Published online Jul 26, 2016. doi: 10.4330/wjc.v8.i7.401
Peer-review started: February 26, 2016
First decision: April 15, 2016
Revised: May 2, 2016
Accepted: May 31, 2016
Article in press: June 2, 2016
Published online: July 26, 2016
In advanced heart failure (HF), chronic inotropic therapy with intravenous milrinone, a phosphodiesterase III inhibitor, is used as a bridge to advanced management that includes transplantation, ventricular assist device implantation, or palliation. This is especially true when repeated attempts to wean off inotropic support result in symptomatic hypotension, worsened symptoms, and/or progressive organ dysfunction. Unfortunately, patients in this clinical predicament are considered hemodynamically labile and may escape the benefits of guideline-directed HF therapy. In this scenario, chronic milrinone infusion may be beneficial as a bridge to introduction of evidence based HF therapy. However, this strategy is not well studied, and in general, chronic inotropic infusion is discouraged due to potential cardiotoxicity that accelerates disease progression and proarrhythmic effects that increase sudden death. Alternatively, chronic inotropic support with milrinone infusion is a unique opportunity in advanced HF. This review discusses evidence that long-term intravenous milrinone support may allow introduction of beta blocker (BB) therapy. When used together, milrinone does not attenuate the clinical benefits of BB therapy while BB mitigates cardiotoxic effects of milrinone. In addition, BB therapy decreases the risk of adverse arrhythmias associated with milrinone. We propose that advanced HF patients who are intolerant to BB therapy may benefit from a trial of intravenous milrinone as a bridge to BB initiation. The discussed clinical scenarios demonstrate that concomitant treatment with milrinone infusion and BB therapy does not adversely impact standard HF therapy and may improve left ventricular function and morbidity associated with advanced HF.
Core tip: Heart failure (HF) patients requiring chronic inotropic support are considered hemodynamically labile and may escape the benefits of evidence based HF therapy (HFTx). Chronic milrinone infusion may be beneficial as a bridge to introduction of HFTx. We discuss evidence that intravenous milrinone support may allow introduction of beta blocker (BB). We propose that HF patients who are intolerant to BB therapy may benefit from intravenous milrinone as a bridge to BB initiation. When used together, BB mitigates cardiotoxic effects and decreases the risk of arrhythmias associated with milrinone. Whereas, milrinone does not attenuate the clinical benefits of BB therapy.
- Citation: Jaiswal A, Nguyen VQ, Le Jemtel TH, Ferdinand KC. Novel role of phosphodiesterase inhibitors in the management of end-stage heart failure. World J Cardiol 2016; 8(7): 401-412
- URL: https://www.wjgnet.com/1949-8462/full/v8/i7/401.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i7.401
Heart failure (HF) is a chronic progressive disease with high morbidity and in advanced stages with an annual mortality > 50%; and prevalence is projected to rise[1-3]. Although the long-term benefit of beta-blocker (BB) in advanced HF is well established[4], many patients may be intolerant due to the negative hemodynamic impact of acute therapy and escape the benefits of HF therapy[4-7]. In such patients with advanced HF, chronic inotrope support is used as a bridge to transplantation, ventricular assist device, or palliation strategy for clinical and hemodynamic improvement. However, the use of chronic inotrope therapy as a bridge to introduction of HF therapy, specifically BB therapy, has not been effectively explored. Furthermore, chronic inotropic support is discouraged in advanced HF patients due to increased sudden death and accelerated disease progression[8,9]. In inotrope dependent advanced HF patients, combination therapy with intravenous milrinone infusion and BB provide a unique opportunity.
Concomitant therapy with BB and inotropes has been reported; however only type IIIA phosphodiesterase inhibitors (PDEI) such as milrinone and enoximone (an PDEI agent available in oral and intravenous formulations in Europe) have demonstrated a positive impact on hospitalization and functional status[10-15]. Both milrinone and enoxamone have shown to improve left ventricular ejection fraction (LVEF) when used in combination with BBs[12,16,17]. However, latest HF management guidelines do not comment on this dual therapy approach and recommends intravenous milrinone infusion only as bridge to advanced management or palliation in refractory end-stage HF[2,18,19].
This review discusses the beneficial effects of combining milrinone infusion and BB therapy in advanced HF. When used together, BB attenuates the cardiotoxicity and accentuates the hemodynamic effect of milrinone. Wherein, milrinone provides the hemodynamic support for introduction of BB therapy. Further, BB therapy decreases the risk of adverse arrhythmias associated with chronic PDEI. Finally, molecular pathways supporting beneficial effects of combination therapy with milrinone infusion and BB therapy are discussed. The index cases to be discussed demonstrate improvement in LVEF after concomitant treatment with carvedilol and chronic milrinone infusion in end-stage HF with severe functional limitation.
Intravenous milrinone is typically used in patients with acute systolic HF with signs or symptoms of end organ hypoperfusion[2,18,19]. However, inotropic support may be difficult or impossible to wean and prolonged support may be required.
The earliest use of chronic inotropic infusion as viable management option in end-stage HF patients was in 1987[20]. Mehra et al[21] reported a 72% survival on long-term milrinone support with a mean duration of 160 d in advanced HF patients awaiting transplantation. Brozena et al[22] found similar results in a study of 60 patients committed to home milrinone with an 88.3% survival rate to heart transplantation. In a prospective randomized study that included 19 hospitalized patients who received milrinone therapy, Aranda et al[23] showed that 84% survived to receive heart transplantation with a mean waiting of 60 ± 45 d.
In advanced HF patients who are transplant ineligible, success of long-term inotrope therapy has been modest. Harjai et al[24] reported a decrease in the number of hospital admissions from 2.7 ± 2.6 to 1.3 ± 1.3 (P = 0.056) and length of hospital stay from 20.9 ± 12.7 to 5.5 ± 5.4 d (P = 0.0004) with improvement in NYHA functional class from 4.0 ± 0.0 to 2.7 ± 0.9 (P < 0.0001) in 24 patients with LVEF < 30%, chronic inotrope-dependence and intolerance to oral HF agents. The benefit of therapy was at the expense of eight deaths (38%) after 2.8 ± 1.7 mo of home IV inotropic therapy. Hershberger et al[25] showed a 3, 6 and 12 mo mortality of 51%, 26% and 6%, respectively, in 36 inotrope-dependent patients with refractory HF on high-dose milrinone (mean dose: 0.6 ± 0.3 mcg/kg per minute). Additionally, using Medicare data, Hauptman et al[26] reported reductions in hospital days at all time points (30, 60 and 180 d) but was negatively counterbalanced by a mortality rate exceeding 40% at 6 mo in 331 patients on chronic inotrope therapy. In a single center retrospective analysis of 56 inotrope dependent, transplant ineligible HF patients, Gorodeski et al[27] reported 62% mortality and 48% hospitalization during a median follow-up of 130 d. However, in a recent single center study of 197 contemporary HF patients, Hashim et al[28] reported an overall median survival of 18 mo on continuous inotropic therapy. Median survival was 9 mo in whom inotrope therapy was intended as palliation, with a 1-year actuarial survival of 48% and a 2-year actuarial survival of 38%. Among all patients placed on inotropes, those on milrinone had a better survival than on dobutamine. The authors proposed that the modest improvement in survival compared to prior studies may be related to utilization of HF medical therapy and electrophysiologic devices that treat arrhythmias.
In the largest study to date, the PROMISE (Prospective Randomized Milrinone Survival Evaluation) trial randomized 1088 HF patients with NYHA functional class III or IV to placebo or oral milrinone[29]. The milrinone group had 28% higher mortality at 6 mo. However, it is noteworthy that patients did not have defibrillators, and those requiring BB were excluded. Moreover, the study did not evaluate hemodynamics at enrollment with milrinone therapy. Secondary analysis of the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study revealed a neutral to beneficial effect of milrinone on 60 d cardiovascular hospitalizations and composite of death and readmission in nonischemic cardiomyopathy but harmful effect in ischemic cardiomyopathy[30]. In addition, it is not clear whether the mortality on chronic inotropic therapy is above and beyond that of patients with end-stage HF where medical options are limited, specifically those with resting hemodynamic decompensation who are not candidates for advanced management[9].
In the light of existing evidence (Table 1), the American Heart Association/American College of Cardiology HF management consensus guideline classifies chronic inotrope infusion in refractory HF as a class IIb indication/level of evidence B due to a lack of randomized controlled trials supporting morbidity and mortality benefits[2,18].
| Ref. | Aim of study | Background beta blocker therapy | Study size n (total) | HF symptoms | Trial duration | Major findings/conclusion | Impact of therapy on LVEF | Complications/adverse events | Inotrope weaning rate |
| Packer et al[29], 1991 | Effect of oral milrinone on mortality of pts with symptomatic chronic HF on conventional therapy | No | 1088 | 100% NYHA III-IV 42% NYHA IV | Median F/U duration 6.1 mo (stopped early due to adverse effects) | 28% increased mortality with milrinone (30% vs 24%) | Not reported | Syncope palpitations hypotension headache blurry vision | Not reported |
| Böhm et al[16], 1997 | Metoprolol restores the reduction of the inotropic effect of the cAMP-phosphodiesterase inhibitor milrinone, independent of beta-adrenoceptor | Yes (100%) | 15 | NYHA II or III | 6 mo | Treatment with metoprolol increased LVEF, fractional shortening and submaximal exercise tolerance and reduced heart rate, plasma norepinephrine concentrations | Addition of metoprolol improved EF (%) from 24.6 ± 1.5 to 40.3 ± 3.6 | Not reported | Not reported |
| After metoprolol treatment, milrinone increased fractional shortening but had no effect before beta-blocker treatment | |||||||||
| Effect of dobutamine was completely antagonized by treatment with metoprolol | |||||||||
| Shakar et al[12], 1998 | Clinical impact of combined therapy with enoximone and beta blocker | Yes (80%) | 30 | NYHA IV | Mean duration of combination therapy was 9.4 ± 1.8 mo; mean length of F/U was 20.9 ± 3.9 mo | Combination therapy with enoximone and beta blocker improved EF and functional status in severe HF | LVEF increased from 17.7 ± 1.6% to 27.6 ± 3.4% (P = 0.01) NYHA improved from 4 to 2.8 (P = 0.0001) | 2 sudden deaths | 48% were weaned off enoximone |
| Yamani et al[67], 2001 | Clinical outcome and economic cost of dobutamine-based and milrinone-based therapy in patients with ADHF | Yes 20% (18% milrinone grp) | 329 (60 milrinone grp) | 100% NYHA IV | Retrospective review of ADHF admissions | No difference in the in-hospital mortality rate or clinical outcomes | Not reported | No difference in adverse effects between the grps (20% pts in milrinone grp with either NSVT or VT) | Not reported |
| Lowes et al[32], 2001 | Efficacy of milrinone vs dobutamine in patients with decompensated heart failure on chronic carvedilol therapy | Yes (100%) | 20 | 100% NYHA II-IV | Acute therapy | Dobutamine has less favorable hemodynamic effects in patients treated chronically with carvedilol | Not reported | Not reported | Not reported |
| Kumar et al[33], 2001 | Carvedilol titration in NYHA class IIIb/IV on milrinone therapy as compared to class II/IIIa CHF without milrinone | Yes (90%) | 32 | Class II-IV | Mean: 24 wk | Successful carvediolol uptitration in NYHA III-b/IV can be achieved at similar rates as in NYHA II/IIIa in the presence of stable chronic milrinone therapy | Not reported | No statistical difference in adverse events among the two grps | 53% patients were weaned off milrinone infusions in a mean of 8.4 ± 8.4 wk |
| Metra et al[13], 2002 | Hemodynamic effects of dobutamine and enoximone before and after 9-12 mo of beta-blocker therapy with metoprolol or carvedilol in chronic HF | Yes (100%) | 34 | NYHA II-IV | 9-12 mo | Beta blockers significantly inhibit the favorable hemodynamic response to dobutamine. No attenuation occurred with beta blockers and enoximone | Not reported | Not reported | Not reported |
| Cuffe et al[68], 2002 | Short-term milrinone in addition to standard therapy to improve outcomes in pts with ADHF | Yes (22%) | 949 | 93% NYHA III-IV | Treatment for up to 72 h, 60 d F/U | Milrinone was associated with higher rate of treatment failure at 48 h due to AE (12.6% vs 2.1%) | Not reported | Hypotension, (SBP < 80 mmHg); 10.7% with milrinone Significant atrial arrhythmias during index hospitalization; 4.6% | Not reported |
| Felker et al[30], 2003 | To assess the interaction between HF etiology and response to milrinone in ADHF | Yes (23%) | 949 | 93% NYHA III-IV | Treatment up to 72 h with 60 d F/U | In ischemic HF, milrinone was associated with worse outcomes: 60 d mortality or hospitalization: 42% vs 36% placebo; in-hospital mortality 5% vs 1.6% placebo | Not reported | No difference in atrial or ventricular arrhythmias and hypotension in both grps | Not reported |
| In nonischemic HF, benefit was derived from milrinone: | |||||||||
| 60 d mortality or hospitalization: 28% vs 35% placebo; in-hospital mortality 2.6% vs 3.1% placebo | |||||||||
| Aranda et al[23], 2003 | Clinical outcomes and costs associated dobutamine vs milrinone in hospitalized pts awaiting cardiac transplantation | Yes (41% in dobutamine grp; 74% in milrinone grp) | 36 | Not reported presumably NYHA III-IV | Enrollment 17 mo | No difference between milrinone and dobutamine with respect to clinical outcomes or hemodynamic measures | Not reported | No difference in death of length of hospital stay | Not reported |
| Beta blocker use in dobutamine grp was associated with worsened pulmonary pressures and PCWP | |||||||||
| Brozena et al[22], 2004 | Feasibility and safety of continuous IV milrinone therapy administered at home in pts listed as status IB for heart transplant | Yes (73%) | 60 | NYHA II-III Peak VO2 11.4 mL/kg per minute | 43 mo F/U | 88.3% of pts underwent OHT 3.2% died before transplant | Not reported | 8% hospitalized for IV line infection | 1 pt weaned off based on clinical improvement |
| Abraham et al[69], 2005 | In-hospital mortality in ADHF pts receiving treatment with 1 of 4 vasoactive meds (NTG, nesiritide, milrinone, dobutamine) | Yes (56% milrinone grp) | 2021 (milrinone) | 100% NYHA IV | 10/01-7/03 | Worse inpatient mortality and longer LOS with IV inotropes | N/A | N/A | N/A |
| Feldman et al[70], 2007 | Whether low-dose oral enoximone could wean pts with end-stage HF from IV inotropic support | Yes (40%) | 201 | 100% NYHA III-IV | 26 wk | 30 d after weaning, 51% of placebo pts and 61.40% enoximone pts were alive and free of IV inotropic therapy | Not reported | Dyspnea, 5% enoximone vs 0% placebo, P < 0.05 | |
| At 60 d, the wean rate was 30% in placebo grp and 46.5% in enoximone grp Kaplan-Meier curves demonstrated a trend towards decreased in time to death or reinitiation of IV inotropic therapy over the 182-d study period and a reduction at 60 d and 90 d after weaning in the enoximone grp | |||||||||
| Elkayam et al[71], 2007 | Six month risks of all-cause mortality and all-cause mortality plus rehospitalization associated with the use of vasodilators, inotropes, and their combinations | Yes (62%) | 433; 75 (vasodilator); 133 (IV inotrope); 47 (both); 178 (neither inotrope/vasodilator) | Mean peak VO2 10.0 | N/A | Worse 6 mo mortality and either mortality/re-hospitalization with inotropes (whether alone or with vasodilator) | Not reported | N/A | N/A |
| Gorodeski et al[27], 2009 | Relationship between choice of dobutamine or milrinone and mortality in inotrope dependent stage D HF pts | Yes [5% (dob) vs 34% (mil)] | 112 | Not reported presumably NYHA III-IV | Median F/U of 130 d | Higher mortality in the dobutamine grp; No difference in mortality between inotrope type in propensity matched cohort | Not reported | Not reported | Not reported |
| Metra et al[37], 2009 | Effects of low dose enoximone on symptoms, exercise capacity, and major clinical outcomes in pts with advanced HF who were also treated with beta blockers and other guideline recommended background therapy | ESSENTIALI Yes (83%) ESSENTIALII Yes (90%) | ESSENTIALI: 904 ESSENTIALII: 950 | 100% NYHA III-IV | Median F/U duration 16.6 mo | No difference in first co-primary endpoints: All cause mortality, all-cause mortality and CV hospitalizations | Not reported | Palpitations 8% enoximone vs 5% placebo, P = 0.01 | N/A |
Patients whose BB dosages have to be reduced or stopped have worse clinical outcomes than those in whom BB is maintained[31]. The use of intravenous PDEI permits successful initiation and up titration of BBs in HF patients who are intolerant to BB therapy[13,32-34]. Milrinone provides hemodynamic support by improving systolic and diastolic function, along with decreasing afterload and filling pressures, correcting some of the adverse effects of acute BB therapy[14]. Whether these hemodynamic benefits translate into clinical improvement has not been extensively studied. Kumar et al[33] assessed the tolerability of carvedilol titration and ability to wean inotrope support in a retrospective review of 32 patients with HF. Seventeen patients with NYHA functional class IIIb/IV HF (group I) who received intermittent milrinone infusion were compared to 15 patients with NYHA functional class II/IIIa symptoms (group II) who did not. Both groups were started on carvedilol 3.125 mg twice daily and titrated to 25 mg twice daily every 2 wk as tolerated. Milrinone infusion had no impact on carvedilol titration (88% vs 93%). At 8 wk, 53% patients in group I were successfully weaned off milrinone infusion. Those who could not be weaned had a 50% decrease in the frequency of infusions. The majority (63%) of group I patients improved by one or more functional class at the end of follow-up. Another retrospective review assessed BB tolerability in 16 patients with stage D HF on continuous milrinone infusion[35]. Twelve patients were started on metoprolol tartrate or carvedilol and the remaining four received only milrinone. After 6 mo, 92% of patients on milrinone were able to tolerate dual therapy with a BB. No significant changes in blood pressure and heart rate after were noted BB initiation. One patient in each group died, and rates of hospitalization for HF were similar (0.83/pt in combination group vs 0.5/pt in BB alone). While these studies suggest tolerability and symptomatic improvement with dual therapy, results cannot be unequivocally extrapolated due to the small sample sizes.
In a retrospective analysis, Zewail et al[36] reported hemodynamic and clinical outcomes of long-term combination therapy with intravenous milrinone and BB in 65 patients with severe HF (NYHA class IV and LVEF < 25%) refractory to oral medical therapy. Fifty-one patients (78%) successfully tolerated BB therapy while on intravenous milrinone, while 14 patients did not and thus received milrinone monotherapy. Functional class improved from NYHA class IV to II-III with combination therapy. While no patients in the milrinone-only arm could be weaned off, 47% patients (24/51) in the combination arm were successfully weaned off. The corrected QT interval was significantly prolonged in the monotherapy group (mean ± 436 ± 13 ms before vs 469 ± 28 ms after; P = 0.002), whereas the interval remained unchanged in the combination group. Most notably, survival at 3 years was 59% higher in the combination group vs the milrinone monotherapy group (P < 0.001). One died of sudden cardiac death on treatment day 116 in the combination group. Jiménez et al[10] carried out an observational study of 26 inotrope dependent patients (> 8 wk home inotrope support) with end stage HF, with 17 patients as bridge to transplantation and 9 patients as destination therapy. They reported an 85% survival at an average of 10 mo home inotropic therapy. The reported mortality rates in the above nonrandomized studies were consistent with randomized studies of similar HF patients[37].
Gattis et al[38] conducted a post-hoc analysis comparing patients receiving BB at the time of hospitalization to those who did not using the OPTIME-CHF study. The 949 patients with acute HF exacerbation were randomized to receive 48-72 h of intravenous milrinone vs placebo. In patients who were continued on BB on admission, there was no difference in the primary endpoint regardless of assignment to milrinone or placebo. Patients whose BB were withdrawn upon randomization to milrinone had worse outcomes (mortality 28.6% vs 7.7%, P-value not reported). Furthermore, patients who received both milrinone and BB during hospitalization had the lowest 60-d mortality (5.8%).
The findings of above studies suggest that combination therapy may reduce mortality and facilitate discontinuation of inotropic support in advanced HF. However, retrospective design and small sample sizes preclude firm conclusions on the impact of combination therapy on mortality, hospitalization, and symptomatic improvement. Further, as there is substantial evidence on BBs in mortality reduction, it would be unjustified to randomize BB vs placebo in milrinone treated patients with refractory HF. Larger observational studies would further elucidate the potential clinical benefits of combining BB with milrinone.
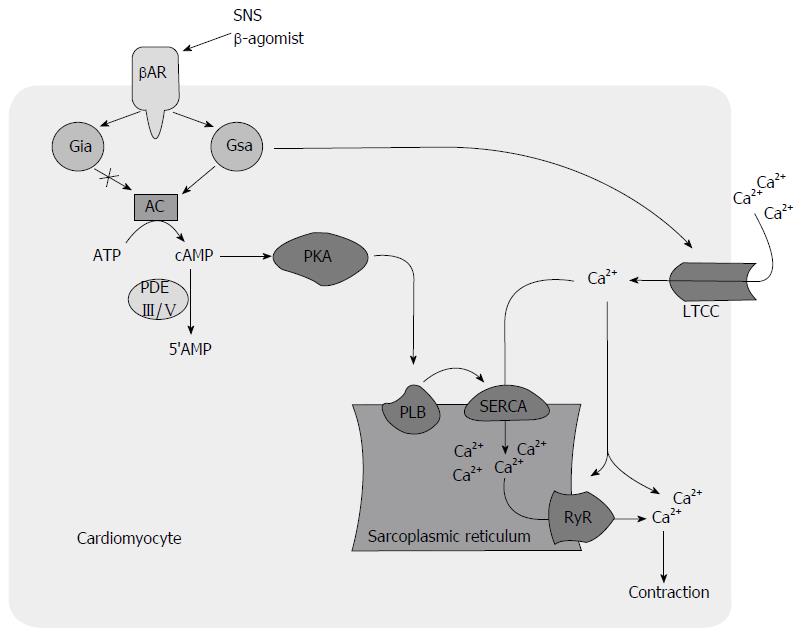
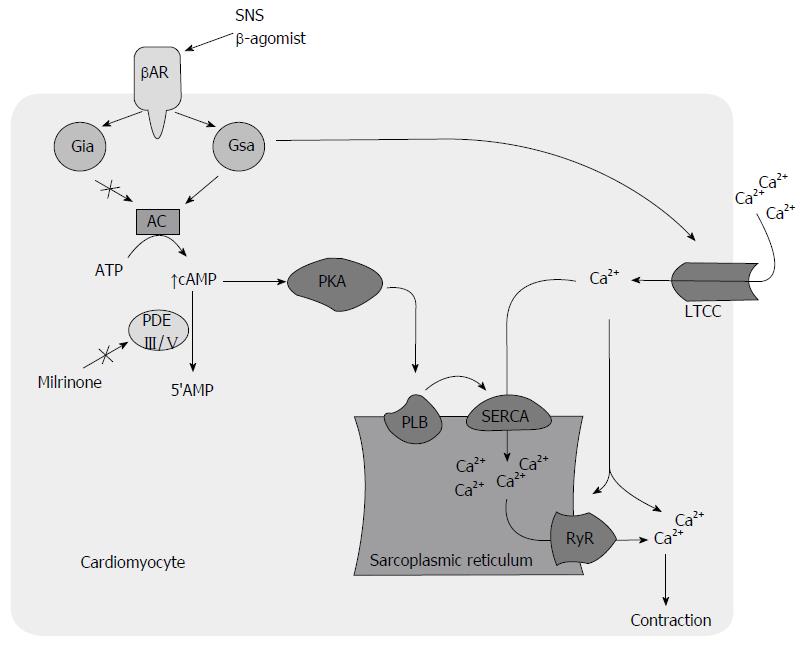
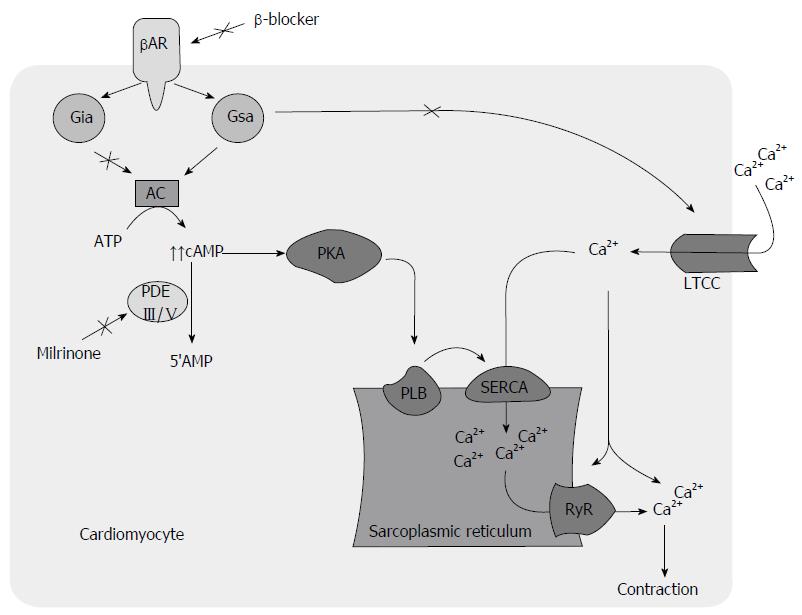
Defective calcium (Ca+) handling is thought to be a major contributor to mechanical and electrical dysfunction in HF (Figure 1)[39]. The increased mortality associated with PDEI therapy in HF is attributed to a proarrhythmic effect[29,40,41], contributing to increased sudden cardiac death and direct cardiomyocyte toxicity related to cyclic adenosine monophosphate (cAMP) mediated Ca+ overload and sustained beta-1-receptor pathway signaling (Figure 2)[21]. Recent investigations suggest that modulation of Ca+ handling may result in improvements in inotropy and lusitropy without increasing arrhythmogenesis and cardiotoxicity[39,42-44]. BBs have shown to attenuate these molecular responses[45-48] and may attenuate adverse effects associated with PDEIs (Figure 3)[49,50].
In the presence of BB, the harmful sustained B-receptor pathway signaling associated with HF, mediated through cAMP-independent G-a-stimulating protein coupling of Ca+ channels[51], is eliminated. The inotropic effect of PDEIs is still maintained through the phosphorylation of phopholamban on the sarcoplasmic reticulum (SR)[52-54]. Inotropic agents that act through inhibition of phospholamban are desirable and best tolerated[14,55]. Phospholamban phosphorylation causes decreased inhibition of SR calcium ATPase (SERCA) activity, resulting in its increased SR calcium uptake in diastole and subsequent increased release in cytosol in systole for augmented myocardial performance. This, in turn, results in increased diastolic and systolic functions[14]. Improvement in Ca+ handling, through targeted SERCA gene expression has shown to retard development of action potential duration alternans and hence decreased arrhythmogenesis[56]. This is further supported by an improved systolic and diastolic function without increase in heart rate in phospholamban knockout models, a maneuver that mimics phospholamban phosphorylation[57,58]. In addition, the delivery of pseudo-phosphorylated mutant of phopholamban into sheep heart using a viral vector reversed chronic pacing induced HF[59]. On the contrary, phosphorylation of L-type Ca+ channel leads to an increased Ca+ influx during the plateau phase of the action potential, resulting in increased intracellular Ca+ during both diastole and systole that causes a detrimental effect on diastolic function and arrhythmogenesis[14].
Using an extracorporeal circulation cardioplegia reperfusion model, Usta et al[60] showed evidence of decreased apoptosis with low dose milrinone on ex vivo human right auricle cardiomyocytes compared with controls. At lower concentrations, the most likely pharmacological target of PDEI is phopholamban as both are localized to SR[61,62]. A twelve-week treatment with lower dose of enoximone (≤ 50 mg three times daily) increased exercise capacity without increasing ventricular arrhythmias. This approach demonstrated favorable effects on degree of dyspnea and physician assessments of clinical status compared to placebo[61]. A contemporary observational study suggested better survival on low dose intravenous milrinone at 0.296 ± 0.092 mcg/kg per minute[28]. Although the short-term benefits have been documented, long-term efficacy and safety of low-dose PDEI remains to be demonstrated in controlled trials. In patients with advanced HF who do not tolerate BB therapy, we choose intravenous milrinone continuous infusion at low dose (< 0.5 μg/kg per minute) as this strategy is shown to augment cardiac function to permit BB therapy[61].
In addition, when used in combination, BB may enhance hemodynamic effects related to PDEI therapy by decreasing activity of upregulated inhibitory G-alpha-inhibitory protein activity[12,63]. The choice of BB to use in combination with a PDEI is uncertain. The use of B1-selective agent is suggested to be preferable as its blockade leads to increased B2-receptor-mediated signal transduction through cross-regulatory mechanisms[64], which is less cardiomyopathic[65] and may even prevent apoptosis[66]. The vasodilator effect of carvedilol can be additive to that of milrinone. However, this combination may be not desirable in patients with marginal blood pressures. The vasodilator property is less pronounced and response to milrinone is not compromised by additional vasodilation once the patient becomes stable[17].
Case1: A 67-year-old man with chronic cardiomyopathy with severely reduced systolic function with LVEF < 15% without significant epicardial coronary artery disease was impaired by six hospitalizations in five months and New York Heart Association (NYHA) class IV functional status. Due to inability to tolerate HF medicines and inadequate diuretic response, invasive hemodynamic assessment was performed. Elevated biventricular filling pressures and decreased cardiac output were noted, both of which improved 20% after milrinone bolus (0.5 mcg/kg per minute over 10 min) (Table 2). Due to refractory cardiomyopathy and hemodynamic findings, he was started on long-term continuous home milrinone infusion. Consequently, the patient tolerated carvedilol initiation and up-titration on outpatient follow-up. His functional class improved to NYHA class II-III and HF hospitalizations decreased to three in the subsequent nine months. Defibrillator interrogation throughout did not reveal significant arrhythmias. Nine months into treatment, LVEF improved to 35%-40% and milrinone was discontinued (Video core tip). The patient continued to thrive independent of milrinone therapy.
| Hemodynamic parameters | Patient 1 | Patient 2 | Reference values | ||
| Baseline | Post-milrinone loading | Baseline | Post-milrinone loading | ||
| RA (mmHg) | 15 | 15 | 5-7 | ||
| RV (mmHg) | 54/15 | Dec-58 | 15-30/1-5 | ||
| PA (mmHg) | 53/33 (40) | 56/21 (34) | 61/37 (45) | 15-30/4-10; mean < 20 | |
| PA O2 saturation | 49.50% | 57% | 60%-80% | ||
| PCWP (mmHg) | 29 | 15 | 30 | < 12 | |
| Cardiac output (L/min) | 5.1 | 7.1 | 3.3 | 6 | 4-8 |
| Cardiac index (L/min per meter squared) | 2.1 | 2.95 | 1.64 | 3.03 | 2.6-4.2 |
| PVR (WU) | 2.68 | 2.16 | 4.54 | < 3 WU | |
| Hemoglobin (g/dL) | 10.2 | 10.2 | 11.7 | 13.5-17.5 | |
Case 2: A 50-year-old man with chronic cardiomyopathy with severely reduced LVEF 10%-15% without significant epicardial coronary artery disease was admitted for decompensated HF with acute renal insufficiency and inadequate diuretic response. Invasive hemodynamics revealed elevated biventricular pressure with severely decreased cardiac output (Table 2). Intravenous mlrinone was initiated, permitting diuresis that led to a net 40-pound weight loss during the two-week hospitalization. The patient also underwent biventricular pacemaker implantation for cardiac resynchronization therapy. Over the ensuing year post-milrinone therapy, his ambulatory status improved from < 100 feet to > 6 city blocks. Defibrillator interrogation throughout the treatment duration did not reveal significant arrhythmias. Repeat LVEF after 10 mo improved to 20%-25% (Video core tip).
In patients with advanced HF, use of a combination therapy with low-dose intravenous milrinone infusion and BB offers an appealing strategy. In the treatment of advanced HF, we propose that chronic milrinone infusion be regarded as a “bridge to BB” in addition to the traditional bridge to advanced options or palliation strategy. Attempt at initiation and up-titration of BBs should be underscored in such patients. Milrinone provides hemodynamic support to initiate and up-titrate BB in the presence of BB-intolerance. Moreover, dual therapy improves symptoms and decreases hospitalization. Lastly, LVEF may improve with this approach without any ill-effects and significant arrhythmias, suggesting that this is a safe and effective therapeutic strategy in advanced refractory HF. Our experience with cases discussed above shows improvement in LVEF after concomitant use of BB and intravenous continuous low-dose milrinone. It is possible that the cases might not have been adherent to prescribed HF medications prior to use of intravenous milrinone, and the increased LVEF is purely a reflection of medical compliance. Systematic exploration involving large cohorts is required for further understanding as the population with advanced HF continues to expand.
Manuscript source: Invited manuscript
P- Reviewer: Bonanno C, den Uil CA, Landesberg G, Ueda H S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1230] [Cited by in F6Publishing: 1317] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 2. | Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1935] [Cited by in F6Publishing: 1923] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 3. | Chen-Scarabelli C, Saravolatz L, Hirsh B, Agrawal P, Scarabelli TM. Dilemmas in end-stage heart failure. J Geriatr Cardiol. 2015;12:57-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 19] [Reference Citation Analysis (0)] |
| 4. | Krum H, Sackner-Bernstein JD, Goldsmith RL, Kukin ML, Schwartz B, Penn J, Medina N, Yushak M, Horn E, Katz SD. Double-blind, placebo-controlled study of the long-term efficacy of carvedilol in patients with severe chronic heart failure. Circulation. 1995;92:1499-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 295] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Cohn JN, Fowler MB, Bristow MR, Colucci WS, Gilbert EM, Kinhal V, Krueger SK, Lejemtel T, Narahara KA, Packer M. Safety and efficacy of carvedilol in severe heart failure. The U.S. Carvedilol Heart Failure Study Group. J Card Fail. 1997;3:173-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 153] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Macdonald PS, Keogh AM, Aboyoun CL, Lund M, Amor R, McCaffrey DJ. Tolerability and efficacy of carvedilol in patients with New York Heart Association class IV heart failure. J Am Coll Cardiol. 1999;33:924-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3654] [Cited by in F6Publishing: 3187] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 8. | Francis GS, Bartos JA, Adatya S. Inotropes. J Am Coll Cardiol. 2014;63:2069-2078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Pinney SP, Stevenson LW. Chronic Inotropic Therapy in the Current Era: Old Wines With New Pairings. Circ Heart Fail. 2015;8:843-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Jiménez J, Jara J, Bednar B, Bauerlein J, Mallon S. Long-term (& gt; 8 weeks) home inotropic therapy as destination therapy in patients with advanced heart failure or as bridge to heart transplantation. Int J Cardiol. 2005;99:47-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Berger R, Strecker K, Hülsmann M, Frey B, Pacher R, Stanek B. Experience with beta-blocker therapy in patients with advanced heart failure evaluated for HTx. J Heart Lung Transplant. 2000;19:1081-1088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Shakar SF, Abraham WT, Gilbert EM, Robertson AD, Lowes BD, Zisman LS, Ferguson DA, Bristow MR. Combined oral positive inotropic and beta-blocker therapy for treatment of refractory class IV heart failure. J Am Coll Cardiol. 1998;31:1336-1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Metra M, Nodari S, D’Aloia A, Muneretto C, Robertson AD, Bristow MR, Dei Cas L. Beta-blocker therapy influences the hemodynamic response to inotropic agents in patients with heart failure: a randomized comparison of dobutamine and enoximone before and after chronic treatment with metoprolol or carvedilol. J Am Coll Cardiol. 2002;40:1248-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Shakar SF, Bristow MR. Low-level inotropic stimulation with type III phosphodiesterase inhibitors in patients with advanced symptomatic chronic heart failure receiving beta-blocking agents. Curr Cardiol Rep. 2001;3:224-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Hauptman PJ, Woods D, Prirzker MR. Novel use of a short-acting intravenous beta blocker in combination with inotropic therapy as a bridge to chronic oral beta blockade in patients with advanced heart failure. Clin Cardiol. 2002;25:247-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Böhm M, Deutsch HJ, Hartmann D, Rosée KL, Stäblein A. Improvement of postreceptor events by metoprolol treatment in patients with chronic heart failure. J Am Coll Cardiol. 1997;30:992-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Lowes BD, Simon MA, Tsvetkova TO, Bristow MR. Inotropes in the beta-blocker era. Clin Cardiol. 2000;23:III11-III16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-e239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4116] [Cited by in F6Publishing: 4473] [Article Influence: 406.6] [Reference Citation Analysis (0)] |
| 19. | Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 858] [Cited by in F6Publishing: 915] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 20. | Applefeld MM, Newman KA, Sutton FJ, Reed WP, Roffman DS, Talesnick BS, Grove WR. Outpatient dobutamine and dopamine infusions in the management of chronic heart failure: clinical experience in 21 patients. Am Heart J. 1987;114:589-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Mehra MR, Ventura HO, Kapoor C, Stapleton DD, Zimmerman D, Smart FW. Safety and clinical utility of long-term intravenous milrinone in advanced heart failure. Am J Cardiol. 1997;80:61-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Brozena SC, Twomey C, Goldberg LR, Desai SS, Drachman B, Kao A, Popjes E, Zimmer R, Jessup M. A prospective study of continuous intravenous milrinone therapy for status IB patients awaiting heart transplant at home. J Heart Lung Transplant. 2004;23:1082-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Aranda JM, Schofield RS, Pauly DF, Cleeton TS, Walker TC, Monroe VS, Leach D, Lopez LM, Hill JA. Comparison of dobutamine versus milrinone therapy in hospitalized patients awaiting cardiac transplantation: a prospective, randomized trial. Am Heart J. 2003;145:324-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Harjai KJ, Mehra MR, Ventura HO, Lapeyre YM, Murgo JP, Stapleton DD, Smart FW. Home inotropic therapy in advanced heart failure: cost analysis and clinical outcomes. Chest. 1997;112:1298-1303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Hershberger RE, Nauman D, Walker TL, Dutton D, Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;9:180-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Hauptman PJ, Mikolajczak P, George A, Mohr CJ, Hoover R, Swindle J, Schnitzler MA. Chronic inotropic therapy in end-stage heart failure. Am Heart J. 2006;152:1096.e1-1096.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail. 2009;2:320-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Hashim T, Sanam K, Revilla-Martinez M, Morgan CJ, Tallaj JA, Pamboukian SV, Loyaga-Rendon RY, George JF, Acharya D. Clinical Characteristics and Outcomes of Intravenous Inotropic Therapy in Advanced Heart Failure. Circ Heart Fail. 2015;8:880-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325:1468-1475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1632] [Cited by in F6Publishing: 1544] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 30. | Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, Gheorghiade M, O’Connor CM. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol. 2003;41:997-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 31. | Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of Beta-Blocker Withdrawal in Acute Decompensated Heart Failure: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2015;3:647-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 32. | Lowes BD, Tsvetkova T, Eichhorn EJ, Gilbert EM, Bristow MR. Milrinone versus dobutamine in heart failure subjects treated chronically with carvedilol. Int J Cardiol. 2001;81:141-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Kumar A, Choudhary G, Antonio C, Just V, Jain A, Heaney L, Papp MA. Carvedilol titration in patients with congestive heart failure receiving inotropic therapy. Am Heart J. 2001;142:512-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Constantinescu AA, Caliskan K, Manintveld OC, van Domburg R, Jewbali L, Balk AH. Weaning from inotropic support and concomitant beta-blocker therapy in severely ill heart failure patients: take the time in order to improve prognosis. Eur J Heart Fail. 2014;16:435-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Earl GL, Verbos-Kazanas MA, Fitzpatrick JM, Narula J. Tolerability of beta-blockers in outpatients with refractory heart failure who were receiving continuous milrinone. Pharmacotherapy. 2007;27:697-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Zewail AM, Nawar M, Vrtovec B, Eastwood C, Kar MN, Delgado RM. Intravenous milrinone in treatment of advanced congestive heart failure. Tex Heart Inst J. 2003;30:109-113. [PubMed] [Cited in This Article: ] |
| 37. | Metra M, Eichhorn E, Abraham WT, Linseman J, Böhm M, Corbalan R, DeMets D, De Marco T, Elkayam U, Gerber M. Effects of low-dose oral enoximone administration on mortality, morbidity, and exercise capacity in patients with advanced heart failure: the randomized, double-blind, placebo-controlled, parallel group ESSENTIAL trials. Eur Heart J. 2009;30:3015-3026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Gattis WA, O’Connor CM, Leimberger JD, Felker GM, Adams KF, Gheorghiade M. Clinical outcomes in patients on beta-blocker therapy admitted with worsening chronic heart failure. Am J Cardiol. 2003;91:169-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Lou Q, Janardhan A, Efimov IR. Remodeling of calcium handling in human heart failure. Adv Exp Med Biol. 2012;740:1145-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 40. | Cowley AJ, Skene AM. Treatment of severe heart failure: quantity or quality of life? A trial of enoximone. Enoximone Investigators. Br Heart J. 1994;72:226-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 88] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Uretsky BF, Jessup M, Konstam MA, Dec GW, Leier CV, Benotti J, Murali S, Herrmann HC, Sandberg JA. Multicenter trial of oral enoximone in patients with moderate to moderately severe congestive heart failure. Lack of benefit compared with placebo. Enoximone Multicenter Trial Group. Circulation. 1990;82:774-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 248] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013;123:46-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 43. | Bristow MR. Treatment of chronic heart failure with β-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res. 2011;109:1176-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 44. | Györke S, Carnes C. Dysregulated sarcoplasmic reticulum calcium release: potential pharmacological target in cardiac disease. Pharmacol Ther. 2008;119:340-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Mann DL, Kent RL, Parsons B, Cooper G. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992;85:790-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 614] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 46. | Shivalkar B, Van Loon J, Wieland W, Tjandra-Maga TB, Borgers M, Plets C, Flameng W. Variable effects of explosive or gradual increase of intracranial pressure on myocardial structure and function. Circulation. 1993;87:230-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 281] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Kendall MJ, Lynch KP, Hjalmarson A, Kjekshus J. Beta-blockers and sudden cardiac death. Ann Intern Med. 1995;123:358-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 186] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 48. | Mochizuki M, Yano M, Oda T, Tateishi H, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J Am Coll Cardiol. 2007;49:1722-1732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Gilbert EM, Olsen SL, Renlund DG, Bristow MR. beta-adrenergic receptor regulation and left ventricular function in idiopathic dilated cardiomyopathy. Am J Cardiol. 1993;71:23C-29C. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Bristow MR. Changes in myocardial and vascular receptors in heart failure. J Am Coll Cardiol. 1993;22:61A-71A. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 141] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Lader AS, Xiao YF, Ishikawa Y, Cui Y, Vatner DE, Vatner SF, Homcy CJ, Cantiello HF. Cardiac Gsalpha overexpression enhances L-type calcium channels through an adenylyl cyclase independent pathway. Proc Natl Acad Sci USA. 1998;95:9669-9674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Hoeper MM, Boeker KH. Overdose of metoprolol treated with enoximone. N Engl J Med. 1996;335:1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Travill CM, Pugh S, Noble MI. The inotropic and hemodynamic effects of intravenous milrinone when reflex adrenergic stimulation is suppressed by beta-adrenergic blockade. Clin Ther. 1994;16:783-792. [PubMed] [Cited in This Article: ] |
| 54. | Galie N, Branzi A, Magnani G, Melandri G, Caldarera I, Rapezzi C, Grattoni C, Magnani B. Effect of enoximone alone and in combination with metoprolol on myocardial function and energetics in severe congestive heart failure: improvement in hemodynamic and metabolic profile. Cardiovasc Drugs Ther. 1993;7:337-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Bristow MR, Shakar SF, Linseman JV, Lowes BD. Inotropes and beta-blockers: is there a need for new guidelines? J Card Fail. 2001;7:8-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol. 2009;2:686-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75:401-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 465] [Cited by in F6Publishing: 445] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 58. | del Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ. Targeting phospholamban by gene transfer in human heart failure. Circulation. 2002;105:904-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 59. | Kaye DM, Preovolos A, Marshall T, Byrne M, Hoshijima M, Hajjar R, Mariani JA, Pepe S, Chien KR, Power JM. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007;50:253-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 60. | Usta E, Mustafi M, Scheule AM, Ziemer G. Suppressing apoptosis with milrinone simulating extracorporeal circulation: a pilot study. Thorac Cardiovasc Surg. 2010;58:285-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Lowes BD, Higginbotham M, Petrovich L, DeWood MA, Greenberg MA, Rahko PS, Dec GW, LeJemtel TH, Roden RL, Schleman MM. Low-dose enoximone improves exercise capacity in chronic heart failure. Enoximone Study Group. J Am Coll Cardiol. 2000;36:501-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Dage RC, Okerholm RA. Pharmacology and pharmacokinetics of enoximone. Cardiology. 1990;77 Suppl 3:2-13; discussion 27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Sigmund M, Jakob H, Becker H, Hanrath P, Schumacher C, Eschenhagen T, Schmitz W, Scholz H, Steinfath M. Effects of metoprolol on myocardial beta-adrenoceptors and Gi alpha-proteins in patients with congestive heart failure. Eur J Clin Pharmacol. 1996;51:127-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Hall JA, Ferro A, Dickerson JE, Brown MJ. Beta adrenoreceptor subtype cross regulation in the human heart. Br Heart J. 1993;69:332-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, Yatani A, Dorn GW. Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101:1707-1714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 66. | Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210-2212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 380] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 67. | Yamani MH, Haji SA, Starling RC, Kelly L, Albert N, Knack DL, Young JB. Comparison of dobutamine-based and milrinone-based therapy for advanced decompensated congestive heart failure: Hemodynamic efficacy, clinical outcome, and economic impact. Am Heart J. 2001;142:998-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Cuffe MS, Califf RM, Adams KF, Benza R, Bourge R, Colucci WS, Massie BM, O’Connor CM, Pina I, Quigg R. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 915] [Cited by in F6Publishing: 824] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 69. | Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, Cheng ML, Wynne J. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 566] [Cited by in F6Publishing: 536] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 70. | Feldman AM, Oren RM, Abraham WT, Boehmer JP, Carson PE, Eichhorn E, Gilbert EM, Kao A, Leier CV, Lowes BD. Low-dose oral enoximone enhances the ability to wean patients with ultra-advanced heart failure from intravenous inotropic support: results of the oral enoximone in intravenous inotrope-dependent subjects trial. Am Heart J. 2007;154:861-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Elkayam U, Tasissa G, Binanay C, Stevenson LW, Gheorghiade M, Warnica JW, Young JB, Rayburn BK, Rogers JG, DeMarco T. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153:98-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |