Published online Jul 26, 2016. doi: 10.4330/wjc.v8.i7.425
Peer-review started: March 24, 2016
First decision: April 15, 2016
Revised: April 26, 2016
Accepted: May 17, 2016
Article in press: May 27, 2016
Published online: July 26, 2016
AIM: To determine the effect of procedural and clinical factors upon C reactive protein (CRP) dynamics following transcatheter aortic valve implantation (TAVI).
METHODS: Two hundred and eight consecutive patients that underwent transfemoral TAVI at two hospitals (Imperial, College Healthcare NHS Trust, Hammersmith Hospital, London, United Kingdom and San Raffaele Scientific Institute, Milan, Italy) were included. Daily venous plasma CRP levels were measured for up to 7 d following the procedure (or up to discharge). Procedural factors and 30-d safety outcomes according to the Valve Academic Research Consortium 2 definition were collected.
RESULTS: Following TAVI, CRP significantly increased reaching a peak on day 3 of 87.6 ± 5.5 mg/dL, P < 0.001. Patients who developed clinical signs and symptoms of sepsis had significantly increased levels of CRP (P < 0.001). The presence of diabetes mellitus was associated with a significantly higher peak CRP level at day 3 (78.4 ± 3.2 vs 92.2 ± 4.4, P < 0.001). There was no difference in peak CRP release following balloon-expandable or self-expandable TAVI implantation (94.8 ± 9.1 vs 81.9 ± 6.9, P = 0.34) or if post-dilatation was required (86.9 ± 6.3 vs 96.6 ± 5.3, P = 0.42), however, when pre-TAVI balloon aortic valvuloplasty was performed this resulted in a significant increase in the peak CRP (110.1 ± 8.9 vs 51.6 ± 3.7, P < 0.001). The development of a major vascular complication did result in a significantly increased maximal CRP release (153.7 ± 11.9 vs 83.3 ± 7.4, P = 0.02) and there was a trend toward a higher peak CRP following major/life-threatening bleeding (113.2 ± 9.3 vs 82.7 ± 7.5, P = 0.12) although this did not reach statistical significance. CRP was not found to be a predictor of 30-d mortality on univariate analysis.
CONCLUSION: Careful attention should be paid to baseline clinical characteristics and procedural factors when interpreting CRP following TAVI to determine their future management.
Core tip: Transcatheter aortic valve implantation (TAVI) results in increases in serum C reactive protein (CRP) levels reaching a peak at day 3 in all patients. CRP increase is further increased in patients with diabetes mellitus, those that underwent pre-TAVI balloon aortic valvuloplasty and patients that suffered major vascular complications. In addition to the bedside evaluation of patients, careful attention should be paid to baseline clinical characteristics and procedural factors when interpreting CRP to aid in the management and risk assessment of patients following TAVI.
- Citation: Ruparelia N, Panoulas VF, Frame A, Ariff B, Sutaria N, Fertleman M, Cousins J, Anderson J, Bicknell C, Chukwuemeka A, Sen S, Malik IS, Colombo A, Mikhail GW. Impact of clinical and procedural factors upon C reactive protein dynamics following transcatheter aortic valve implantation. World J Cardiol 2016; 8(7): 425-431
- URL: https://www.wjgnet.com/1949-8462/full/v8/i7/425.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i7.425
Aortic stenosis (AS) is the most common valvular pathology in the elderly population with a prevalence of approximately 4.6% in patients greater than 75 years of age[1]. Whilst asymptomatic AS is associated with a low mortality[2], in those who develop symptoms, prognosis is very poor with a mortality of 50% within 2 years without treatment[3]. Whilst surgical aortic valve replacement (SAVR) remains the “gold standard” treatment, many of these elderly patients present with many co-existent comorbidities that render them inoperable or high-risk for SAVR. The emergence of transcatheter aortic valve implantation (TAVI) has revolutionised the treatment of these patients[4-8]. The transfemoral (TF) route is now the preferable TAVI vascular route due to shorter procedure and recovery times, and better clinical outcomes[9].
In spite of TAVI being less invasive, these frail, elderly patients are at increased risk of developing complications resulting in adverse outcomes. Post-procedural infection is a potentially life-threatening complication and has been reported to occur in approximately 20% of all patients[10,11]. In combination with the clinical evaluation of patients, the C reactive protein (CRP), an acute phase protein synthesized and released by the liver is commonly measured to aid in diagnosis. However, the CRP is non-specific for infection and misinterpretation can result in misdiagnosis, inappropriate antibiotic therapy (and associated adverse effects) and prolonged in-hospital stay.
CRP is also a measure of inflammation that is thought to play a critical role in both the underlying pathogenesis of AS[12,13] with persistently high levels of circulating plasma inflammatory proteins following aortic valve intervention associated with increased cardiovascular and all-cause mortality[14,15]. SAVR results in greater activation of inflammatory pathways in comparison to TAVI with the TF access route being associated with the most attenuated inflammatory response[16]. Understanding CRP dynamics following TF TAVI is therefore critical in both the post-procedural management of these patients and predicting outcome.
The aim of this study was therefore to characterise CRP dynamics following TF TAVI and to identify clinical or procedural factors that may impact upon them.
Consecutive patients that underwent TF TAVI at two hospitals (Hammersmith Hospital, Imperial College Healthcare NHS Trust, London, United Kingdom and San Raffaele Scientific Institute, Milan, Italy) were included. All patients were treated for native severe AS with patients treated with TAVI devices for aortic regurgitation and for bioprosthesis degeneration excluded. A dedicated multidisciplinary “Heart Team” consisting of interventional cardiologist, cardiac surgeons, imaging specialists, general physicians and cardiac anaesthetists, discussed the management of all patients. All patients included in the study were of high surgical risk or inoperable on the basis of surgical risk scores (e.g., Euroscore) and clinical judgement to allow for other patient factors including frailty.
Daily venous plasma CRP levels were measured for up to 7 d following the procedure (or up to discharge) using.
Informed consent was provided by all patients for the procedure, subsequent clinical follow-up and analysis of data collected.
Pre-operatively, all patients were evaluated with multi-slice computed tomography or invasive angiography to determine the presence or absence of coronary artery disease and for the characterisation of the peripheral vasculature. The choice of prosthesis (balloon expandable Edwards Sapien XT or Sapien 3 (Edwards LifeSciences, Irvine, CA, United States) or self-expandable Medtronic CoreValve or Evolut R (Medtronic, Minnesota, MN, United States) and size was at the operator’s discretion. Patients treated with other devices were excluded due to their unavailability at both sites. At the time of TAVI, no patients had any clinical signs, symptoms of biochemical evidence of infection. All procedures were carried out under general anaesthesia or conscious sedation provided by a cardiac anaesthetist and were performed when possible by a fully percutaneous approach utilizing the cross-over technique and suture-mediated closure devices (Proglide and Prostar, Abbott Laboratories, IL, United States). Antibiotics were not administered to any patient routinely during the peri-operative period.
Procedural outcomes in-hospital clinical outcomes were prospectively collected in a dedicated TAVI database. Longer-term follow-up was conducted by clinic visits. All definition of the clinical endpoints used were in concordance with the Valve Academic Research Consortium 2 (VARC-2) definitions[17]. Patients were deemed to have infection on the basis of clinical symptoms (e.g., dysuria), signs of infection (e.g., fever) and objective evidence (e.g., elevated white cell count, positive blood culture). The administration and choice of antibiotics was at the discretion of the treating physician.
Continuous variables are presented as the mean ± standard error of the mean. Normality of each continuous variable was tested with the Kolmogorov-Smirnov test and differences were compared using the paired t-test. Categorical variables are presented as numerical values and percentages and were compared using the Fisher’s exact test. Cox proportional hazards regression analysis was performed to determine predictors of mortality. Receiver-operator characteristic (ROC) analysis was performed to identify the threshold for CRP as a binary classifier. All reported P-values were 2-sided, and values of P < 0.05 were regarded as statistically significant. Analyses were performed with SPSS version 21.0 (SPSS Inc., Chicago IL, United States) and GraphPad Prism version 5.0 (GraphPad, San Diego, CA, United States).
Two hundred and eight patients underwent TF TAVI at both institutions during the study period and were included in the final analysis. The baseline characteristics of all patients are summarised in Table 1. As expected, the patient group were elderly (age: 81.4 ± 8.5 years) and of high surgical risk standard Euroscore 14.8% ± 10.4%.
| Variable | All patients (n = 208) |
| Age (yr) | 81.4 ± 0.9 |
| Female (%) | 57 (27.4) |
| Diabetes mellitus (%) | 34 (16.3) |
| Hypertension (%) | 122 (58.7) |
| Dyslipidemia (%) | 65 (31.3) |
| History of smoking (%) | 34 (16.3) |
| NYHA III or IV (%) | 94 (45.2) |
| Previous MI (%) | 24 (11.5) |
| Previous CABG (%) | 37 (17.8) |
| Previous PCI (%) | 40 (19.2) |
| Cerebrovascular disease (%) | 19 (9.1) |
| eGFR < 60 mL/min per 1.73 m2 (%) | 42 (20.2) |
| Logistic EuroScore (%) | 14.8 ± 1.4 |
All patients underwent TF TAVI with an overall procedural success rate of 98.1%. Procedural characteristics are summarised: Forty-nine percent of patients received a balloon expandable device [Edwards Sapien XT (27.4%) and Edwards Sapien 3 (21.6%)] and 51% of patient received a self-expandable prosthesis [Medtronic Corevalve (37.5%) and Medtronic Evolut R (13.9%)]. Seventy-three (35.1%) patients underwent pre-TAVI balloon aortic valvuloplasty (BAV). Thirty-seven patients (17.8%) required post-dilatation following TAVI for AR with 27 patients (13%) with residual grade ≥ 2 AR at the end of the procedure. Four patients (1.9%) required emergency cardiac surgery, one patient for coronary artery obstruction, two patients for left ventricular perforation and one patient for right ventricular perforation following temporary wire placement. There were 10 (4.8%) peri-procedural deaths. Thirty-day outcomes according to the VARC-2 criteria are summarised in Table 2.
| All patients (n = 208) | |
| All-cause death | 12 (5.8) |
| Coronary obstruction (%) | 1 (0.05) |
| Stroke | 9 (4.3) |
| PPM implantation | 38 (18.3) |
| Minor vascular complication | 8 (3.8) |
| Major vascular complication | 8 (3.8) |
| Minor bleed | 34 (16.3) |
| Major bleed | 23 (11.1) |
| Life-threatening bleeding | 8 (3.8) |
| Valve related dysfunction | 0 (0) |
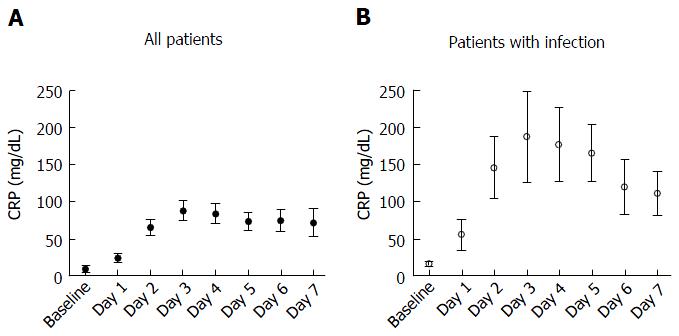
The baseline CRP (measured in 87.7% of patients) was 8.9 ± 2.5 mg/dL for total study population. Following TAVI this significantly increased reaching a peak on day 3 of 87.6 ± 5.5 mg/dL (measured in 77.6% of patients), P < 0.001 (Figure 1A). As would be expected, patients who developed clinical signs and symptoms of sepsis had significantly increased levels of CRP (n = 8) when compared to all patients which at day 3 was 187.7 ± 6.1 representing a 21-fold increase when compared to baseline levels (P < 0.001, Figure 1B).
Following exclusion of patients with clinical evidence of infection, peak (day 3) CRP levels were compared to determine the impact of baseline clinical factors upon maximal CRP release following TAVI. The presence of diabetes mellitus was associated with a significantly higher peak CRP level at day 3 (78.4 ± 3.2 vs 92.2 ± 4.4, P < 0.001). The presence of hypertension (75.2 ± 4.1 vs 93.1 ± 3.2, P = 0.22), previous PCI (70.6 ± 3.9 vs 82.2 ± 5.2, P = 0.39), previous cardiac surgery (87.4 ± 3.1 vs 93.9 ± 3.4, P = 0.65) or smoking (99.6 ± 3.7 vs 78.1 ± 3.3, P = 0.31) did not impact upon the peak CRP following TAVI.
There was no difference in peak CRP release following balloon-expandable or self-expandable TAVI implantation (94.8 ± 9.1 vs 81.9 ± 6.9, P = 0.34) or if post-dilatation was required (86.9 ± 6.3 vs 96.6 ± 5.3, P = 0.42). There was a difference in maximal CRP release when pre-TAVI balloon aortic valvuloplasty was performed (110.1 ± 8.9 vs 51.6 ± 3.7, P < 0.001). Peak CRP was not found to be different between patients with residual ≥ 2 AR and those that had residual < 2 AR (71.9 ± 7.4 vs 88.9 ± 7.9, P = 0.28). The development of a major vascular complication did result in a significantly increased maximal CRP release (153.7 ± 11.9 vs 83.3 ± 7.4, P = 0.02) and there was a trend toward a higher peak CRP following major/life-threatening bleeding (113.2 ± 9.3 vs 82.7 ± 7.5, P = 0.12) although this did not reach statistical significance.
Both CRP levels at baseline [hazard ratio (HR) per unit increase 0.98, 0.94-1.03, P = 0.42] and peak levels at day 3 (HR per unit increase: 1.01, 0.98-1.02, P = 0.18) were not found to be predictors of 30-d mortality on univariate analysis. We also did not find the magnitude of change in CRP (the difference between peak and baseline levels) to be a predictor of 30-d mortality (HR per unit increase: 0.92, 0.83-1.14, P = 0.33). ROC analysis further confirmed that both baseline [area under the curve (AUC): 0.42] and peak levels (AUC: 0.48) of CRP was a poor predictive tool for 30-d mortality in this study population.
The principal findings are: (1) CRP universally increases following TAVI reaching a peak at day 3; (2) the presence of diabetes mellitus was associated with a significant increase in the peak CRP following TAVI; (3) procedurally, the use of balloon aortic valvuloplasty during the procedure and the development of a major vascular complication resulted in a significant increase in the peak CRP; and (4) the peak CRP did not predict 30-d adverse outcomes.
Inflammation plays a central role in the pathogenesis and progression of AS[13,18,19]. The treatment of AS also results in activation of inflammatory pathways with more invasive treatment options (e.g., SAVR) associated with more inflammation in comparison to less invasive treatment options (e.g., TF TAVI)[16]. In addition to the magnitude of inflammation, persistently elevated markers of inflammation have been shown to be negatively associated with outcomes including mortality[14,20,21]. In agreement with previous reports, we found that CRP increased in all patients following TF TAVI reaching a peak level at day 3[16,22].
CRP in diabetes mellitus has been shown to be a predictor of cardiovascular events and outcomes[23,24]. After excluding patients with clinical signs and symptoms of infection we found that the presence of diabetes mellitus resulted in a significantly increased peak release in CRP following TAVI. This may explain worse outcomes in this patient sub-group[25] and should be considered when interpreting CRP results following TAVI and also when counselling patients with regards to risk pre-procedurally. We did not find any other baseline clinical characteristic (e.g., smoking, hypertension) to have an impact upon CRP dynamics following TAVI.
The impact of specific procedural factors upon CRP dynamics is poorly characterised in patients undergoing TF TAVI. We did not find a difference in the peak CRP between patients that were treated with a BE or SE valve possibly suggesting that they are both equally traumatic. Interestingly, the use of pre-implantation BAV was associated with a significant increase in the peak CRP at day 3, whilst the requirement for post-dilatation or the extent of residual AR did not impact upon maximal CRP release. This finding may represent the increased trauma to the native valvular apparatus and systemic debris shower resulting in greater release of CRP, although this may also reflect disease severity of the native valve that required a BAV rather than direct valvular implantation. Nonetheless, it is important for physicians managing patients following TAVI to be aware of the procedural specifics when interpreting CRP results in the post-operative period.
Unsurprisingly, the development of a major vascular complication resulted in a greater release of CRP, likely reflecting further trauma associated with peripheral vessel intervention and also longer procedural times. The requirement for a blood transfusion, in our study population, did not impact upon CRP dynamics in the post-procedural period.
CRP has been shown to be a useful prognostic tool[22] following TAVI, however in our study population, this was not found to be the case, possibly due to the relative small numbers of patients and short follow-up.
This study has some limitations. This was a retrospective study with treatment strategy (e.g., prosthesis selection, use of BAV) at the operator’s discretion and so the effect of selection bias cannot be excluded. Patient numbers were relatively small with limited follow-up and so the study may be underpowered to detect the predictive value of CRP upon outcomes. Finally, we did not measure the role of other markers of inflammation that in combination with CRP may have augmented its usefulness, although this study reflects routine clinical practice and makes the results directly applicable to a contemporary TAVI service.
In conclusion, TAVI results in increases in serum CRP levels reaching a peak at day 3 in all patients. CRP increase is further increased in patients with diabetes mellitus, those that underwent pre-TAVI BAV and patients that suffered major vascular complications. In addition to the bedside evaluation of patients, careful attention should be paid to baseline clinical characteristics and procedural factors when interpreting CRP to aid in the management and risk assessment of patients following TAVI.
Transcatheter aortic valve implantation (TAVI) is now the established treatment of choice for the management of patients presenting with severe symptomatic aortic stenosis (AS) who are deemed inoperable or of high surgical risk. The post-procedural management of these patients is complex due to their concomitant co-morbidities. In combination with the clinical evaluation of patients, the C reactive protein (CRP), is commonly measured to aid in diagnosis. However, the CRP is non-specific for infection and misinterpretation can result in misdiagnosis, inappropriate antibiotic therapy (and associated adverse effects) and prolonged in-hospital stay. Understanding CRP dynamics following TAVI is therefore critical in the post-procedural management of these patients.
The role of inflammation in both the pathogenesis of AS and its roles in repair, recovery and predicting outcomes following TAVI are currently important areas of investigation in this area.
This study demonstrates that CRP levels increase in all patients following TAVI but we here identify both specific patient and procedural factors that may result in a greater magnitude of change in CRP, that should be considered in the management of these complex patients in the post-operative period.
The findings of this study highlight the importance of taking into consideration not only the clinical condition of the patient but also baseline patient characteristics and procedural factors when interpreting CRP levels following TAVI. Into the future, research will focus on interventions to reduce inflammation peri- and post-procedurally to investigate if this will have an effect on outcomes.
TAVI is a technique by which a bioprosthetic aortic valve can be implanted in a minimally invasive fashion by delivering a catheter-mounted valve to the aortic annulus. The CRP is an acute phase protein synthesized and released by the liver.
The study was nicely executed and the text is well written.
Manuscript source: Invited manuscript
P- Reviewer: den Uil CA, Lin GM S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3079] [Cited by in F6Publishing: 3023] [Article Influence: 167.9] [Reference Citation Analysis (0)] |
| 2. | Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, Barnes ME, Tajik AJ. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111:3290-3295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 562] [Cited by in F6Publishing: 533] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 3. | Ross J, Braunwald E. Aortic stenosis. Circulation. 1968;38:61-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 480] [Cited by in F6Publishing: 516] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5086] [Cited by in F6Publishing: 5148] [Article Influence: 367.7] [Reference Citation Analysis (0)] |
| 5. | Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, Webb JG, Mack MJ, Douglas PS, Thourani VH. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485-2491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 586] [Cited by in F6Publishing: 607] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 6. | Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477-2484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1153] [Cited by in F6Publishing: 1218] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 7. | Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4547] [Cited by in F6Publishing: 4639] [Article Influence: 356.8] [Reference Citation Analysis (0)] |
| 8. | Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Kleiman NS. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1971] [Cited by in F6Publishing: 2023] [Article Influence: 202.3] [Reference Citation Analysis (0)] |
| 9. | Conrotto F, D’Ascenzo F, Francesca G, Colaci C, Sacciatella P, Biondi-Zoccai G, Moretti C, D’Amico M, Gaita F, Marra S. Impact of access on TAVI procedural and midterm follow-up: a meta-analysis of 13 studies and 10,468 patients. J Interv Cardiol. 2014;27:500-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | van der Boon RM, Nuis RJ, Benitez LM, Van Mieghem NM, Perez S, Cruz L, van Geuns RJ, Serruys PW, van Domburg RT, Dager AE. Frequency, determinants and prognostic implications of infectious complications after transcatheter aortic valve implantation. Am J Cardiol. 2013;112:104-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Falcone M, Russo A, Mancone M, Carriero G, Mazzesi G, Miraldi F, Pennacchi M, Pugliese F, Tritapepe L, Vullo V. Early, intermediate and late infectious complications after transcatheter or surgical aortic-valve replacement: a prospective cohort study. Clin Microbiol Infect. 2014;20:758-763. [PubMed] [Cited in This Article: ] |
| 12. | Mohler ER, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522-1528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 656] [Cited by in F6Publishing: 660] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 13. | Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kiliç R, Sarikoç A, Piñol R, Hagl S, Lang S. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14:80-87. [PubMed] [Cited in This Article: ] |
| 14. | Schewel D, Frerker C, Schewel J, Wohlmuth P, Meincke F, Thielsen T, Kreidel F, Kuck KH, Schäfer U. Clinical impact of paravalvular leaks on biomarkers and survival after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2015;85:502-514. [PubMed] [Cited in This Article: ] |
| 15. | Husser O, Núñez J, Núñez E, Holzamer A, Camboni D, Luchner A, Sanchis J, Bodi V, Riegger GA, Schmid C. Tumor marker carbohydrate antigen 125 predicts adverse outcome after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2013;6:487-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Erdoes G, Lippuner C, Kocsis I, Schiff M, Stucki M, Carrel T, Windecker S, Eberle B, Stueber F, Book M. Technical Approach Determines Inflammatory Response after Surgical and Transcatheter Aortic Valve Replacement. PLoS One. 2015;10:e0143089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438-1454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1345] [Cited by in F6Publishing: 1414] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 18. | Imai K, Okura H, Kume T, Yamada R, Miyamoto Y, Kawamoto T, Watanabe N, Neishi Y, Toyota E, Yoshida K. C-Reactive protein predicts severity, progression, and prognosis of asymptomatic aortic valve stenosis. Am Heart J. 2008;156:713-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Galante A, Pietroiusti A, Vellini M, Piccolo P, Possati G, De Bonis M, Grillo RL, Fontana C, Favalli C. C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J Am Coll Cardiol. 2001;38:1078-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Dahl JS, Videbæk L, Poulsen MK, Rudbaek TR, Christensen NL, Pellikka PA, Rasmussen LM, Møller JE. Relation of osteoprotegerin in severe aortic valve stenosis to postoperative outcome and left ventricular function. Am J Cardiol. 2013;112:1433-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Sinning JM, Scheer AC, Adenauer V, Ghanem A, Hammerstingl C, Schueler R, Müller C, Vasa-Nicotera M, Grube E, Nickenig G. Systemic inflammatory response syndrome predicts increased mortality in patients after transcatheter aortic valve implantation. Eur Heart J. 2012;33:1459-1468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Krumsdorf U, Chorianopoulos E, Pleger ST, Kallenbach K, Karck M, Katus HA, Bekeredjian R. C-reactive protein kinetics and its prognostic value after transfemoral aortic valve implantation. J Invasive Cardiol. 2012;24:282-286. [PubMed] [Cited in This Article: ] |
| 23. | Pfützner A, Forst T. High-sensitivity C-reactive protein as cardiovascular risk marker in patients with diabetes mellitus. Diabetes Technol Ther. 2006;8:28-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Tabák AG, Kivimäki M, Brunner EJ, Lowe GD, Jokela M, Akbaraly TN, Singh-Manoux A, Ferrie JE, Witte DR. Changes in C-reactive protein levels before type 2 diabetes and cardiovascular death: the Whitehall II study. Eur J Endocrinol. 2010;163:89-95. [PubMed] [Cited in This Article: ] |
| 25. | Conrotto F, D’Ascenzo F, Giordana F, Salizzoni S, Tamburino C, Tarantini G, Presbitero P, Barbanti M, Gasparetto V, Mennuni M. Impact of diabetes mellitus on early and midterm outcomes after transcatheter aortic valve implantation (from a multicenter registry). Am J Cardiol. 2014;113:529-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |