Published online Dec 26, 2016. doi: 10.4330/wjc.v8.i12.695
Peer-review started: July 13, 2016
First decision: August 4, 2016
Revised: October 11, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: December 26, 2016
Advanced heart failure has been traditionally treated via either heart transplantation, continuous inotropes, consideration for hospice and more recently via left ventricular assist devices (LVAD). Heart transplantation has been limited by organ availability and the futility of other options has thrust LVAD therapy into the mainstream of therapy for end stage heart failure. Improvements in technology and survival combined with improvements in the quality of life have made LVADs a viable option for many patients suffering from heart failure. The question of when to implant these devices in those patients with advanced, yet still ambulatory heart failure remains a controversial topic. We discuss the current state of LVAD therapy and the risk vs benefit of these devices in the treatment of heart failure.
Core tip: Heart failure remains the most common diagnosis in patients discharged from the hospital. In its most advanced stages, it bears a grim prognosis and there are only a limited number of treatments that can truly change the course of the disease. Advancements in left ventricular assist device technology have enticed clinicians to expand their role in earlier ambulatory, but advanced heart failure. Here, we describe the current equilibrium between early implantation and risks of the current technology.
- Citation: Cerier E, Lampert BC, Kilic A, McDavid A, Deo SV, Kilic A. To ventricular assist devices or not: When is implantation of a ventricular assist device appropriate in advanced ambulatory heart failure? World J Cardiol 2016; 8(12): 695-702
- URL: https://www.wjgnet.com/1949-8462/full/v8/i12/695.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i12.695
Approximately 5.7 million people in the United States have heart failure (HF) and more than half of those who develop heart failure die within five years of the diagnosis[1]. As the population ages, the incidence of HF is expected to concurrently increase highlighting the importance of a continuation of need for developing more effective therapies. In the current spectrum of options, heart transplantation remains the gold standard for those with advanced heart failure[2]. The limitation of organ availability and unpredictability of rapidly advancing multi-system organ deterioration in patients with advanced heart failure have contributed to the rapid rise of left ventricular assist device (LVAD) implantation.
Since their first inception, there have been marked improvements in LVAD technology making them now a reliable therapeutic option for patients with advanced heart failure. There have been over 15000 mechanical circulatory support devices implanted since 2006 in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) registry[3]. In addition to improvements in technology, better understanding of patient selection, peri-operative management strategies, and long term management have led to reduced complications with improvements in survival and quality of life in HF patients[4].
Despite tremendous advancements, however, there remain important limitations to LVADs. Gastrointestinal bleeding, infections, thromboembolic events such as stroke, pump thrombosis and right heart failure remain barriers to earlier use of this therapy. Even with these improved clinical outcomes and significant decreases in size of LVADs, many patients and clinicians still view them as bulky machines associated with significant morbidity, mortality and need for life-long hospitalization. Patients with advanced disease who have not quite reached “end-stage heart failure” present lower surgical risk with less end organ dysfunction, better functional capacity, and enhanced capacity to rehabilitate from major surgery. Many experts contend that these “less sick” ambulatory advanced heart failure patients could benefit from earlier LVAD implantation, but in clinical practice this has yet to commonly occur. (Cite intermacs report and can find other opinion pieces about early implantation).
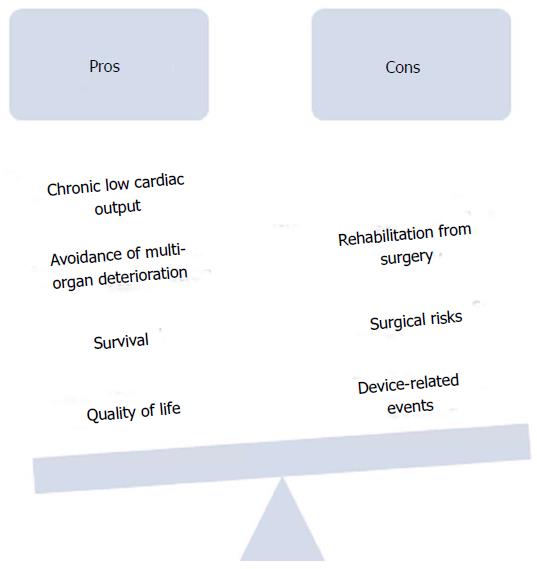
This paper aims to review the current advantages and disadvantages of LVAD implantation in patients with advanced, ambulatory heart failure and discuss the pertinent issues in establishing an equilibrium between early surgical and/or device-related risks and benefits of quality and/or quantity of life with earlier implantation (Figure 1).
When asked about their decision to pursue optimal medical management over LVAD, patients stated reasons such as “they didn’t like the idea of a major device implantation surgery”, “they are worried about the possible complication”, and they don’t think an LVAD will improve quality of life and survival[5]. Moreover, many patients are never referred for advanced mechanical support due to inadequate understanding of LVAD outcomes by their medical providers and unavailability of the technology locally. However, one-year survival with the current pump technology is near 80%, which is markedly higher compared to the original data that established LVADs as a form of heart failure therapy[3]. To parallel the great advancements in LVAD therapy, it seems natural that the number of patients offered this therapy will continue to increase to the more than 10% of the HF population that will progress to advanced heart failure.
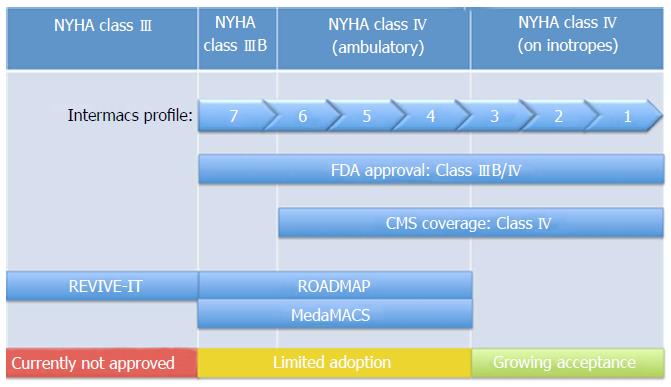
Even with tremendous improvements in survival and device related adverse events over the past decade, considerable debate persists regarding the optimal timing of LVAD implantation. The benefits of LVAD implantation in inotrope dependent patients and those in cardiogenic shock are generally accepted. However, for patients with advanced heart failure who have not yet progressed to inotrope dependency the decision is more challenging. A single effective model for risk stratification is currently lacking for this large, heterogeneous, group. Traditionally patients have been classified according to the New York Heart Association (NYHA) functional classification, but this system is somewhat subjective and limited by significant inter-reporter variability[6]. While current FDA approval exists for LVAD implantation in NYHA class IIIB and class IV patients, the vast majority (81%) of LVADs are implanted in those identified as class IV on chronic inotropic therapy or in cardiogenic shock[5]. Implantation of LVADs has led to improved symptom burden and quality of life in those with advanced heart failure. In the HeartMate II destination therapy trial, 80% of patients who received a continuous flow LVAD went from NYHA class III or IV to NYHA class I or II. Furthermore, these patients also had a significant increase in a 6-min walk distance by 1 year[7].
As previously stated, the one-year survival with the current pump technology is near 80%[5]. The greatest risk for mortality following LVAD implantation falls during the early post-operative period and reaches a low by 3 mo following the procedure[8]. When analyzing factors that are related to survival following LVAD implantation, the 7th INTERMACS Annual Report found that patients with an INTERMACs profile of 2-3, and thus less severe disease, have better survival than those with an INTERMACS profile 1[5]. However, while INTERMACS levels 1-3 have been associated with lower survival rates 3 years post-LVAD implantation when compared to levels 4-7, no graded mortality risk has been demonstrated to help further discriminate the potential benefit between levels 4-7, which could be associated with the subjectivity of assignment in these levels[9]. Per Shah et al[8], other factors that have a great impact on LVAD perioperative mortality include age, female sex, prior stroke, mechanical ventilation, LVAD for destination therapy, hepatic or renal dysfunction, right ventricular dysfunction, and prior or concurrent cardiac surgery.
To better characterize patients’ risk to benefit profiles for LVAD implantation, multiple risk assessments have been developed. Unfortunately, few consistent predictors have been identified across models and currently no single model effectively triages potential LVAD patients. In general, however, the predictors that have been recognized in different models are markers of end-organ dysfunction secondary to heart failure or other significant comorbidities, such as age[9,10]. Patients that are “sicker”, as reflected by a more acute INTERMACS profile, are also known to have worse outcomes. Moreover, regardless of INTERMACS profile, mortality increases with increasing age at the time of implantation[3]. With this in mind, there is support for considering LVAD implantation earlier in the disease course theoretically leading to lower operative risk and fewer post-operative complications.
In continuing to lower the morbidity and mortality associated with LVADs the balance of patient risk to benefit for LVAD implantation may suggest sooner application of this technology.
Though LVAD implantation can result in significant improvements in morbidity and mortality, their use is associated with complications including infection, stroke, pump thrombosis, gastrointestinal bleeding, and right ventricular failure. Infection occurs in about 20% of patients following implantation and may present as sepsis or a driveline infection. Infection additionally may predispose to pump thrombosis[11]. Pump thrombosis occurs at an annual incidence of 6%-12%, although the exact incidence varies based on device type and anticoagulation regimen employed. One thing for certain; however, is that pump thrombosis is associated with an increase in neurologic events as well as a higher rate of mortality. Cerebrovascular complications occur with an annual incidence of greater than 6%[8]. Furthermore, 30% of patients have major bleeding in the first month, and then following one month, bleeding occurs at a rate of 8%-23% by one year. Overall, 55% of patients will be rehospitalized for any cause[11].
Bleeding, in particular gastrointestinal bleeding, is associated with significant morbidity after LVAD implantation. The cause of increased bleeding is multifactorial and can be attributed to chronic anticoagulation, acquired von Willebrand syndrome, and chronic low pulse pressure leading to increased risk for angiodysplasia. Therefore, screening patients for angiodysplasia and von Willebrand syndrome prior to implantation may allow for preemptive treatment of these conditions to help avoid complications postoperatively[12]. With further understanding of the pathogenesis of bleeding post implantation and research on the prevention and appropriate management, its hopeful the risk of bleeding will decrease to support the earlier implantation of LVADs.
As stated before, Pump thrombosis occurs at an annual incidence of 6%-12% raising awareness that LVAD therapy is not without inherent risks[8]. The lack of equipoise in many physicians’ minds of benefit vs risk of LVAD for NYHA Class III patients that were highlighted by pump thrombosis led to early termination of the Registry Evaluation of Vital Information for VADs in Ambulatory Life (REVIVE-IT) trial. The PREVENT (Prevention of Heartmate II Pump Thrombosis through Clinical Management) study was designed to analyze the impact of clinical practices developed to decrease the risk of Heartmate II pump thrombosis. The study followed the “PREVENT protocol” which were recommendations on LVAD implantation, anticoagulation and antiplatelet protocols, and pump management. Preliminary results have been positive and show that the protocol is associated with lower rates of thrombosis without increased incidence in bleeding complications[13].
Furthermore, in the case that pump exchange must occur, the morbidity and mortality of the exchange has decreased. Soleimani et al[14] found that off-pump minimally invasive exchange of the Heartmate II can be safely accomplished with low morbidity and mortality, resulting in excellent outcomes. Therefore, will evolving clinical guidelines improving the risk of pump thrombosis and minimizing the risk of adverse events in addition to the decreased morbidity and mortality of pump exchange, this supports the shift to earlier implantation of LVADs.
In particular, the risk of right heart failure following LVAD implantation has been extremely difficult to predict. With improved left ventricular decompression, pulmonary congestion should decrease resulting in decreased afterload for the right ventricle. However, increased cardiac output from LVAD support will result in increased right ventricle preload. Also, leftward shift of the interventricular septum shift and change in motion after LVAD implantation may impair the right ventricle contractility, leading to right ventricle dysfunction, and ultimately right heart failure[15]. Right ventricular failure is likewise often the last manifestation of advanced heart failure. There are no durable treatment options currently available for right ventricular failure emphasizing the need to prevent it in LVAD patients, and identify those who may be at increased risk of developing it with extended time with an LVAD.
A study by Santambrogio et al[16] showed that early right heart failure will develop in about 25% of patients receiving LVAD support. Furthermore, Argiriou et al[15] noted that female sex, existence of pre-operative circulatory failure, presence of end-organ dysfunction, severe right ventricle systolic dysfunction, and presence of pulmonary vascular disease are all pre-operative risk factors for early right heart failure. However, there are limitations to all these risk factor stratification models as has been pointed out by Lampert et al[17] that most of the risk scores were developed primarily in BTT patients with pulsatile devices, and so there is a need for further investigation. The report notes that echocardiography, hemodynamic parameters, and biomarkers including neutrophil gelatinase-associated lipocalin, blood urea nitrogen, aspartate aminotransferase and serum creatinine could be of use in predicting pre-operative risk of early right heart failure.
While much has been studied about early right heart failure following LVAD implantation, less is known about the development of late right ventricular failure, which is an important complication to consider when arguing to implant LVADs in patients earlier. As there is a question of whether late right heart failure is a distinct entity, or just undiagnosed early right heart failure, the risk factors are not as well established, although there is likely significant overlap with the risk factors of early right heart failure[17]. Takeda et al[18] found that late right heart failure occurred in about 11% of patients at a median of 99 d, with significant predictors including diabetes mellitus, body mass index greater than 29 kg/m2, and BUN level greater than 41 mg/dL. These patients had significantly worse survival when compared to those who did not develop late right heart failure, but this could also be attributable to their increased incidence of comorbidities. Currently, treatment for late right heart failure is directed at the underlying causes and management of symptoms, however it is thought that optimization of pump speed, which will avoid excessive leftward septal shift and decrease excessive venous return, may help to avoid this late complication[17]. Further research on the effects of more frequent imaging and hemodynamic measurements in patients with LVADs could help develop appropriate post implantation management guidelines to best screen for and prevent late right heart failure. Additionally, avoiding early and aggressive titration of beta-blockers and use of inotropes to support right ventricular function and pulmonary vasodilators to decrease right ventricular afterload may also help[17].
Thus, if these risk factors could be further developed and taken into consideration when selecting patients for early implantation, the risk of late right heart failure could be minimized. With the shift to earlier implantation of LVADs, there is a clear need for continued research in the screening and management of late right heart failure to better care for patients who do receive LVADs earlier in their course of heart failure. However, the development of bi-ventricular failure in non-transplant eligible patients still warrants special consideration. Advancements in total artificial heart technology and a better understanding of right ventricular failure are needed to better care for these patients who do develop right ventricular failure.
Despite these adverse events, The 7th INTERMACS Annual Report demonstrated that with the improved technology of the continuous-flow pumps, there has been a dramatic decrease in the overall adverse event rate when pumps implanted between 2012 to 2014 are compared to pumps implanted between 2008 to 2011[5].
Appropriate identification of patients with the best chance to benefit from therapy and lowest risk of complications is a perpetual focus of investigation for LVAD implantation. For example, Boyle et al[19] found that patients on inotropes before LVAD implantation trended toward a higher incidence of hemorrhagic stroke post-operatively. Boyle et al[19] also found that patients in INTERMACS 4-7 had significantly shorter length of stay following LVAD implantation and greater survival when compared to both INTERMACS 1, and 2/3 patients[20]. This suggests that selecting patients earlier on in the progression of heart failure, prior to dependence of inotropic therapy, would reduce the LVAD implantation post-operative risk of complications. Furthermore, studies are currently being conducted which directly show the benefit in both quality of life and survival with earlier LVAD implantation (Table 1, Figure 2).
| Study | Objective | Significant findings |
| ROADMAP | Compare outcomes of HeartMate II implantation in destination therapy patients who are not dependent on inotropic support with those on optimal medical management | Early LVAD implantation associated with improved quality of life and more adverse events. Intent to treat analysis showed no survival benefit with early implantation |
| REVIVE-IT | Compare outcomes of HeartMate II implantation in NYHA class III patients not severe enough to qualify for transplant or permanent LVAD therapy with those on optimal medical management | Study discontinued due to difficulty recruiting from observed increase in pump thrombosis (enrolled 0/100 patients (randomized study), 0/2500 patients (screening registry) |
| MedaMACS | Characterize and report on patients with ambulatory advanced heart failure who have not receive an LVAD | Patients desire LVADs and LVAD shows survival benefit compared to medical management for INTERMACS 4 and 5 |
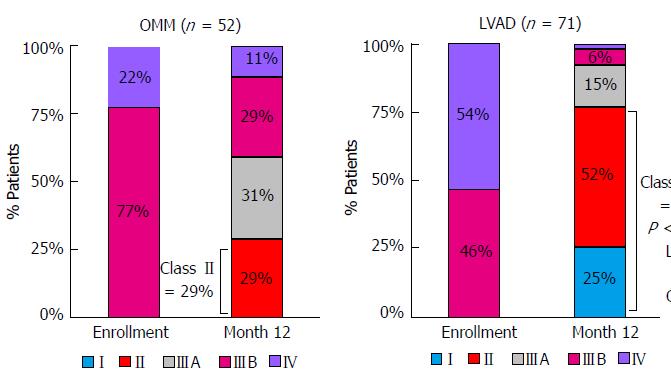
The Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients (ROADMAP) Study attempted to evaluate the effects of LVAD implantation in less sick patients[5]. ROADMAP was a prospective, multi-center, nonrandomized observational study that evaluated outcomes of LVAD implantation in destination therapy patients who are not dependent on inotropic support (INTERMACS profiles 4-7). Currently, these patients make up roughly 20% of all implantations[5]. In ROADMAP, patients and their providers chose to continue on optimal medical therapy (OMM) or proceed with LVAD implantation. The primary composite endpoint was survival on original therapy with increase in 6 min walk distance (6MWD) by at least 75 m. Significantly more patients in the LVAD cohort (n = 97) reached this endpoint than those on OMM (n = 103) (39% vs 21%). Furthermore, the LVAD group had greater improvements in self-reported quality of life and depression. Additionally, the LVAD group had 77% of patients change in their NYHA classifications to class II or I, while the OMM group only had 29% change to class II, and none to class I (Figure 3). This greater improvement in functional status was also supported by the improvements in the 6MWD, as LVAD patients had a significant increase while there was no significant change in the OMM cohort. The LVAD group also had a significantly greater 12-mo as-treated (event-free) survival (80% vs 63%). However, since delayed LVAD implantation counted as a “failure” in OMM patients, the intent-to-treat analysis showed no survival benefit with early LVAD implantation[5].
There were some adverse findings with early LVAD implantation. These patients had more frequent adverse events as compared to the OMM patients. LVAD patients’ adverse events were primarily due to bleeding as opposed to the OMM patients’ adverse events that were primarily due to worsening heart failure[5]. The ROADMAP results suggest that earlier LVAD implantation in select patients may provide significant benefit, but there remains no consensus on a singular way to identify these patients.
A significant limitation to the ROADMAP trial that prevents generalization of the results is the lack of randomization of patients between LVAD and OMM. At baseline, patients who elected to have an LVAD were sicker than those who elected to continue OMM. The LVAD group had more NYHA class IV patients (52% vs 25%), which is a group that is generally already thought to benefit from LVAD implantation. Moreover, the LVAD group in ROADMAP consisted of more INTERMACS profile 4 patients (65% vs 34%), had less beta-blocker use, and a lower predicted Seattle Heart Failure Model 12-mo survival. Also, the LVAD cohort was much less satisfied with their quality of life on average than the OMM group[5]. This could lessen the significance of the greater improvements in self-reported depression and quality of life. Despite these limitations, the LVAD group still showed much more functional improvement in both the 6 min walk test and NYHA classification.
The Medical Arm of the Mechanically Associated Circulatory Support (MedaMACS) project is an ongoing cross-sectional, observational study following patients with ambulatory advanced heart failure (INTERMACS profile 4-7) that aims to characterize and report on the medical outcomes of those patients who have not yet received an LVAD (include citation). In the MedaMACS screening pilot study, a majority (56%) of patients reported they would “definitely” or “probably” want an LVAD given the alternative was their current symptomatic state. Interestingly, 93% of these patients were at a low or intermediate implant risk based on the HeartMate II Risk Score. Furthermore, many patients were willing to consider LVAD surgery despite expectation of a long survival with OMM suggesting that more than HF mortality influences preference for mechanical support[21]. This suggests that patients value the improved quality of life made possible by LVADs and may be willing to take on the risk of adverse events associated with them. Hence an argument can be made for LVAD implantation in the ambulatory heart failure patient by individualized patient desire.
In terms of survival, MEDAMACS showed a one-year survival for patients on medical management of 78% in INTERMACS level 6/7, 67% in INTERMACS level 5, and 39% in INTERMACS level 4[8]. Therefore, when compared to the 80% one-year survival after LVAD implantation, this data would suggest an increase in survival for patients in INTERMACS level 4/5 who undergo LVAD implantation and further supports a shift towards earlier implantation of LVADs and an expansion in their utilization.
The REVIVE–IT study, like the ROADMAP study, also planned to test the theory that patients with less advanced heart failure will benefit in both survival and quality of life with LVAD implantation as opposed to optimal medical management. This trial however was to analyze LVAD implantation in moderate NYHA class III patients with marked limitation of physical activity and LVEF of 35% or less[22]. However, this study was discontinued as it met great challenges with recruiting patients due to the observed increase from 2.2% at 3 mo post-implantation to 8.4% in pump thrombosis in the pump used in the study discovered by Starling et al[23]. Therefore, in combination with the perceived increased risk of thrombosis, a renewed hesitancy for wider adoption of LVAD technology grew. As some were already risk aware in patients with NYHA class IV/INTERMACS profile 4-6 patients, it became clear that routine consideration of patients for NYHA Class III/INTERMACS profile 7 were too far out of reach. However, it is clear that controversy persists as, the ROADMAP study has shown the benefits of earlier implantation with regards to quality of life and once again shifting the equilibrium towards early implantation.
When considering the earlier implantation of LVADs, its critical for one to account for the extended amount of time these patients will have using the LVADs and how that will impact the potential for adverse events. The increased chance of adverse events will need to be weighed against the increase in quantity and quality of life.
Although LVADs are currently being used to improve quality and quantity of life for those in NYHA class IV end-stage heart failure, there is anticipation that a much larger group of patients may benefit from this potentially life-saving therapy. Although we are not quite there yet, we are moving towards a balance where the improvement of quality and quantity of life outweigh the risks of adverse events for patients who aren’t quite yet at NYHA class IV end-stage heart failure. Patients who are implanted earlier may experience much greater benefits with lower risks of complication than those currently being treated. Earlier implantation of LVADs, prior to the onset of end organ dysfunction, may have benefits when compared to optimal medical management and could be considered as an alternative for less advanced heart failure patients, who do not have risk factors for adverse events. In continuing to reduce the morbidity and long-term risks of LVAD implantation, LVADs will likely be used earlier in the treatment of advanced heart failure as the technology progresses. In fact the next generation of devices, the HeartMate III (St. Jude Medical, St. Paul, MN) and the MVAD (HeartWare International Inc, Framingham, MA) have been developed with this very goal in mind – to push the boundaries of reducing surgical morbidity and long-term reduction of device related adverse events. With continued research in the early implantation of LVADs we can better identify what to expect with extended time on LVAD support. Additionally, with continued research on incidence, management and prevention of adverse events, we can better select patients for early implantation and be more prepared in the case that adverse events occur. As we continue to learn from trials such as the ROADMAP trial and the MedaMACS registry, we hope to clarify the delicate balance between implantation of devices in patients who are too sick to benefit from the therapy and those who are too well to undergo the morbidity of the procedure.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Amiya E, Bonanno C, Cebi N, Kirali K, Skobel E S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3462] [Cited by in F6Publishing: 4431] [Article Influence: 443.1] [Reference Citation Analysis (0)] |
| 2. | Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435-1443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3136] [Cited by in F6Publishing: 2872] [Article Influence: 124.9] [Reference Citation Analysis (0)] |
| 3. | Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495-1504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1054] [Cited by in F6Publishing: 1005] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 4. | Maciver J, Ross HJ. Quality of life and left ventricular assist device support. Circulation. 2012;126:866-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Estep JD, Starling RC, Horstmanshof DA, Milano CA, Selzman CH, Shah KB, Loebe M, Moazami N, Long JW, Stehlik J. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: Results From the ROADMAP Study. J Am Coll Cardiol. 2015;66:1747-1761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 264] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 6. | Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, Mayet J, Francis DP. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93:476-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 286] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 7. | Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, Long JW. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241-2251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2404] [Cited by in F6Publishing: 2293] [Article Influence: 152.9] [Reference Citation Analysis (0)] |
| 8. | Shah SP, Mehra MR. Durable left ventricular assist device therapy in advanced heart failure: Patient selection and clinical outcomes. Indian Heart J. 2016;68 Suppl 1:S45-S51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, Jaski B, Farrar DJ, Slaughter MS. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol. 2013;61:313-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Matthews JC, Pagani FD, Haft JW, Koelling TM, Naftel DC, Aaronson KD. Model for end-stage liver disease score predicts left ventricular assist device operative transfusion requirements, morbidity, and mortality. Circulation. 2010;121:214-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | McIlvennan CK, Magid KH, Ambardekar AV, Thompson JS, Matlock DD, Allen LA. Clinical outcomes after continuous-flow left ventricular assist device: a systematic review. Circ Heart Fail. 2014;7:1003-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Harvey L, Holley CT, John R. Gastrointestinal bleed after left ventricular assist device implantation: incidence, management, and prevention. Ann Cardiothorac Surg. 2014;3:475-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 25] [Reference Citation Analysis (0)] |
| 13. | Emani S, Keebler M, Ransom JM, Kilic A, Egnacyzk G, Gallegos R, Mather P, Stulak J, Gregoric I, Katz J. Prevention of HeartMate II Pump Thrombosis - Recommendations and Preliminary Observations From the PREVENT Study. Circulation. 2015;132:A16405; Published online before print November 6, 2015 Available from: http://circ.ahajournals.org/content/132/Suppl_3/A16405. [Cited in This Article: ] |
| 14. | Soleimani B, Pietras C, Stehpenson E, High K, Pae W. Outcomes of Off-Pump Minimally Invasive Exchange of the HeartMate II (HMII) Left Ventricular Assist Device (LVAD). J Heart Lung Transplant. 2015;34:S127-S128. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | Argiriou M, Kolokotron SM, Sakellaridis T, Argiriou O, Charitos C, Zarogoulidis P, Katsikogiannis N, Kougioumtzi I, Machairiotis N, Tsiouda T. Right heart failure post left ventricular assist device implantation. J Thorac Dis. 2014;6 Suppl 1:S52-S59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 67] [Reference Citation Analysis (0)] |
| 16. | Santambrogio L, Bianchi T, Fuardo M, Gazzoli F, Veronesi R, Braschi A, Maurelli M. Right ventricular failure after left ventricular assist device insertion: preoperative risk factors. Interact Cardiovasc Thorac Surg. 2006;5:379-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant. 2015;34:1123-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 18. | Takeda K, Takayama H, Colombo PC, Yuzefpolskaya M, Fukuhara S, Han J, Kurlansky P, Mancini DM, Naka Y. Incidence and clinical significance of late right heart failure during continuous-flow left ventricular assist device support. J Heart Lung Transplant. 2015;34:1024-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Boyle AJ, Jorde UP, Sun B, Park SJ, Milano CA, Frazier OH, Sundareswaran KS, Farrar DJ, Russell SD. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol. 2014;63:880-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 20. | Boyle AJ, Ascheim DD, Russo MJ, Kormos RL, John R, Naka Y, Gelijns AC, Hong KN, Teuteberg JJ. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 2011;30:402-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 21. | Stewart GC, Kittleson MM, Cowger JA, Johnson FL, Patel CB, Mountis MM, Patel PC, Rame JE, Testani J, Guglin ME. Who wants a left ventricular assist device for ambulatory heart failure? Early insights from the MEDAMACS screening pilot. J Heart Lung Transplant. 2015;34:1630-1633. [PubMed] [Cited in This Article: ] |
| 22. | REVIVE-IT heart failure study resumes after safety modification (University of Michigan Health System, 13 May 2014. [accessed 2016 Jul 28]). Available from: http://www.umcvc.org/news/archive/201404/revive-it-heart-failure-study-resumes-after-safety. [Cited in This Article: ] |
| 23. | Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, Rame JE, Acker MA, Blackstone EH, Ehrlinger J. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:33-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 630] [Cited by in F6Publishing: 596] [Article Influence: 59.6] [Reference Citation Analysis (0)] |