Published online Mar 26, 2017. doi: 10.4330/wjc.v9.i3.248
Peer-review started: July 29, 2016
First decision: September 8, 2016
Revised: October 7, 2016
Accepted: December 27, 2016
Article in press: December 28, 2016
Published online: March 26, 2017
To investigate validity of electrocardiographic (ECG) criteria for left ventricular hypertrophy (LVH) in young adults.
Retrospectively, echocardiograms showing LVH and concomitant electrocardiograms were collected in patients 18 to 39 years old. A control group of patients without LVH was collected. Using echocardiogram as the gold standard, electrocardiograms were analyzed using common voltage criteria.
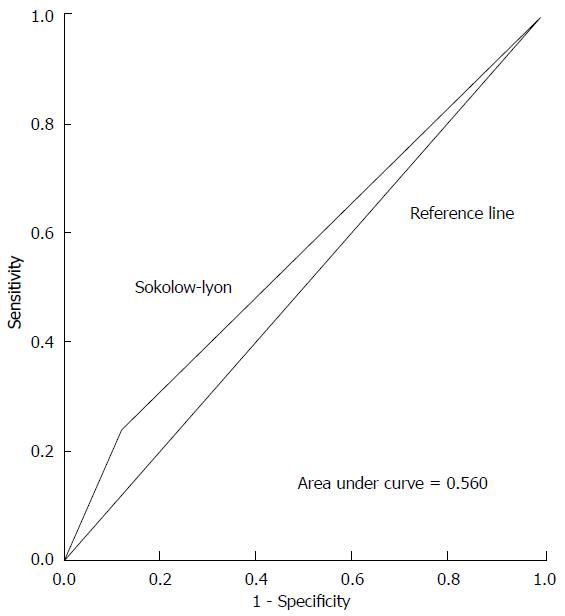
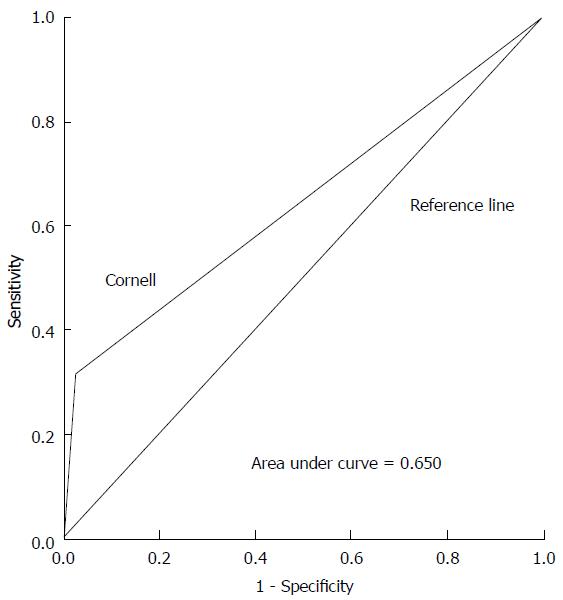
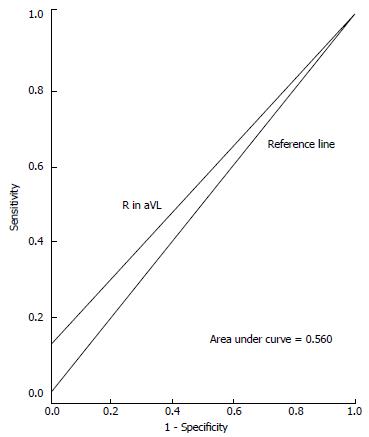
Study included 100 subjects (52% male, mean age = 28 ± 6.8 years, 96% Hispanic or African-American) with 50% LVH prevalence. Sensitivity and specificity for Sokolow-Lyon criteria were 24% (95%CI: 13.5%-38.4%) and 88% (95%CI: 74.9%-95%). For Cornell criteria, sensitivity was 32% (95%CI: 19.9%-46.8%) and specificity 98% (95%CI: 87.9%-99.8%). For R in aVL criteria, sensitivity was 12% (95%CI: 4.9%-25%) and specificity 100% (95%CI: 91.1%-100%).
In young adults common ECG voltage criteria have low sensitivities and high specificities similar to other age groups. Low sensitivities preclude these ECG criteria from serving as effective screening tests.
Core tip: The electrocardiographic (ECG) has been used for years to diagnose left ventricular hypertrophy (LVH). However, to the best of our knowledge, there were no prior studies validating most common ECG criteria for LVH in young adults. The authors believe that this is important group of population, as athletes screening for pre participation to professional sport falls into this category. ECG is one of the proposed screening tools and we think that it should be validated for diagnosis of LVH. This study showed that common ECG criteria for LVH can be used in young adults with similar sensitivity and specificity to other age groups.
- Citation: Sklyar E, Ginelli P, Barton A, Peralta R, Bella JN. Validity of electrocardiographic criteria for increased left ventricular mass in young patients in the general population. World J Cardiol 2017; 9(3): 248-254
- URL: https://www.wjgnet.com/1949-8462/full/v9/i3/248.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i3.248
Epidemiologic studies have demonstrated that increased left ventricular mass (LVM) is a risk factor for cardiovascular disease and death[1-4]. Left ventricular hypertrophy (LVH) has a significant prevalence in the general population with some estimates approaching 16%-20% in population based samples[5,6], and up to 50% in those with hypertension[7-9]. As a result of the obesity epidemic, even the adolescent population has had a higher incidence of hypertension and LVH[10], with some estimates showing LVH in nearly 30% of younger hypertensive[11]. Thus, with the high prevalence of LVH that extends even into the young population and the increased cardiovascular risk it confers, it is important to identify patients with increased LVM so that they may receive appropriate care.
Another important indication for detecting LVH, specifically in younger individuals in the general population, is in the setting of pre-participation screening prior to partaking in athletic activities. Current screening methods often involve looking for evidence of LVH on electrocardiogram (ECG) and transthoracic echocardiogram (TTE) to help identify those who may be at risk for sudden cardiac death[12]. Unfortunately, LVH is often misdiagnosed during these pre-participation screenings which can lead to unwanted outcomes[13]. Due to the immense importance of detecting increased LVM in the screening process of this younger population, a reliable screening tool is vital for this age group.
The ECG is the simplest and most commonly used method to detect LVH, but to date there has been no study evaluating the correlation between the established ECG criteria and increased LVM detected by TTE in adults from the general population with a mean age of 18 to 39 years old. Although numerous investigations have been conducted to validate the ECG criteria for LVH in individuals of the pediatric and older populations, the data for younger adults have been limited. Thus, with the growing number of young individuals with hypertension and LVH, along with the need for effective pre-participation screening, the quick and simple electrocardiographic methods for detecting increased LVM and obtaining prognostic information need to be validated for use in the younger members of the general population. Therefore, the aim of this study is to examine this particular subset of the population in order to determine the efficacy of this potentially valuable tool for identifying patients at increased risk for cardiovascular morbidity and mortality.
This was a single-center, retrospective study involving ECG and TTE data conducted in the Cardiology Division of the Department of Medicine at Bronx Lebanon Hospital Center, a large teaching medical center in Bronx, New York. After receiving approval from the Institutional Review Board, the hospital’s electronic database was used to collect all consecutive TTEs with the finding of LVH performed between January 2010 and July 2011 on male and female patients aged 18-39 years old who were referred or admitted to the hospital. A control group of age-matched patients without LVH on TTE was also collected in the same manner from the database. Subjects were also required to have a standard 12-lead ECG within 30 d before or after the reference TTE. Excluded were patients with ECGs showing myocardial infarction, bundle branch block, paced rhythm, pre-excitation, or any intraventricular conduction delay, as these ECG findings can interfere with voltage measurements and were not included in the original studies from which the ECG criteria for detecting increased LVM were derived[14,15]. After creating the study and control groups based on the presence or absence of increased LVM using TTE as the gold standard for diagnosis, the subjects’ ECGs were analyzed using several ECG criteria for detecting LVH in order to determine their efficacy in this cohort of patients.
Study subjects had previously completed the prerequisite two-dimensional (2-D) rest echocardiogram using a standard, commercially available ultrasound transducer and machine (M3S probe, Vivid 7, GE Medical Systems). In each patient, standard parasternal views (long and short axis) and apical views (4- and 2-chamber) were obtained. TTE images were saved digitally in raw data format for off-line analysis using GE Medical Systems’ EchoPAC PC software. These TTEs were previously read by board-certified cardiologists on the day of acquisition, and it was these interpretations that were used to extract the study population from the hospital database. In order to ensure uniformity of the echocardiographic data used in this study, all subjects’ TTEs were reread by a single board-certified noninvasive cardiologist who was blinded to the study arm in which the TTE’s belonged. This reader remeasured left-ventricular end-diastolic dimension (LVEDd), end-diastolic posterior wall thickness (PWTd), and end-diastolic septal wall thickness (SWTd) in the standard 2-D parasternal long axis view as detailed by the American Society of Echocardiography (ASE)[16]. These measurements were then used to calculate LVM using the ASE recommended formula for estimation of LVM from left ventricular linear dimensions[16]: LVM = 0.8 × {1.04[(LVEDd + PWTd + SWTd)3 - (LVEDd)3]} + 0.6 grams. The LVM was then indexed to body surface area (BSA) which was obtained from the original reference TTE reports. The ASE gender specific cut-off values for LVM[16] were used to classify patients as having increased LVM if the LVM indexed to BSA was greater than 88 g/m2 for women and greater than 102 g/m2 for men.
All subjects had previously undergone a standard 12-lead rest ECG at 25 mm/s speed, 10 mm/mV sensitivity, and 0.05 Hz to 150 Hz frequency within 30 d of the index TTE. Of the various ECG criteria for LVH including, Sokolow-Lyon voltage[14], Sokolow-Lyon product[17], R in aVL voltage[14], Cornell voltage[15], Cornell product[17], and Gubner voltage[18], three of the most commonly used criteria in many clinical trials[19-21], Sokolow-Lyon voltage, Cornell voltage, and R in aVL voltage, were then selected to analyze the ECGs. For Sokolow-Lyon voltage, the amplitude of the S wave in lead V1 was added to the largest amplitude of the R wave in either lead V5 or V6, with a value greater than or equal to 35 mm meeting criteria for LVH. For Cornell voltage, the amplitude of the S wave in lead V3 was added to the amplitude of the R wave in lead aVL, with a value greater than 28 mm for men and greater than 20 mm for women signifying LVH. For R in aVL voltage, an amplitude of the R wave in lead aVL greater than or equal to 11 mm was indicative of LVH. All study ECGs were evaluated for these three criteria using manual calipers by each of two trained readers who were blinded to the study group in which the ECGs belonged. Any discrepancies in measurements between the two readers were evaluated by a board-certified electrophysiologist who made the final decision on the ECG findings.
Data management and descriptive analysis were performed with IBM SPSS 20 (Statistical Packages for the Social Sciences). Data are presented as mean (SD) for continuous variables and proportions for categorical variables. For measurements of sensitivity and specificity, increased LVM as detected by TTE was used as the reference standard against which the performance of the ECG criteria was compared. Mean values of continuous variables were compared by using an independent sample t-test. Linear correlations were evaluated with the Pearson’s r correlation. Receiver operating characteristic (ROC) curves were constructed for each ECG criteria to evaluate test performance over a wide range of possible partition values. A two-tailed value of P < 0.05 was considered statistically significant.
The initial database query revealed 1107 subjects who had a TTE performed during the search period. Of these 1107 patients, 239 had LVH documented in their TTE report. Using the aforementioned inclusion and exclusion criteria, 84 subjects were then found for the increased LVM group. After left ventricular dimensions were re-measured by our expert reader, there were 50 remaining subjects with increased LVM by ASE criteria. Fifty subjects without increased LVM were found for the control group, resulting in a prevalence of increased LVM by TTE of 50%. The total study population had a mean age of 28 ± 6.8 years and consisted of 52 men and 48 women, of which 96% were either Hispanic or African-American. The 1007 excluded subjects had similar demographics with a mean age of 29 ± 6.2 years, 38% men, and 96% Hispanic or African-American. There were no significant differences noted in age, gender, or BSA between the increased LVM and control arms (Table 1). As expected, the increased LVM group had a mean LVM indexed to BSA of 130.35 ± 36.76 g/m2 which was significantly higher (P < 0.001) than the control group’s value of 63.61 g/m2± 11.73 (Table 1). The increased LVM group also had significantly higher values (P < 0.001) for SWTd, PWTd, and LVEDd as compared to the control group (Table 1).
| Parameter | Increased LVM (n = 50) | Controls (n = 50) | P value |
| Age (yr) | 29.7 ± 5.9 | 26.9 ± 7.4 | 0.05 |
| Male/female | 27/23 | 25/25 | 0.69 |
| BSA (m2) | 1.95 ± 0.34 | 1.88 ± 0.32 | 0.30 |
| SWTd (cm) | 1.09 ± 0.16 | 0.79 ± 0.08 | < 0.001 |
| LVEDd (cm) | 5.70 ± 0.68 | 4.69 ± 0.43 | < 0.001 |
| PWTd (cm) | 1.05 ± 0.11 | 0.76 ± 0.09 | < 0.001 |
| LVM/BSA (g/m2) | 130.35 ± 36.76 | 63.61 ± 11.73 | < 0.001 |
Sensitivities and specificities for detecting increased LVM with each ECG criteria are displayed in Table 2. Sensitivity and specificity for the Sokolow-Lyon criteria were 24% (95%CI: 14%-38%) and 88% (95%CI: 75%-95%), respectively. For the Cornell voltage criteria, sensitivity was 32% (95%CI: 19%-47%) and specificity was 98% (95%CI: 88%-99%). For the R in aVL criteria, sensitivity was 12% (95%CI: 5%-25%) and specificity was 100% (95%CI: 91%-100%). Positive predictive values (PPV) and negative predictive values (NPV) are also shown in Table 2. PPVs were 67% (95%CI: 41%-86%) for Sokolow-Lyon, 94% (95%CI: 69%-99%) for Cornell, and 100% (95%CI: 52%-100%) for R in aVL. NPVs were 54% (95%CI: 42%-65%) for Sokolow-Lyon, 59% (95%CI: 48%-69%) for Cornell, and 53% (95%CI: 43%-63%) for R in aVL.
| ECG criteria | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) |
| Sokolow-lyon | 0.24 (0.14-0.38) | 0.88 (0.75-0.95) | 0.67 (0.41-0.86) | 0.54 (0.42-0.65) |
| Cornell | 0.32 (0.19-0.47) | 0.98 (0.88-0.99) | 0.94 (0.69-0.99) | 0.59 (0.48-0.69) |
| R in aVL | 0.12 (0.05-0.25) | 1 (0.91-1) | 1 (0.52-1) | 0.53 (0.43-0.63) |
ROC curves illustrating the performance of each ECG criteria in detecting increased LVM are shown in Figures 1-3. Areas under the ROC curve were 0.560 for Sokolow-Lyon, 0.650 for Cornell, and 0.560 for R in aVL. All three ECG criteria also demonstrated good statistical correlation with increased LVM by TTE (Table 3).
| ECG criteria | Pearson’s r correlation | P valuea |
| Sokolow-lyon | 0.28 | 0.005 |
| Cornell | 0.51 | < 0.001 |
| R in aVL | 0.26 | 0.01 |
This investigation found that three frequently used ECG voltage criteria are effective in identifying increased LVM in the subset of the general population aged 18 to 39 years old. Sensitivity was highest with the Cornell criteria at 32%, as compared to 24% with the Sokolow-Lyon criteria and 12% with the R in aVL criteria. The highest specificity was found with the R in aVL criteria at 100%, while the Cornell and Sokolow-Lyon criteria had somewhat lower specificities of 98% and 88%, respectively. Although there is some minor variation among these values, all three criteria demonstrated a low sensitivity but high specificity for detecting increased LVM in this young adult population. These findings are similar to those previously published for other age groups and populations.
Prior studies examining the accuracy of these ECG criteria in older individuals of the general population encompassing mean ages from 45 to 70 years old[21-24] have shown similar findings with values for sensitivity ranging from 4% to 52% for the Sokolow-Lyon criteria and 2% to 41% for the Cornell criteria, and specificities ranging from 53% to 100% for the Sokolow-Lyon criteria and 89% to 100% for the Cornell criteria, as reported in a large meta-analysis[21]. In another large study in patients with mean age of 65 years old, the Sokolow-Lyon criteria had a sensitivity of 17% and specificity of 90%, the Cornell criteria had a sensitivity of 5.9% and a specificity of 99%, and the R in aVL criteria had a sensitivity of 8% and specificity of 94%[25]. The original Cornell criteria study[15] and a follow-up paper[26] by the same authors also found similarly low sensitivities and high specificities in the older population. Evaluation of the Sokolow-Lyon criteria in a pediatric population encompassing infants to 15 year olds also yielded a low sensitivity of 25% and high specificity of 95%[27].
In young adults from the general population there have been no data for ECG voltage criteria until the present study was conducted and demonstrated that these commonly used criteria perform equally well as in other age groups. Prior studies involving young adults were conducted in very distinct subsets of the population. In a recent study of healthy male Air Force candidates, the Sokolow-Lyon and Cornell criteria were found to have a sensitivity of 55% and specificity of 87%[28]. Another study involving healthy young male military recruits found the Sokolow-Lyon criteria to have a sensitivity of 50% and specificity of 71%, and the Cornell criteria to have a sensitivity of 25% and specificity of 88%[29]. Other studies in the young adult population involved highly trained athletes and found poor correlation between the Sokolow-Lyon criteria and increased LVM[30,31].
Hypertension has become more prevalent in the young adult population[10], and has been shown to cause increased LVM and even heart failure if left untreated[32]. With proper antihypertensive therapy LVH can regress and left ventricular dysfunction can improve[33,34]. Thus, with our findings that ECG voltage criteria are highly specific for increased LVM in young adults, it is reasonable to conclude that such a patient meeting ECG criteria for LVH may benefit from further testing, including TTE, to identify and treat increased LVM, a known and modifiable risk factor for cardiovascular disease and death[1-4]. Our results also suggest that ECG voltage criteria may not be suitable for pre-participation screening prior to partaking in athletic activities. Some screening methods often involve looking for evidence of LVH on ECG to identify those who may be at risk for sudden cardiac death[12], but with this study demonstrating such low sensitivities for detecting increased LVM, ECG voltage criteria may not perform adequately as screening tools. This finding is in agreement with the current United States guidelines for pre-participation screening, as laid forth by the American Heart Association, which do not recommend performing an ECG as part of pre-athletic screening[35]. Despite their low sensitivities, the ECG voltage criteria showed rather high specificity for detecting increased LVM in young adults. This result brings into question the notion that the presence of ECG voltage criteria in young adults is merely a normal variant[36]. Regardless of the initial indication, if an ECG performed in a young adult meets voltage criteria for LVH, the finding should not be assumed normal until completing further investigation with an imaging modality such as TTE.
In conducting a retrospective analysis it was necessary to accept certain limitations inherent with this type of design, the most significant being the lack of randomization. In analyzing the study and control groups, however, there were no significant differences in baseline characteristics as shown in Table 1. Although this was a study of the general population, the majority of subjects were Hispanic or African-American, with few Caucasian individuals. It is likely that the ECG voltage criteria tested would also show efficacy in other races, but this cannot be concluded from this study alone. This study examined only three of the numerous ECG criteria that have been developed for detecting LVH, but this was done intentionally as those chosen are among the most commonly used and simplest to perform, with no difficult calculations or point systems.
In conclusion, three commonly used ECG voltage criteria, Sokolow-Lyon, Cornell, and R in aVL, show efficacy in detecting increased LVM in young adults of the general population and have sensitivities and specificities that are similar to those found in other age groups. Although their low sensitivities preclude these ECG criteria from serving as effective screening tests, their relatively high specificities would necessitate further evaluation if the criteria were present in a young individual. Our results provide evidence that these simple diagnostic tools can be utilized in a population subset that may benefit from the valuable prognostic information that they provide.
Electrocardiographic (ECG) is a very common test to evaluate for structural heart disease, including left ventricular hypertrophy (LVH). The ECG criteria for LVH are widely studies in older patients; its utility in young adults is unknown. ECG was proposed to be a screening test for detection of structural abnormality of the heart, however in order to be a screening test is should have high sensitivity for detection of pathology. Thus, it’s important to evaluate sensitivity and specificity of ECG criteria for LVH in young adults.
ECG has been used for years to diagnose LVH. However, to the best of the authors’ knowledge, there were no prior studies validating most common ECG criteria for LVH in young adults.
The results of this study contribute to clarifying the ECG diagnostic criteria for LVH in young adults in compression to older patients and its sensitivity and specificity.
In young adults common ECG voltage criteria have low sensitivities and high specificities similar to other age groups. Although their low sensitivities preclude these ECG criteria from serving as effective screening tests, their relatively high specificities would necessitate further evaluation if the criteria were present in a young individual. The results provide evidence that these simple diagnostic tools can be utilized in a population subset that may benefit from the valuable prognostic information that they provide.
The authors have done a good job analyzing retrospectively common electrocardiographic criteria for LVH in young adults.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bonanno C, Kettering K, Nam GB, Said SAM, Soliman EZ S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Ghali JK, Liao Y, Simmons B, Castaner A, Cao G, Cooper RS. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med. 1992;117:831-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 353] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1660] [Cited by in F6Publishing: 1589] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 3. | Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561-1566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4052] [Cited by in F6Publishing: 3912] [Article Influence: 115.1] [Reference Citation Analysis (1)] |
| 4. | Wachtell K, Palmieri V, Gerdts E, Bella JN, Aurigemma GP, Papademetriou V, Dahlöf B, Aalto T, Ibsen H, Rokkedal JE. Prognostic significance of left ventricular diastolic dysfunction in patients with left ventricular hypertrophy and systemic hypertension (the LIFE Study). Am J Cardiol. 2010;106:999-1005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987;59:956-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 585] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Bella JN, Devereux RB, Roman MJ, O’Grady MJ, Welty TK, Lee ET, Fabsitz RR, Howard BV. Relations of left ventricular mass to fat-free and adipose body mass: the strong heart study. The Strong Heart Study Investigators. Circulation. 1998;98:2538-2544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 166] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Savage DD, Drayer JI, Henry WL, Mathews EC, Ware JH, Gardin JM, Cohen ER, Epstein SE, Laragh JH. Echocardiographic assessment of cardiac anatomy and function in hypertensive subjects. Circulation. 1979;59:623-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 258] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Kaplan NM, Lieberman E, Neal W. Kaplan’s clinical hypertension. Philadelphia: Lippincott, Williams & Wilkins 2002; . [Cited in This Article: ] |
| 9. | Hammond IW, Devereux RB, Alderman MH, Lutas EM, Spitzer MC, Crowley JS, Laragh JH. The prevalence and correlates of echocardiographic left ventricular hypertrophy among employed patients with uncomplicated hypertension. J Am Coll Cardiol. 1986;7:639-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 324] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Movahed MR, Bates S, Strootman D, Sattur S. Obesity in adolescence is associated with left ventricular hypertrophy and hypertension. Echocardiography. 2011;28:150-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Cuspidi C, Meani S, Sala C, Valerio C, Negri F, Mancia G. Age related prevalence of severe left ventricular hypertrophy in essential hypertension: echocardiographic findings from the ETODH study. Blood Press. 2012;21:139-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Corrado D, Drezner J, Basso C, Pelliccia A, Thiene G. Strategies for the prevention of sudden cardiac death during sports. Eur J Cardiovasc Prev Rehabil. 2011;18:197-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Hill AC, Miyake CY, Grady S, Dubin AM. Accuracy of interpretation of preparticipation screening electrocardiograms. J Pediatr. 2011;159:783-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | SOKOLOW M, LYON TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1314] [Cited by in F6Publishing: 1288] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 15. | Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 373] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8282] [Cited by in F6Publishing: 8585] [Article Influence: 476.9] [Reference Citation Analysis (0)] |
| 17. | Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol. 1992;20:1180-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 281] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Gubner R UH. Electrocardiographic criteria of left ventricular hypertrophy. Arch Intern Med. 1943;196-206. [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 155] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Devereux RB, Bella J, Boman K, Gerdts E, Nieminen MS, Rokkedal J, Papademetriou V, Wachtell K, Wright J, Paranicas M. Echocardiographic left ventricular geometry in hypertensive patients with electrocardiographic left ventricular hypertrophy: The LIFE Study. Blood Press. 2001;10:74-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343-2349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 511] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 21. | Pewsner D, Jüni P, Egger M, Battaglia M, Sundström J, Bachmann LM. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ. 2007;335:711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 22. | Crow RS, Prineas RJ, Rautaharju P, Hannan P, Liebson PR. Relation between electrocardiography and echocardiography for left ventricular mass in mild systemic hypertension (results from Treatment of Mild Hypertension Study). Am J Cardiol. 1995;75:1233-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Dada A, Adebiyi AA, Aje A, Oladapo OO, Falase AO. Standard electrocardiographic criteria for left ventricular hypertrophy in Nigerian hypertensives. Ethn Dis. 2005;15:578-584. [PubMed] [Cited in This Article: ] |
| 24. | Vottonen P, Husso M, Sipola P, Vanninen R, Peuhkurinen K, Magga J. Electrocardiographic left ventricular hypertrophy has low diagnostic accuracy in middle-aged subjects. Blood Press. 2007;16:328-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Casiglia E, Schiavon L, Tikhonoff V, Bascelli A, Martini B, Mazza A, Caffi S, D’Este D, Bagato F, Bolzon M. Electrocardiographic criteria of left ventricular hypertrophy in general population. Eur J Epidemiol. 2008;23:261-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75:565-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 386] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Rijnbeek PR, van Herpen G, Kapusta L, Ten Harkel AD, Witsenburg M, Kors JA. Electrocardiographic criteria for left ventricular hypertrophy in children. Pediatr Cardiol. 2008;29:923-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Grossman A, Prokupetz A, Koren-Morag N, Grossman E, Shamiss A. Comparison of usefulness of Sokolow and Cornell criteria for left ventricular hypertrophy in subjects aged & lt; 20 years versus & gt; 30 years. Am J Cardiol. 2012;110:440-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Sohaib SM, Payne JR, Shukla R, World M, Pennell DJ, Montgomery HE. Electrocardiographic (ECG) criteria for determining left ventricular mass in young healthy men; data from the LARGE Heart study. J Cardiovasc Magn Reson. 2009;11:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Rawlins J, Carre F, Kervio G, Papadakis M, Chandra N, Edwards C, Whyte GP, Sharma S. Ethnic differences in physiological cardiac adaptation to intense physical exercise in highly trained female athletes. Circulation. 2010;121:1078-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Somauroo JD, Pyatt JR, Jackson M, Perry RA, Ramsdale DR. An echocardiographic assessment of cardiac morphology and common ECG findings in teenage professional soccer players: reference ranges for use in screening. Heart. 2001;85:649-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Di Bella MA, Carbone MC, De Leo G. Aspects of cell production in mantle tissue of Ciona intestinalis L. (Tunicata, Ascidiacea). Micron. 2005;36:477-481. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Wachtell K, Bella JN, Rokkedal J, Palmieri V, Papademetriou V, Dahlöf B, Aalto T, Gerdts E, Devereux RB. Change in diastolic left ventricular filling after one year of antihypertensive treatment: The Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Circulation. 2002;105:1071-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Solomon SD, Verma A, Desai A, Hassanein A, Izzo J, Oparil S, Lacourciere Y, Lee J, Seifu Y, Hilkert RJ. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension. 2010;55:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Maron BJ, Thompson PD, Ackerman MJ, Balady G, Berger S, Cohen D, Dimeff R, Douglas PS, Glover DW, Hutter AM. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115:1643-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 707] [Cited by in F6Publishing: 608] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 36. | Weiner RB, Hutter AM, Wang F, Kim JH, Wood MJ, Wang TJ, Picard MH, Baggish AL. Performance of the 2010 European Society of Cardiology criteria for ECG interpretation in athletes. Heart. 2011;97:1573-1577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |