 years. In-hospital mortality was not different between the two groups (7.8% vs 10.3%, P = 0.49). Early postoperative morbidity included myocardial infarction (4.2% vs 3.8%, P = 0.80), stroke (1.2% vs 3.8%, P = 0.14), and mediastinitis (5.3% vs 2.3%, P = 0.11). At 8-year follow-up, Kaplan-Meier-estimated survival (74.2% vs 58.9%, P = 0.02) and Kaplan-Meier-estimated event-free survival (all cause deaths, myocardial infarction, stroke, target vessel revascularization, heart failure) (61.7% and 41.1%, P < 0.01) were significantly higher in the BIMA group compared with the LIMA-SVG group in univariate analysis. The propensity score matching analysis confirmed that BIMA grafting is a safe revascularization procedure but there was no long term survival (P = 0.40) and event-free survival (P = 0.13) in comparison with LIMA-SVG use.
Our longitudinal analysis suggests that BIMA grafting can be performed with acceptable perioperative mortality in patients with left ventricular dysfunction.
Core tip: This study reports a daily practice observation of patients with multivessel coronary artery disease and left ventricular (LV) dysfunction undergoing surgical revascularization. We evaluate the periprocedural safety of bilateral internal mammary artery grafting (BIMA) in this high risk population and its long-term survival benefit compared with the left internal mammary artery grafting (LIMA) to left anterior descending artery with additional saphenous venous grafting (SVG). Our longitudinal analysis suggests that BIMA grafting can be performed with acceptable perioperative mortality in patients with LV dysfunction but there was no survival difference in our follow up in comparison with LIMA-SVG use.
- Citation: Popovic B, Maureira P, Juilliere Y, Danchin N, Voilliot D, Vanhuyse F, Villemot JP. Bilateral vs unilateral internal mammary revascularization in patients with left ventricular dysfunction. World J Cardiol 2017; 9(4): 339-346
- URL: https://www.wjgnet.com/1949-8462/full/v9/i4/339.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i4.339
Coronary artery disease is an important contributor to the rise in the prevalence of heart failure and its associated morbidity and mortality[1,2]. Severe left ventricular (LV) dysfunction caused by extensive coronary artery disease usually carries a poor prognosis, although surgical revascularization is thought to be the most effective treatment strategy[2-4]. Myocardial revascularization in patients with severe LV dysfunction preserves the remaining myocardium, prevents further myocardial damage, and induces the recovery of systolic function in ischemic LV myocardial segments. However, although advances in surgical techniques and myocardial protection have improved outcomes, cardiac arterial bypass grafting (CABG) in patients with LV impairment is still associated with high perioperative risk and the long-term survival remains unsatisfactory overall.
The use of a single internal mammary artery rather than vein graft to the left anterior descending coronary artery has become the standard operation, largely due to excellent long-term graft patency into the first and second postoperative decade[5]. The superior outcome associated with left internal mammary artery (LIMA) grafting has quickly encouraged the use of bilateral internal mammary artery (BIMA)[6,7]. However, the widespread adoption of BIMA grafting is hindered because it might be associated with increased early morbidity.
We report here our experience in patients with multivessel coronary artery disease and LV dysfunction undergoing surgical revascularization. We evaluate the periprocedural safety of BIMA grafting in this high risk population and its long-term survival benefit compared with the conventional standard-of-care CABG using the LIMA to left anterior descending artery with additional saphenous venous grafting (SVG).
From April 1996 to December 2009, 4210 patients underwent isolated CABG at our university center. From this group, we identified 430 patients with left ventricular ejection fraction (LVEF) of ≤ 40% who underwent primary isolated multivessel CABG with a least 2 grafts. Among them, 167 procedures were performed using BIMA grafting ± SVG and 263 procedures were performed with LIMA and SVG. Exclusion criteria were patients older than 80 years, surgical myocardial revascularization of only one coronary artery, concomitant repair/replacement of valve, cardiac rupture, ventricular aneurysm or ascending aortic aneurysm.
Coronary artery disease was defined as a reduction of the vessel diameter by ≥ 70% in 1 view on coronary angiography. The presence of stenosis ≥ 70% in the left anterior descending, circumflex, or right coronary system was used as the criterion for single, double, or triple-vessel disease.
The preoperative measurement of LV chamber size at end-diastole and end-systole as well as the assessment of mitral regurgitation were performed by transthoracic two-dimensional echocardiographic images in the parasternal long-axis view (including M-mode) and by apical four-chamber view. LVEF was measured using Simpson’s method with two views. LV chamber dilatation was defined by LV diastolic diameter > 54 mm and > 60 mm in women and men respectively[8]. Mitral regurgitation was quantified according to the prevailing guidelines at the time of the study and was taken into account as reported by the expert cardiologist who performed the examination. Before myocardial revascularization, the heart team, including cardiologists and surgeons, systematically identified the target vessels according to myocardial viability. Revascularization was considered complete if every significant target vessel was grafted.
Our selection of patients for LIMA-SVG and BIMA grafting was not random but was influenced by the heart team decision and was decided from the in situ graft size. CABG was performed using standard on-pump or off-pump bypass techniques at the discretion of the operating surgeon. Myocardial preservation during cardiopulmonary bypass involves normothermic, intermittent, anterograde and retrograde blood cardioplegia.
The LIMA was harvested as a pedicle and grafted exclusively to the left coronary system. The right internal mammary artery (RIMA) was mostly harvested as a pedicle and grafted to the left coronary system or the right coronary artery, and in a minority of cases, used as a free graft. A free RIMA was used when the length of the conduit was too short, or if the distal anastomotic site was unreachable with a pedicled RIMA. The composite graft included an end-to-side anastomosis of the free RIMA onto an in situ LIMA. The right gastroepiploic artery or radial artery was not used as a third arterial conduit in our study.
The in-hospital course was studied in terms of procedural characteristics, vital status, renal status, peri- and post-operative red blood cell transfusions, infectious complications, myocardial infarction, cerebrovascular events, mesenteric ischemia, emergency repeat coronary and/or peripheral revascularization procedures and arrhythmias.
Patients did not undergo systematic control angiography during the follow-up period. Perioperative myocardial infarction (i.e., within 7 d after intervention) was defined as a creatine kinase-MB ≥ 5 times the upper limit of normal, with new Q waves in 2 contiguous leads on the postoperative electrocardiogram or the new development of bundle branch block. In the case of elevated creatine kinase levels at baseline, myocardial infarction was defined as an increase of > 50% over baseline values after intervention. After this postoperative period, myocardial infarction was defined as the presence of new pathologic Q waves or the new development of left bundle branch block with increased cardiac marker levels (i.e., creatine kinase-MB ≥ 3 times the upper limit of normal). Cardiovascular death included death resulting from an acute myocardial infarction, sudden cardiac death, hospitalization for heart failure, stroke, pulmonary embolism or digestive ischemia.
Event free survival is a composite end point of all cause deaths, myocardial infarction, stroke, target vessel revascularization and first hospitalization for heart failure.
Follow up was obtained through comprehensive questionnaires and by telephone with the patient’s personal physician. If subsequent hospitalization, death, or other events had occurred, the patient’s physician or appropriate hospital record department were interviewed to document the events.
This study was conducted according the principles of the Helsinki declaration. The retrospective study of patients’ files was approved by the Commission Nationale Informatique et Libertés (CNIL), in keeping with French law for single-center usual care observational studies.
Continuous variables are presented as means ± standard deviation (SD), categorial variables as frequencies (percentages). Comparison of patients characteristics between groups were carried out using Student’s t tests, Wilcoxon tests or Pearson’s χ2 tests as required. Kaplan-Meier survival curves were compared using the log-rank test.
The associations between mortality and age, sex, risk factors, previous history, clinical presentation and LV ejection fraction were performed using Cox regression models. Assumptions of log-linearity, absence of interaction between surgical strategy and adjustment covariate mentioned above, absence of collinearity and proportionality of hazards were thoroughly verified. Cox proportionality assumption was verified using interaction between time and each covariate. A propensity score was developed to control the selection bias potentially related to surgery strategy. Propensity scores were constructed using logistic model with surgery strategy as predicted event and independent covariates which were selected a priori on the basis of their known or suspected association with both surgery strategies and mortality and cardiovascular events. A value of P < 0.05 was used to determine the statistical significance of all tests.
The patients’ baseline and operative characteristics are presented in Tables 1 and 2.
| Characteristics [n (%)] | Unmatched groups | Propensity score-matched groups | ||||
| BIMA (n = 167) | LIMA-SVG (n = 263) | P | BIMA (n = 130) | LIMA-SVG (n = 130) | P | |
| Age (yr) | 58.5 ± 10.3 | 64.3 ± 9.0 | 0.01 | 60.6 ± 9.9 | 60.5 ± 10.0 | 0.95 |
| Female sex | 20 (12.0) | 30 (11.5) | 0.88 | 16 (12.3) | 19 (14.6) | 0.71 |
| Hypertension | 87 (52.4) | 163 (61.5) | 0.07 | 70 (53.8) | 73 (56.2) | 0.80 |
| Diabetes mellitus | 66 (39.5) | 97 (36.9) | 0.61 | 53 (40.8) | 54 (41.5) | 0.90 |
| Hypercholesterolemia1 | 99 (59.6) | 183 (69.6) | 0.04 | 79 (60.8) | 82 (63.1) | 0.79 |
| History of smoking | 116 (69.5) | 173 (65.8) | 0.53 | 84 (64.6) | 83 (63.8) | 0.89 |
| Body mass index (kg/m2) | 27.6 ± 4.4 | 27.4 ± 4.3 | 0.85 | 27.6 ± 4.5 | 27.3 ± 4.3 | 0.56 |
| Other comorbid conditions | 16 (9.6) | 31 (11.8) | 0.53 | 12 (9.2) | 9 (6.9) | 0.65 |
| Chronic renal insufficiency COPD2 | 28 (16.8) | 53 (20.5) | 0.38 | 24 (18.5) | 23 (17.7) | 0.87 |
| Peripheral artery disease | 55 (33.1) | 111 (42.2) | 0.07 | 44 (33.8) | 45 (34.6) | 0.89 |
| Prior ischemic cardiopathy | 83 (49.7) | 154 (58.5) | 0.11 | 68 (52.3) | 67 (51.5) | 0.90 |
| Previous PCI | 48 (28.7) | 70 (26.6) | 0.66 | 36 (28.5) | 38 (29.2) | 0.89 |
| EuroSCORE logistic3 (%) | 7.5 ± 6.9 | 8.0 ± 6.0 | 0.21 | 7.5 ± 7.1 | 8.0 ± 6.1 | 0.90 |
| Symptoms | ||||||
| Stable angina pectoris | 50 (30.1) | 70 (26.6) | 0.90 | 36 (28.5) | 36 (27.7) | 0.90 |
| Acute coronary syndrome | 71 (42.5) | 117 (44.5) | 0.86 | 58 (44.6) | 55 (42.3) | 0.90 |
| ambulatory Q wave MI | 22 (13.2) | 40 (15.2) | 0.95 | 18 (13.8) | 21 (16.2) | 0.85 |
| silent ischemia | 33 (13.8) | 36 (13.7) | 0.98 | 17 (13.1) | 18 (13.8) | 0.90 |
| NYHA class 3-4 | 47 (28.1) | 112 (42.8) | 0.03 | 45 (34.6) | 46 (35.4) | 0.99 |
| Angiographic parameters: Three coronary arteries narrowed (≥ 70%) | 112 (67.1) | 165 (62.7) | 0.35 | 83 (63.8) | 85 (65.4) | 0.90 |
| Mitral insufficiency grade ≥ 2 | 24 (14.6) | 46 (18.0) | 0.19 | 22 (16.9) | 24 (18.5) | 0.80 |
| Left ventricular chamber dilatation | 55 (32.9) | 100 (38.0) | 0.29 | 45 (34.6) | 47 (36.1) | 0.42 |
| Ejection fraction (%) | 35.2 ± 5.9 | 33.5 ± 5.8 | 0.58 | 34.5 ± 6.0 | 34.6 ± 5.6 | 0.80 |
| Characteristics [n (%)] | BIMA group (n = 167) | LIMA-SVG group (n = 263) | P |
| On-pump surgery | 164 (98.8) | 245 (93.1) | 0.80 |
| Mean number of grafts per patient | 2.5 ± 0.6 | 2.5 ± 0.7 | 0.91 |
| Complete coronary revascularization | 117 (70.1) | 175 (66.5) | 0.50 |
| Extracorporeal circulation time (min) Median (IQR) | 79.5 (65.2-100) | 80.0 (64-100) | 0.86 |
| Length of stay in ICU (d) Median (IQR) | 3.1 (2-4) | 3.2 (2-5) | 0.11 |
| Length of stay in hospital (d) Median (IQR) | 8.2 (7-11) | 9.3 (6-12) | 0.12 |
| Ventilation time < 24 h | 160 (95.8) | 210 (79.9) | < 0.01 |
| IABP | 12 (7.2) | 24 (9.1) | 0.80 |
| Vasopressors or inotropic drugs (> 24 h) | 45 (27.3) | 107 (41.1) | < 0.01 |
| Transfusion (red blood cell units ≥ 3) | 53 (31.7) | 123 (46.7) | < 0.01 |
From April 1996 to December 2009, we identified 430 patients with LVEF ≤ 0.4 who underwent isolated CABG including patients with LVEF ≤ 30% in 34% of cases.
Patients who received LIMA-SVG grafting were significantly older than BIMA group (64 ± 9 years vs 58.5 ± 10 years, P = 0.01), with more frequent dyslipidemia (59.6% vs 69.6%, P = 0.04) and trend for a more frequent peripheral artery disease (42.2% vs 33.1%, P = 0.07).
Patients who received BIMA grafting were significantly younger than LIMA-SVG (58.5 ± 10 years vs 64 ± 9 years, P = 0.01) with less frequent dyslipidemia (59.6% vs 69.6%, P = 0.04). Although acute coronary syndrome and ambulatory myocardial infarction were the most common clinical presentations in both groups, NYHA class 3 and 4 in the preoperative period was more frequent in group 2 (P = 0.03).
The analysis of echocardiographic parameters showed that mean LVEF, LV chamber dilatation and the presence of mitral insufficiency ≥ 2 were not significantly different between two groups.
The number of grafts per patient was similar in both groups, most surgeries were performed on-pump, and complete revascularization was obtained in 70% of group 1 compared with 66.5% of group 2 (P = 0.50).
In group 1, the LIMA was harvested almost exclusively as a pedicle and grafted onto the left coronary system. The LIMA was grafted onto the circumflex as a free graft in 4 patients. A sequential anastomosis technique for the left anterior descending artery and diagonal branch was used in 30 patients. The RIMA graft was anastomosed to the left coronary system in 141 (82%) patients and the right coronary artery in 31 (18%) patients. The RIMA was harvested as a pedicle in most cases and was grafted onto the circumflex artery as a free graft or on an in situ LIMA in 39 patients (23% of cases).
The older age of patients and worse initial clinical status in LIMA group influenced the post-operative period with longer ventilation time, higher vasopressor use and transfusion.
The overall in-hospital mortality rate in our study was 9.5%, and cardiovascular mortality represented 75% of deaths. The analysis of in-hospital course showed that all-cause mortality (7.8% vs 10.3%, P = 0.49) and early postoperative morbidity including myocardial infarction (4.2% vs 3.8%, P = 0.80), stroke (1.2% vs 3.8%, P = 0.14), and digestive ischemia (1.8% vs 1.9%, P = 0.94) were not significantly different between two groups (Table 3).
| Characteristics [n (%)] | BIMA group (n = 167) | LIMA-SVG group (n = 263) | P |
| All-cause mortality | 13 (7.8) | 27 (10.3) | 0.49 |
| Cardiovascular mortality | 8 (4.8) | 23 (8.7) | 0.10 |
| Myocardial infarction | 7 (4.2) | 10 (3.8) | 0.80 |
| Redo CABG | 2 (1.2) | 2 (0.8) | 0.64 |
| Stroke | 2 (1.2) | 10 (3.8) | 0.14 |
| Mesenteric ischemia | 3 (1.8) | 5 (1.9) | 0.94 |
| Mediastinitis | 9 (5.3) | 6 (2.3) | 0.11 |
| Cardiac rehabilitation | 79 (47.3) | 104 (39.5) | 0.40 |
| 1-yr follow up LVEF | 44.2% ± 10.1% | 41.3% ± 10.2% | 0.81 |
Mediastinitis requiring sternal re-opening and antibiotics occurred in 15 patients without significant difference between two groups group (5.3% vs 2.3%, P = 0.11). Among patients who experienced mediastinitis, 8 (53.3%) patients were diabetic and 7 (43%) were obese.
The long-term follow-up (mean follow up: 6.2 ± 3.8 years; median follow up: 5.6 years) revealed 135 deaths, including 75 cardiovascular deaths.
The evidence-based medical postoperative treatment didn’t significantly differ between LIMA-SVG group and BIMA group: Statin therapy (88.3% vs 94.7%, P = 0.4), beta blockers (77.1% vs 82.0%, P = 0.4), angiotensin-converting enzyme inhibitor (84.3% vs 88.6%, P = 0.6) and aspirin (90.5% vs 94.7%, P = 0.7). During the follow up, 18 patients received an implantable cardiac defibrillator without significant difference between two groups.
At 1-year follow-up, LVEF value was obtained in 93.0% of cases, and mean LVEF in the overall population was 43.1% ± 6.2%. In comparison with the preoperative LVEF estimation, 1-year LVEF was improved in 72.5% of cases (in LIMA-SVG and BIMA groups: 71.3% and 74.9%, respectively, P = 0.32) and unchanged or worsened in 27.5% of cases. A LVEF of > 50% was achieved in 49.1% of cases.
Figure 1 show Kaplan-Meier estimated overall survival in unmatched cohorts. The Kaplan-Meier 8-year estimated overall survival for patients in the BIMA group compared with patients in the LIMA-SVG group were 74.2% and 58.9%, respectively (P = 0.02) but this significant difference became insignificant after multivariate adjustment hazard ratio (HR) [95% confidence interval (CI)] 1.02 (0.68-1.56), P = 0.8.
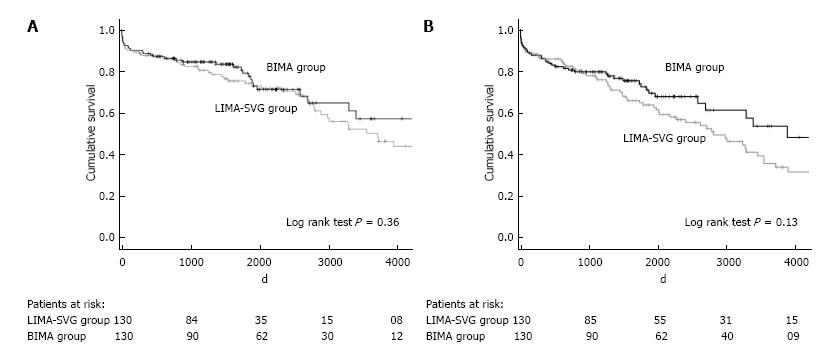
We performed 1:1 propensity score matching to select patients receiving BIMA or LIMA SVG group with comparable preoperative characteristics (Figure 2).
The propensity score matching analysis revealed that BIMA grafting is a safe revascularization procedure without on long term survival (P = 0.40) or event free survival (P = 0.13) difference in comparison with LIMA-SVG use.
We present a large, single-center observational study of surgical coronary revascularization in patients with reduced LVEF, which represent a sizeable proportion of all procedures performed at our hospital during this period (11% of cases).
Our analysis of the overall postoperative course is consistent with large contemporary reports on the topic, which indicate an in-hospital mortality ranging from about 4% to greater than 20% according to the co-morbid conditions and the degree of LV dysfunction[4,9,10].
One of the most interesting points of our study is to confirm that BIMA grafting does not modify the postoperative mortality in patients with LV impairment compared to LIMA-SVG grafting. Furthermore, although BIMA grafting appears to be the technically more challenging of the two techniques, the duration of surgical procedures did not differ between the two groups. This similarity may explain the relatively good postoperative outcome. The safety of BIMA grafting was also demonstrated by relatively low rates of periprocedural stroke, myocardial infarction and mesenteric ischemia, with no significant differences from the control group.
Sternal deep wound infection is a major complication after cardiac surgery with high in-hospital mortality (30% in our study). This complication concerned 3.5% of our overall population without significant difference between both surgical revascularization strategies. Among patients who experienced mediastinitis in our report, diabetes and obese patients represent well known high-risk subgroups (54% and 43% respectively)[11,12]. There is now good evidence in the contemporary literature that the use of both internal mammary arteries doesn’t significantly increase the rate of sternal deep wound complication, especially with the skeletonized technique[13,14]. Moreover, high-risk subgroups as diabetic patients have the most to gain from this technique thereby preserving collaterals ad sternal blood supply[14,15]. Galbut et al[16] also confirms that BIMA grafting doesn’t significantly increase the rate of sternal wound infection whatever the preoperative LV function.
Late survival, although reduced when compared with patients with no decrease in LVEF, remains relatively high with estimated 5- and 8-year overall survival rates of 77% and 64%, respectively, in our study. This prognosis is largely influenced by the reduction of ischemia and the preservation of the remaining myocardium leading to the improvement of postoperative LVEF. In our study, we noted that LVEF was improved by surgical revascularization in 72.5% of cases at 1 year follow-up, and LVEF was > 50% in 49.1% of cases. This benefit on LVEF is especially shown in patients with ischemic but viable myocardium who subsequently underwent revascularization[17,18]. However, the lack of interaction between myocardial viability status and benefit from CABG in the Stich trial indicates that assessment of myocardial viability alone should not be the deciding factor in selecting the best therapy for these patients[8]. Other structural predictive parameters such as LV volumes and ischemic mitral regurgitation should be taken into account for intervention planning.
Long-term survival after CABG is presumed to be directly correlated with late patency of the selected conduits and grafts. The internal mammary artery upregulates eNOS and therefore has higher concentration of NO than other conduits, which not only explain its improved patency but also likely has a significant impact on vascular endothelium and resistance to atherosclerosis in the grafted coronaries or in the coronaries that were not bypassed[19].
However, the benefit of arterial conduits seems to concern internal mammary artery conduits in a majority of cases. Ruttmann et al[20] demonstrated the superiority of the internal mammary artery graft over the radial artery as a second graft, and the benefit of all-arterial revascularization using additional radial artery is still debated.
Several large studies recently added to the growing literature that confirms the superiority of BIMA grafting over LIMA use, particularly in terms of improved long-term mortality with no significant increase in peri-operative complications[7,13,21]. Recently, Locker et al[7] showed that multi-artery grafting compared with LIMA-SVG improved long-term survival in almost all co-morbid conditions, including impaired LV function.
In our analysis, the difference between the BIMA group and the LIMA-SVG group concerning long-term survival and event-free survival was significant in a univariate analysis. The older age of patients in LIMA-SVG group and the difference in baseline characteristics between the two groups may explain in part these results. However, the propensity score matching analysis revealed that BIMA grafting is a safe revascularization procedure but no significant difference on long term survival in comparison with LIMA-SVG was noted. These results reflect possibly the small size of two groups after propensity score matching analysis and therefore a type II error. It also suggests that the difference between these surgical strategies remains small in patients with low to moderate LEVF (≤ 40%) despite a long term follow up.
Galbut et al[16] recently confirmed also that the benefit of BIMA grafting on long term survival concerned especially patients with normal LV function and moderate LV dysfunction (LVEF from 30% to 50%). However, this revascularization strategy should be considered with more caution in patients with low LV dysfunction.
It seems that the advantage of BIMA grafting compared with LIMA-SVG grafting in patients with reduced ejection fraction increases over time and the real benefit depends on survival probability determinated by age and comorbidities. Comorbid factors, especially postoperative atrial fibrillation also increase the risk of long-term mortality and should be given important consideration when evaluating the benefits of the surgical revascularization strategy[22]. Therefore, further stratified analyses with larger number of patients and a follow up of one or two decades should be encouraged to identify the exact benefit from BIMA grafting in this situation.
Our study was a non-randomised, retrospective, and observational study with inherent bias.
There was a definite patient selection bias, demonstrated by a younger population in BIMA group that led to overestimate the benefits of this procedure in univariate analyses. The cut-off value of ejection fraction ≤ 40% has also influenced the results of our study. Further stratified analysis with larger number of patients should identify the exact benefit of BIMA grafting according to different level of LV dysfunction. Other variables not included in the multivariate models, however, may also influence the results of surgical revascularization as the quality of coronary vessels requiring bypass grafting and the location of both grafts. Likewise, we cannot exclude that the degree of viability or extent of fibrosis/necrosis might have led to the preferred choice of one or the other operative technique.
Medications including beta blockers, statins or ACE inhibitors at the time of discharge but also LV remodelling[23] and mitral regurgitation[24] might have affected the long term results. However, multiple adjustments using many factors could not be performed because of limited number of events.
Patients with severe coronary artery disease and markedly reduced LVEF represent a high-risk group that can undergo CABG safely. Our longitudinal analysis suggests that BIMA grafting can be performed with acceptable perioperative mortality in patients with LV dysfunction but there was no survival difference in our follow up in comparison with LIMA-SVG use. Further studies, assessing the long-term impact hybrid approaches using BIMA and elective PCI using drug eluting stents will determine whether these new approaches constitute a true improvement in the field of myocardial revascularization[25].
Patients with severe coronary artery disease and markedly reduced left ventricular ejection fraction represent a high-risk group that can undergo cardiac arterial bypass grafting (CABG) safely. The excellent outcome associated with left internal mammary artery (LIMA) grafting has quickly encouraged the use of bilateral internal mammary artery (BIMA). However, the widespread adoption of BIMA grafting is hindered because it might be associated with increased early morbidity. The aim of this study was to review the authors’ institutional experience over 13 years with 430 patients with left ventricular (LV) dysfunction who underwent surgical revascularization.
To our knowledge, this is the largest single center observational report on patients with LV dysfunction who underwent myocardial surgical revascularization.
This longitudinal analysis demonstrates that BIMA grafting can be performed with acceptable perioperative mortality in patients with LV dysfunction but there was no survival difference in our follow up in comparison with LIMA-SVG use.
Appropriate patient selection.
BIMA grafting (bilateral internal mammary artery grafting) and LIMA-SVG (left internal mammary artery with saphenous venous) grafting: Two surgical strategies used to revascularize heart with impaired function.
The manuscript is well written and addresses interesting and important facts regarding the revascularisation in left ventricular dysfunction.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Barili F, Ranstam J, Skobel E, Teragawa H S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787-1847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3411] [Cited by in F6Publishing: 3448] [Article Influence: 287.3] [Reference Citation Analysis (0)] |
| 2. | Gheorghiade M, Sopko G, De Luca L, Velazquez EJ, Parker JD, Binkley PF, Sadowski Z, Golba KS, Prior DL, Rouleau JL. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114:1202-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 264] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Alderman EL, Fisher LD, Litwin P, Kaiser GC, Myers WO, Maynard C, Levine F, Schloss M. Results of coronary artery surgery in patients with poor left ventricular function (CASS). Circulation. 1983;68:785-795. [PubMed] [Cited in This Article: ] |
| 4. | Appoo J, Norris C, Merali S, Graham MM, Koshal A, Knudtson ML, Ghali WA. Long-term outcome of isolated coronary artery bypass surgery in patients with severe left ventricular dysfunction. Circulation. 2004;110:II13-II17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Lytle BW, Blackstone EH, Sabik JF, Houghtaling P, Loop FD, Cosgrove DM. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg. 2004;78:2005-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 352] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 6. | Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358:870-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 566] [Cited by in F6Publishing: 563] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 7. | Locker C, Schaff HV, Dearani JA, Joyce LD, Park SJ, Burkhart HM, Suri RM, Greason KL, Stulak JM, Li Z. Multiple arterial grafts improve late survival of patients undergoing coronary artery bypass graft surgery: analysis of 8622 patients with multivessel disease. Circulation. 2012;126:1023-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2471] [Cited by in F6Publishing: 2542] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 9. | Kunadian V, Zaman A, Qiu W. Revascularization among patients with severe left ventricular dysfunction: a meta-analysis of observational studies. Eur J Heart Fail. 2011;13:773-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Nardi P, Pellegrino A, Scafuri A, Colella D, Bassano C, Polisca P, Chiariello L. Long-term outcome of coronary artery bypass grafting in patients with left ventricular dysfunction. Ann Thorac Surg. 2009;87:1401-1407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Taggart DP, Altman DG, Gray AM, Lees B, Nugara F, Yu LM, Campbell H, Flather M. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART). Eur Heart J. 2010;31:2470-2481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 12. | Matsa M, Paz Y, Gurevitch J, Shapira I, Kramer A, Pevny D, Mohr R. Bilateral skeletonized internal thoracic artery grafts in patients with diabetes mellitus. J Thorac Cardiovasc Surg. 2001;121:668-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Dorman MJ, Kurlansky PA, Traad EA, Galbut DL, Zucker M, Ebra G. Bilateral internal mammary artery grafting enhances survival in diabetic patients: a 30-year follow-up of propensity score-matched cohorts. Circulation. 2012;126:2935-2942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Itagaki S, Cavallaro P, Adams DH, Chikwe J. Bilateral internal mammary artery grafts, mortality and morbidity: an analysis of 1 526 360 coronary bypass operations. Heart. 2013;99:849-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Boodhwani M, Lam BK, Nathan HJ, Mesana TG, Ruel M, Zeng W, Sellke FW, Rubens FD. Skeletonized internal thoracic artery harvest reduces pain and dysesthesia and improves sternal perfusion after coronary artery bypass surgery: a randomized, double-blind, within-patient comparison. Circulation. 2006;114:766-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Galbut DL, Kurlansky PA, Traad EA, Dorman MJ, Zucker M, Ebra G. Bilateral internal thoracic artery grafting improves long-term survival in patients with reduced ejection fraction: a propensity-matched study with 30-year follow-up. J Thorac Cardiovasc Surg. 2012;143:844-853.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607-1616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 832] [Cited by in F6Publishing: 872] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 18. | Bonow RO, Maurer G, Lee KL, Holly TA, Binkley PF, Desvigne-Nickens P, Drozdz J, Farsky PS, Feldman AM, Doenst T. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 621] [Cited by in F6Publishing: 588] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 19. | Nishioka H, Kitamura S, Kameda Y, Taniguchi S, Kawata T, Mizuguchi K. Difference in acetylcholine-induced nitric oxide release of arterial and venous grafts in patients after coronary bypass operations. J Thorac Cardiovasc Surg. 1998;116:454-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Ruttmann E, Fischler N, Sakic A, Chevtchik O, Alber H, Schistek R, Ulmer H, Grimm M. Second internal thoracic artery versus radial artery in coronary artery bypass grafting: a long-term, propensity score-matched follow-up study. Circulation. 2011;124:1321-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 21. | Grau JB, Ferrari G, Mak AW, Shaw RE, Brizzio ME, Mindich BP, Strobeck J, Zapolanski A. Propensity matched analysis of bilateral internal mammary artery versus single left internal mammary artery grafting at 17-year follow-up: validation of a contemporary surgical experience. Eur J Cardiothorac Surg. 2012;41:770-775; discussion 776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | El-Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, Leon AR, Puskas JD. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55:1370-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Oh JK, Velazquez EJ, Menicanti L, Pohost GM, Bonow RO, Lin G, Hellkamp AS, Ferrazzi P, Wos S, Rao V. Influence of baseline left ventricular function on the clinical outcome of surgical ventricular reconstruction in patients with ischaemic cardiomyopathy. Eur Heart J. 2013;34:39-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, Jain AR, Leng Chua Y, Daly R, Senni M. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125:2639-2648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 25. | Halkos ME, Vassiliades TA, Douglas JS, Morris DC, Rab ST, Liberman HA, Samady H, Kilgo PD, Guyton RA, Puskas JD. Hybrid coronary revascularization versus off-pump coronary artery bypass grafting for the treatment of multivessel coronary artery disease. Ann Thorac Surg. 2011;92:1695-1701; discussion 1701-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |