Peer-review started: June 4, 2015
First decision: July 27, 2015
Revised: September 4, 2015
Accepted: November 3, 2015
Article in press: November 4, 2015
Published online: January 26, 2016
Processing time: 235 Days and 17.6 Hours
Ventricular repolarization is a complex electrical phenomenon which represents a crucial stage in electrical cardiac activity. It is expressed on the surface electrocardiogram by the interval between the start of the QRS complex and the end of the T wave or U wave (QT). Several physiological, pathological and iatrogenic factors can influence ventricular repolarization. It has been demonstrated that small perturbations in this process can be a potential trigger of malignant arrhythmias, therefore the analysis of ventricular repolarization represents an interesting tool to implement risk stratification of arrhythmic events in different clinical settings. The aim of this review is to critically revise the traditional methods of static analysis of ventricular repolarization as well as those for dynamic evaluation, their prognostic significance and the possible application in daily clinical practice.
Core tip: The analysis of the role of ventricular repolarization perturbations as potential triggers of malignant arrhythmias has increasingly gained interest, particularly as a potential tool for the risk stratification of arrhythmic events in different clinical settings. Several measures of ventricular repolarization have been developed and tested in clinical practice. This review critically revises the traditional methods of static analysis as well as those for dynamic evaluation, their prognostic significance and the possible application in daily clinical practice.
- Citation: Monitillo F, Leone M, Rizzo C, Passantino A, Iacoviello M. Ventricular repolarization measures for arrhythmic risk stratification. World J Cardiol 2016; 8(1): 57-73
- URL: https://www.wjgnet.com/1949-8462/full/v8/i1/57.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i1.57
The electrocardiogram (ECG) is widely used in clinical practice for the diagnosis of cardiac arrhythmias, conduction disturbance, structural changes of the myocardium, myocardial ischemia, drug effects, and electrolyte and metabolic disorders. The ECG waveforms are the expression of transmembrane action potentials (APs) of atrial and ventricular myocytes[1].
ECG recording in a normal cardiac cycle is composed of two basic processes: Depolarization and repolarization. Ventricular depolarization and activation is represented by the QRS complex, whereas ventricular repolarization (VR) is expressed as the interval from the beginning of the QRS complex to the end of the T wave (QT interval). VR is a complex electrical phenomenon which has been studied in detail[2,3].
It is a crucial step in cardiac electrical activity consisting of a recovery period with the return of the ions to their previous resting state, which corresponds with the relaxation of the myocardial muscle, thus setting the stage for the next depolarization and contraction. On the surface ECG, VR is made up of the J-wave, ST-segments, and T and U waves[1]. Over the years previous studies have emphasized the role of VR alterations in predisposing to lethal arrhythmias[4,5] and thus the analysis of VR has increasingly gained interest, particularly as a potential tool for the risk stratification of arrhythmic events[6,7], integrating other well-established parameters[8].
This review aims to critically revise the available static and dynamic methods of VR analysis and their application in clinical practice.
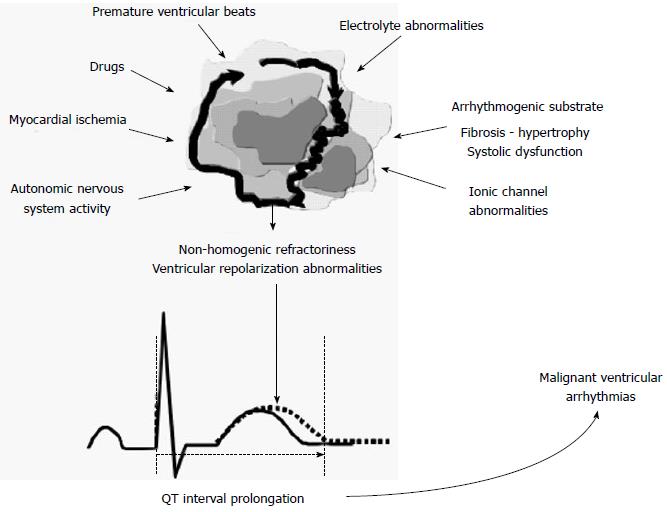
VR measures have been proposed to stratify arrhythmic risk due to their ability to reflect abnormalities in cardiac electrical activity predisposing to the occurrence of malignant arrhythmias. Cardiac structural and electrical alterations may cause abnormalities in APs, and in the refractory period and conduction velocities of adjacent myocardial areas, thus leading to spatial heterogeneity and temporal fluctuations in repolarization and favoring the onset of arrhythmias[9,10]. Furthermore, it has been demonstrated that autonomic nervous system (ANS) activity can interact with structural heart diseases, affecting the VR and promoting the onset of arrhythmias. Moreover, the modulation of VR by ANS is not limited to its influence on sinus node regulation and on heart rate (HR). In fact, there is a direct regulation by ANS on APs through the regulation of the activity of ion channels[11]. Furthermore, the effects of vagal and sympathetic systems should not be considered singularly. Sympathovagal interactions are fundamental in regulating heart function and conditions which alter the sympathovagal balance, facilitate cardiovascular instability and can lead to arrhythmias[8].
Finally, several drugs have been showed to affect VR and prolong the QT interval, inducing alterations which can predispose to ventricular arrhythmias[12,13]. From these considerations, the importance of the analysis of VR and the QT interval in the evaluation of arrhythmic risk, drug effects and liability of the ventricular arrhythmias is clear (Figure 1). Since the earliest demonstrations that a prolonged corrected QT interval (QTc) is associated with an increased risk of ventricular arrhythmias and sudden cardiac death (SCD), in patients with myocardial infarction[14], as well as in patients taking QT-prolonging drugs[15], interest in the assessment of QT prolongation grown. At the same time, the role of QTc prolongation as a marker for arrhythmic risk is controversial[16] and measures of QT dispersion at ECG did not always demonstrate the ability to predict the risk of arrhythmic events[17]. Thus, as an alternative to “static evaluation” of the QT interval, different measures of QT variability and dynamicity have been proposed, better representing the complex interactions between arrhythmic substrate, HR and ANS activity and offering a more complete assessment of VR and estimation of arrhythmic risk[18].
The measurement of the QT interval represents the traditional approach to the analysis of VR.
The QT interval is calculated as the distance between the first deflection of the QRS and the end of the T wave on the surface ECG. Measurement of the QT interval by surface ECG can be performed either manually or automatically[11,19-22].
In the manual measurement, the end of the T wave is determined as the intersection between the tangent to the steepest down-slope of the T wave and the isoelectric line[22], but this method can be time-consuming and is liable to inter-reader variability. Recommendations concerning QT measurement have not proposed a single defined lead (lead II or the lead with the largest T wave) or a mean QT derived from an arbitrary subset of leads. As consequence of this lack of a systematic approach, there is a variation in sensitivity and specificity for single lead measurement of the QT interval in predicting the risk of major arrhythmic events. The interlead QT variation associated with the observation that in healthy individuals the longest QT interval is most frequent in leads V2 to V5 and that the QT interval is not closely related to T wave height makes it difficult assessing ventricular recovery by measuring a single QT interval[23-26]. The measurement of the QT dispersion addresses this challenge as subsequently explained. It is widely recognized that the typical measurement of the QT interval is subject to different interpretations due to different factors, such as autonomic tone, electrolytes imbalance, technical aspects, variations in T wave morphology, presence of U waves, and noisy baseline. The absence of agreement among experts and of a standardized measure of the QT interval contributes to interobserver variability[27,28].
The main problems regarding the automated measurement are related to the T wave morphology (flat, bifid, biphasic) and to the presence or absence of the U wave. Consequently, the measurement of the QT interval requires a lot of experience and a good interpretation of the ECG signal[29]. In addition, some of the automated QTc interval monitoring strategies are labor-intensive, and dependent on expensive technology[30] and experts disagree on the utility and efficacy of automated readings when compared with careful manual measurements[31]. The other relevant aspect in the measurement of the QT interval is its dependence on HR, the main physiological factor influencing VR. The QT interval is inversely correlated with HR prolonging at a slower HR and shortening at faster one. In order to minimise the influence of this factor on the measurement, it is essential to make a correction of the QT interval for HR (QTc). Different formulas have been developed in order to adjust QT for HR. Those most commonly used are Fridericia’s formula: QTc: QTxRR-1/3[32], and Bazett’s formula: QTc: QTxRR-1/2[33], where RR means the time interval and QT means the distance. However different opinions exist as to the best and most useful correction for HR and the evidences remain unclear and contrasting[34-36]. The normal range of QTc has been assessed by Straus et al[37] who established the gender-specific variability of QTc measurement. The authors subdivided the Bazett-corrected QTc interval into gender-specific groupings and further subdivided the QTc interval into normal, borderline, and prolonged categories.
Considering the lack of standardization and recommendations for the best method to measure QT, a broad of experts proposed guidelines for measuring the QT interval[31].
The expert group from the American Heart Association (AHA) and American College of Cardiology Foundation (ACCF) recommends that a QTc over the 99th percentile should be considered abnormally prolonged. Approximate 99th percentile QTc values for otherwise healthy postpubertal individuals are 470 ms for males and 480 ms for females[38].
Marked prolongation of the QT interval is a well-established pro-arrhythmic risk factor in the general population[39] in patients with coronary artery disease[40], hypertrophic cardiomyopathy (HCM)[41], or heart failure (HF)[42] and in patients taking QT-prolonging drugs[43]. However, the clinical usefulness of QT measurement has mainly been demonstrated in both congenital[43] and acquired long QT syndrome (LQTS)[44].
The congenital LQTS was described for the first time in 1957 and since then remarkable efforts have been made to define its pathogenesis, diagnosis and treatment[45]. Genetic studies have shown that LQTS is caused by pathogenic mutations in 15 genes encoding cardiac ion channels or membrane adaptors and thus different LQTS genotypes have been identified. Pathogenic mutations identified in the KCNQ1 and KCNH2 genes as well as in the sodium channel, encoded by SCN5A, are responsible for nearly 80% of all clinically diagnosed cases. All other genes together account for less than 5% of LQTS cases[46]. LQTS 1, 2 and 3 are the most common genotypes, representing 80%-90% of the total cases of inherited LQTS[47,48]. Due to limitations of space and the different purposes of this article, our focus is mainly on the diagnostic criteria of the congenital LQTS. However, details about specific triggers, clinical presentation, prevalence and genetic aspects, risk stratification and therapeutic advances are available in the literature[49-53]. As regards the diagnosis of LQTS, a scoring system, updated in 2011, and including symptoms, family history and ECG findings was proposed by Schwartz et al[54]. In 2013 an expert consensus, incorporating the Schwartz score, drafted the following recommendation for the diagnosis of LQTS[50]. Congenital LQTS is diagnosed when the LQTS risk score is ≥ 3.5, in the absence of a secondary cause for QT prolongation and/or in the presence of an unequivocally pathogenic mutation in one of the LQTS genes, or it can be diagnosed in the presence of a corrected QT interval for HR using Bazett’s formula (QTc) ≥ 500 ms in repeated 12- lead ECG and in the absence of a secondary cause for QT prolongation. Moreover LQTS can be diagnosed in the presence of a QTc between 480 and 499 ms in repeated 12-lead ECGs in a patient with unexplained syncope in the absence of a secondary cause for QT prolongation and in the absence of a pathogenic mutation[50].
Short-QT syndrome (SQTS) is a clinical entity originally described by Gussak et al[55] in 2000 as an inherited syndrome in a family with paroxysmal atrial fibrillation and constantly shortened QT intervals.
Subsequently, a high familial risk for sudden cardiac death associated with a short QT interval was demonstrated by Gaita et al[56] in the members of a family with a history of syncope or palpitations, as well as one case of sudden death resuscitation. A strong family history of sudden death, present in 4 generations, was detected. The family members had a very short QT interval at ECG, which never exceeded 280 and 290 ms when corrected for HR with Bazett’s formula (QTc). The authors provided a definition of SQTS as characterized by familial sudden death, short refractory periods, and inducible ventricular fibrillation. The definition of SQTS became a possible diagnosis for yet unexplained SCD in patients without structural heart disease. Details of possible pathophysiological mechanisms, related genetic mutations and other possible explanations, besides the well-known channelopathy, are available in the literature[57-59].
In daily clinical practice, one of the most frequent application of QTc measurement is the monitoring of drug-induced QTc prolongation, the aim of which is to mitigate the risk of Torsade de Pointes (TdP) and other ventricular arrhythmias and prevent SCD. Several drugs have been shown to induce perturbation of VR and prolongation of QT interval, increasing the risk of ventricular arrhythmias such as TdP and SCD. There has been increased research into the mechanisms involved in drug-induced LQTS in patients in treatment with these drugs. Those generally involved in acquired LQTS are cardiac drugs such as class IA and III antiarrhythmic drugs, while the non-cardiac ones include antibiotics, antihistamines, antidepressants, antipsychotic and methadone. An updated list of drugs that can potentially cause QT prolongation is available on the Internet where they are classified into the following four categories: (1) drugs with known risk of TdP; (2) drugs with possible risk of TdP; (3) drugs with conditional risk of TdP; and (4) drugs to avoid in congenital LQTS (https://http://www.crediblemeds.org/new-drug-list/). Medications that cause QT prolongation generally act by blocking Ikr, a potassium-channel protein that regulates an important rapid delayed repolarizing current in phase 3 of the cardiac AP. This protein is encoded by the human ether-a-go-related gene (HERG), mutated in the LQT2 form of the congenital syndrome[60]. The blocking of Ikr causes a lenghthening of AP in cardiac myocytes and QT interval, thus increasing the risk of ventricular arrhythmias and SCD[16,61]. However many drugs blocking the Ikr current do not seem to cause TdP (e.g., amiodarone). Other mechanisms involved in drug-induced LQTS have been described and concern the loss of K channels (e.g., fluoxetine) and an increased inward sodium current, such as that produced by cisapride and antimony[62-65]. However, drug-induced LQTS is not easy to predict in any given individual and the same medication does not exhibit the same pro-arrhythmic effects in different individuals[16]. Indeed, some individuals taking QT prolonging drugs, may develop QTc prolongation with or without TdP, or may not experience QTc prolongation, while several authors have also demonstrated genetic predisposition to acquired LQTS[61,66]. However, only a small proportion of patients taking QT prolonging drugs experience TdP and, at the same time, TdP can develop in patients with a normal QTc interval[27]. Additional risk factors causing QT prolongation and favoring drug-induced VR alterations and TdP have been identified. They include hypokalemia, drug-drug interactions, the female gender, advancing age, genetic predisposition, hypomagnesemia, heart failure, bradycardia, and baseline QTc interval prolongation. Many risk factors are potentially modifiable and should be corrected in those patients at risk for QT interval prolongation. It has been reported that all patients with TdP secondary to non-cardiac drugs have established risk factors. Most patients manifestating drug-induced LQTS have at least one identifiable risk factor in addition to drug exposure[13,27,67]. Female gender is the most common risk factor[68]. Age has also been detected as a risk factor for QT prolongation, particularly when combined with additional risk factors, such as female gender, personal or family history of pre-syncope or syncope, electrolyte disturbances or cardiovascular disease[69]. Impaired glucose tolerance and diabetes were observed to be associated with an increased likelihood of prolonged QTc independent of age, race, gender, education, and heart rate. In addition, persons with diabetes and multiple cardiovascular disease risk factors were more likely to have a prolonged QTc than those with Normal Glucose Tolerance and no additional risk factors, suggesting that this group may be at increased risk for cardiac arrhythmia and sudden death[70]. Hypokaliemia is a very common QT-prolonging factor and is often detected in patients with drug-induced QT prolongation and TdP and some proposals have been made as to the different mechanisms that may potentially be involved[61,71]. Other metabolic conditions correlated with QT-prolongation are hypothyroidism, hypocalcemia and hypomagnesemia as well as hepatic diseases[72,73]. The “five-year cross-sectional ECG screening Outcome in psychiatry study” revealed that patients with drug-induced LQTS had a significantly higher frequency of hypokaliemia, hepatitis C virus infection and abnormal T wave morphology. The drugs mostly involved in QT prolongation were haloperidol, clotiapine, phenothiazines and citalopram[74]. Another recognized risk factor is represented by the polytherapy and the concomitant use of medications known to prolong QT[13,75]. A prospective observation study carried out in the United States demonstrated the high prevalence of QTc prolongation in patients admitted to cardiac units, with 28% of patients presenting with QTc prolongation (defined as ≥ 470 ms in males and ≥ 480 ms in females) and 18.2% with a QTc > 500 ms at admission. The study revealed that of 251 patients admitted with QTc interval prolongation, 87 (34.7%) were subsequently administered QT interval-prolonging drugs, and 166 of the patients admitted with QTc interval > 500 ms, 70 (42.2%) were subsequently administered QT interval-prolonging drugs. Moreover additional QTc interval prolongation ≥ 60 ms occurred in 57.1% of these patients[76]. The authors also suggested that hospitalized patients are more prone to develop QTc prolongation due to the presence of other risk factors such as heart disease, electrolyte imbalance, and advanced age associated with the administration of QTc prolonging drugs. The statement from the AHA/ACCF underlined the importance of collecting the medical history and the risk factors of each single hospitalized patient in order identify those at higher risk and minimize the possibility of QTc prolongation and TdP. According to the AHA’s practice standards for ECG monitoring in hospital settings, the indications for QT-interval monitoring include the following: (1) initiation of a drug known to cause TdP; (2) overdose from potentially proarrhythmic agents; (3) new-onset bradyarrhythmias; and (4) severe hypokalemia or hypomagnesemia. Due to the lack of clarity regarding the types and amounts of drugs taken in an intentional overdose situation, it is prudent to monitor QT intervals in all overdose victims[38]. To achieve a long-term reduction in the risk of drug-induced QTc prolongation and TdP in hospitalized patients, a risk score has been developed and validated. The authors suggested that, by using easily obtainable risk factors, the risk score can identify patients at highest risk for in-hospital QTc prolongation and, thus, could be incorporated into clinical decision support system to guide monitoring and treatment decisions[30].
Among the drugs known to cause QT-prolongation, antipsychotic agents are probably the most studied. Since the development and marketing of the first molecules, a close relation has been found with QTc prolongation and an increased risk of major arrhythmic events such as TdP[77,78]. Antipsychotic drugs are widely used in the treatment of schizophrenia, mood disorders and somatic symptoms.
Conventional or first generation antipsychotics have been widely recognized as being associated with an increased risk of cardiac arrhythmias, TdP and SCD[79].
Since the early 1960s, sudden unexpected deaths with antipsychotic use have been reported[77].
Among the first generation antipsychotics, the described association between thioridazine and haloperidol with TdP or SCD led to the withdrawal of thioridazine from the market by its producer in 2005 and to the use of label warnings by the Food and Drug Administration for intravenous haloperidol[80,81]. However, the rate of ventricular arrhythmia, sudden death, and unexplained or unattended death did not appear to increase with the dosage for either thioridazine or haloperidol, suggesting that low-doses of these drugs have similar cardiac safety[82]. As in the case of thioridazine and haloperidol, data on the safety profile and possible link of other first generation antipsychotics with severe arrhythmias are contradictory[83-85]. When first introduced, atypical or second generation antipsychotics were considered to be safer than first generation antipsychotics as regards the QTc prolongation and arrhythmic risk[84,86,87]. However these promising data were not univocally confirmed[88]. Among the second generation antipsychotics available, lurasidone and aripiprazole seem to have minimal effect on QTc interval[89-91]. It should be noted that patients with schizophrenia are more likely to experience SCD and QTc prolongation than individuals from the general population because of treatment-related metabolic disorders and autonomic dysfunction linked to the underlying psychiatric illness[92,93]. Given all of these evidence, and considering the contradictory and inconclusive data available, it is easy to imagine how difficult the assessment of the individual risk in patients taking antipsychotic agents must be. In fact, drug-induced LQTS is unpredictable in any given individual. Because of all the possible medical complications associated with antipsychotics, Nachimuthu et al[62] and Shulman et al[94] highlighted the importance of a multidisciplinary approach which takes into account pre-existing heart or other diseases, personal or family history of ventricular arrhythmias or syncope, metabolic and endocrine disorders such as hypothyroidism and hypokalemia in order to reduce the risk of adverse events. Some authors recommend performing an ECG before and shortly after initiation of treatment with an antipsychotic drug in order to screen for existing or emergent prolongation of the QT interval[95]. The majority of authors recommend a baseline ECG for patients with personal or family history of cardiovascular disease or signs of arrhythmias, such as syncope, and for those patients taking another agent known to prolong QTc[96]. De Hert et al[97] performed a systematic review of the practice guidelines for the screening and monitoring of cardiometabolic risk in patients with schizophrenia and related psychotic disorders using the Appraisal of Guidelines for Research and Evaluation. The authors concluded that four European guidelines can be recommended for clinical use in daily clinical practice and proposed a monitoring protocol to manage cardiovascular disease risk. After a careful research of the literature, Trinkley et al[13] concluded that it is necessary to perform a careful and systematic monitoring of ECG and electrolytes, after the initiation of QT-interval-prolonging drugs. Where there are risk factors for QTc prolongation, patients should be trained to go to the emergency room in case of palpitations, lightheadedness, dizziness or syncope. When the QTc interval is 470-500 ms for males, or 480-500 ms for females, or the QTc interval increases by 60 ms or more from pretreatment values, dose reduction or discontinuation of the offending drug should be considered if possible, and electrolytes corrected as needed. Furthermore, if the QTc interval is ≥ 500 ms, the drug should be discontinued, and continuous ECG telemetry monitoring should be performed, or a 12-lead ECG should be repeated every 2-4 h, until the QT interval has normalized[13]. The American Psychiatric Association practice guidelines report that an absolute QTc interval > 500 ms or an increase of 60 ms from baseline requires dosage reduction or discontinuation of the agent. Serum potassium levels and an ECG should be obtained before initiating treatment with thioridazine, mesoridazine and pimozide and in the presence of cardiac risk factors, known heart disease, personal history of syncope, a family history of sudden death under 40 years age or LQTS before treatment with ziprazidone. An ECG should be performed again after a significant change in the dose of thioridazine, mesoridazine and pimozide or in the presence of cardiac risk factors, ziprasidone, or following the addition of another QT-prolonging medication[98,99]. Based on a relevant literature research, Shah et al[100] recently provided clinical practice guidelines for monitoring QTc intervals in patients being treated with antipsychotics. The authors reported that antipsychotics can be prescribed without pre-treatment ECG and without ECG monitoring, with the exception of patients with increased cardiac risk, antipsychotics with known risk of TdP and SCD, patients with an overdose of antipsychotic agents. In these cases ECG monitoring is recommended. If a pre-treatment ECG indicates a prolonged QTc, the ECG should be repeated and risk factors assessed and it is advisable to use lusaridone, which is the agent with minimal QTc prolongation. If QTc prolongation is assessed during antipsychotic treatment, the ECG should be repeated and monitored in case of continuous QTc prolongation in association with evaluation and correction of serum electrolytes. If a patient being treated complains of syncope or palpitations, discontinuation of the antipsychotic can be considered.
As already outlined, several cardiovascular diseases are associated to QTc prolongation. Those known to predispose to QT-prolongation are HF, cardiac arrhythmias, bradycardia, myocardial ischemia, and HCM[40-42]. Prolonged QTc reflects cardiac repolarization prolongation and/or increased repolarization inhomogenity known to be associated with an increased risk of arrhythmias[101].
QTc prolongation has been widely observed in patients with myocardial infarction and it has been suggested as one of the earliest ECG abnormalities in transmural ischemia[102] and a prognostic marker of arrhythmic events[103].
Thus, for some time, the assessment of QTc interval has been considered an interesting tool in the evaluation of the arrhythmic risk[104,105] improving the accuracy of the personalized cardiovascular prognosis when associated to conventional risk models for cardiovascular diseases[106].
However, data available in literature concerning the role of QTc prolongation in the risk assessment are not univocal. The QTc interval did not seem a useful prognostic tool after acute myocardial infarction[107] and reduced left ventricular ejection fraction (LVEF) and frequent ventricular premature complexes were found to be the most important factors for predicting subsequent SCD after acute myocardial infarction[108].
Similarly in patients with HF, baseline QTc interval within normal limits seems to be associated with a marked reduction in mortality, suggesting its possible usefulness in identifying patients who might benefit from prophylactic treatment with antiarrhythmic drugs[109]. Prolonged QTc interval has been found to be a strong, independent predictor of adverse outcomes in patients with advanced HF with BNP levels > 400 pg/mL[110].
At the same time, prolonged QRS interval, but not prolonged QTc interval, was observed to be associated with increased long-term mortality in patients with acute decompensated HF[111].
Moreover, the role of QTc prolongation as a marker of arrhythmic risk is not widely confirmed, sometimes judged “imperfect”, and nowadays its role remains controversial[112,113].
Despite the large number of studies on the evaluation of QTc in cardiovascular diseases, data about its utility are not consistent and the measurement of QTc interval in daily clinical practice is not widely carried out. Based on this background, other strategies to assess abnormalities of VR in cardiovascular diseases have been proposed.
The “simple” measurement of QT interval cannot always permit a complete assessment of the arrhythmic risk. The analysis of the QT dispersion seems to more accurately represent the non-uniform prolongation of APs and heterogeneity of the duration of the refractory periods and the conduction velocities of adjacent myocardial areas, thus better analyzing the perturbation of VR.
Various experimental studies have highlighted the close relationship between the dispersion of the myocardial repolarization and the development of ventricular arrhythmias[114-116].
A non-invasive method to highlight the inhomogeneity of myocardial repolarization time has been proposed, i.e., the measurement of the variability of the QT interval duration in the different leads of the standard 12-lead ECG[117,118].
QT dispersion (QTd) is the measurement of the variability of the QT interval on the 12-lead surface ECG, defined as the difference between the maximum QT and minimum QT calculated[119]. This phenomenon was described by Campbell et al[24] who demonstrated small but significant differences between the QT intervals of different leads measured by a digitizer program. The normal value of QTd is less than 50-70 ms. The standard deviation of repeat measurements is approximately 6 ms when an experienced observer measures from recordings made at 50 mm/s and 10 mV/cm. When the digitizer is used the normal rate-corrected values for QTd are between 20 ms and 50 ms with values after infarction rising to 60-100 ms and to as high as 150-200 ms in patients with LQTS[26].
There are more discrepancies about the determination of the end of the T wave, the lead group to be used, and the use of manual or automatic measurements. Surely, the greatest difficulties in the assessment of electrocardiographic VR are based essentially on the lack of universally accepted criteria for defining the end of the T wave. In particular, Kautzner et al[119] concluded that in the absence of more objective criteria for the separation of the T wave and U wave, measuring the QT dispersion appears to be unstable and its statistical validity is disputable.
New measurements have gradually been added to the traditional measurement of QT dispersion, among which are the following: (1) QTd in the precordial leads: The measurement is carried out only in the precordial leads, considered closer to the heart and, therefore, more reliable; (2) QTc-d in the 12 leads and in the precordial leads: The measurement is performed using the values of QTc (Bazett’s formula)[120]; (3) QT “adjusted”: The value of QT dispersion is adjusted to the number of leads on which the calculation is made (QTd/numbers of leads); (4) and QT “relative” and QTc “relative”: Respectively, the standard deviation of the QT/QT average x 100 ratio and the standard deviation of the QTc/QTc average x 100 ratio on the 12-lead ECG.
The electrophysiological mechanisms through which the dispersion of ventricular repolarization may induce ventricular arrhythmias are different. Kuo et al[116] identified at least three: The formation of an ectopic focus; the creation of a reentry circuit facilitated by conduction from an area with long refractoriness to an area with short refractoriness; and the creation of a reentry circuit facilitated by an area with short refractoriness to an area with prolonged refractoriness. The increase in the QTd has been associated to vulnerability in the development of ventricular tachycardia (VT), particularly in patients with previous myocardial infarction[121]. A cross sectional American study compared 100 patients with coronary artery disease and a history of arrhythmia events with 70 patients with previous myocardial infarction. QTd was measured in all cases.
It was observed that QTc and QTd were consistently higher in patients with susceptibility to episodes of sustained and unsustained VT as well as in the post infarction patients[122].
The mechanism underlying the origin of VT in post infarction has been recognized to be a mechanism of reentry[122-125]. The role of increased dispersion of repolarization in the genesis of ventricular fibrillation has also been generally accepted[126] and infarct scar and reentrant circuits are known to be substrates in the pathogenesis of sustained monomorphic VT[127]. Strong evidence supports the hypothesis that dispersion of refractoriness and repolarization provides a pathophysiologic basis for reentry[128,129]. Furthermore, QTd has been shown to reflect the dispersion of recovery times and repolarization. Thus, increased QTd suggests the presence of a substrate for ventricular tachy-arrhythmias, more realistically by a reentry mechanism[117,118,121,122]. Changes in QTd evaluated in a population of patients after percuteneous coronary intervention were predictors of long-term cardiac mortality, confirming how a defective QTd recovery suggests the persistence of repolarization inhomogeneities[130].
Measures of QT variability and dynamicity have been proposed as an alternative to the “static” evaluation of VR and seem to offer a more complete picture of the complexity of VR components.
Several kinds of softwares have been developed in order to dynamically analyse the QT interval and QTd from 24-h Holter recordings. Compared to the ECG static evaluation, dynamic assessment of VR allows the analysis of the relationship between the duration of the QT interval and HR changes and the effects of the ANS on both these elements. Moreover, through the measurement of the variability of VR duration and not its duration in absolute terms, this kind of analysis allows technical difficulties related to the exact definition of the T wave end to be reduced.
Dynamic behaviour of repolarization may translate in beat-to beat changes in repolarization duration and morphology[18,131].
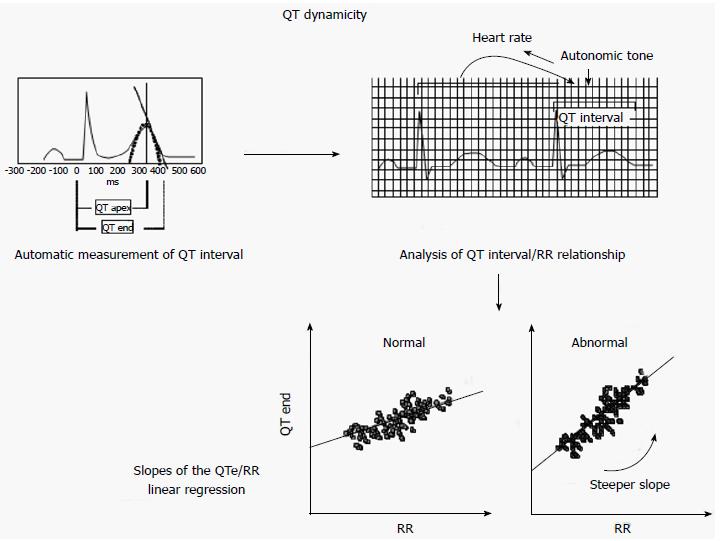
QT-RR relationship and QT variability reflect an increased vulnerability of the myocardium and changes in autonomic HR control, which are conditions related to the increased risk of SCD[132]. The automatic assessment of VR dynamicity is based on the measure of: QT apex (QTa): The interval between the Q wave start and the T wave apex. The T-wave apex is identified by interpolation of a parabola with the peak of the T-wave.
QT end (QTe): The interval between the Q wave start and the T wave end, T-wave end is determined by the intersection of the tangent of the downslope of the T-wave with the isoelectric baseline[7]. It has been shown that the QTa is more influenced by HR changes than QTe. The interval between the apex and the end of the T wave is rate independent and is probably influenced by the ANS and by activity of M cells that seem to be the cells involved in arrhythmogenesis[133,134].
QT dynamicity is generally assessed by 24 h ECG recordings which are analyzed by a specific software able to automatically calculate, in a template of 30 s the QTa, the QTe and the correspondent RR interval. By interpolating each measure obtained the software also computes the slopes of the linear regressions between QTe and QTa and the corresponding RR interval (QTe/RR and QTa/RR). A steeper slope reflects a greater variation of QT interval for changes in RR intervals (Figure 2).
QT-RR slope, i.e., the slope of the regression line between QT end and RR during a 24 h period, is considered an index of QT dynamicity related to arrhythmic events.
The automatic measure of VR by ELA system was validated by Copie et al[135] in a study that did not show significant differences between this kind of measurement and the manual measurement.
The QT-RR slope is highly individual. Generally the QT-RR slope is steeper in women than men and has higher values during the day than at night due to the ANS influence[136]. A steeper QTe slope indicates that the QT interval is more prolonged with longer RR intervals and shortens more with shorter RR intervals. The steeper the QTe-slope, the greater the arrhythmic risk[18]. Another parameter of QT dynamicity, QT/RR variability ratio, was proposed by Jensen et al[137]. This is the ratio between the standard deviation of all QT intervals and the standard deviation of all RR intervals. Whereas QT dynamicity is based on the analysis of QT/RR relationship, “QT variability” is based on the analysis of beat to beat changes in duration and the morphology of VR. HR and ANS can influence QT variability, but do not entirely explain beat to beat repolarization changes. These can depend on fluctuations in ion channel activity and number[18]. QT variability can be measured during a short-term (256 s or 30 beats) or 24-h period, distinguishing into short term and long term variability. Its measurement is not standardized, so several algorithms have been developed in order to quantify it. In 1997, Berger et al[9] proposed a first semiautomated algorithm able to measure temporal beat-to-beat lability of repolarization. In this instance, the operator selects the start and the end of the QT interval of one beat and the algorithm by stretching or compressing the JT segment identifies the QT interval of all other beats. Moreover, Berger et al[9] developed an index, called the QT variability index (QTVI), that is the log ratio between the QT interval and HR variability, both normalized by their mean values.
Most studies use this method in order to evaluate QT variability, but other algorithms have been proposed.
Burattini et al[138] proposed a time domain method which is able to quantify beat-to-beat variability of repolarization morphology without the need to exactly define the T wave end.
Starc et al[139] proposed a fully automated time-shifting algorithm that estimates the QT interval constructing separate QRS and T wave templates and shifting them in time. This algorithm has been shown to be the best method in order to measure QT variability[140]. On the basis of these considerations the assessment of QT dynamicity and/or variability allows a more complete evaluation of ventricular repolarization because they reflect the interaction between the arrhythmic substrate, the increased vulnerability of the myocardium, HR and ANS activity.
The increased QT variability and dynamicity probably build up repolarization heterogeneity inducing the onset of arrhythmias. Therefore a number of studies have investigated the role of an increased QT dynamicity and variability as predictor of arrhythmic events in different clinical settings. QT dynamicity was demonstrated to be able to predict major arrhythmic events in patients with both idiopathic and ischemic dilated cardiomyopathy, improving the accuracy in stratifying the arrhythmic risk of these patients. Our group evaluated the role of the QT slope as a predictor of a greater risk of ventricular arrhythmias in a population of patients affected by non-ischemic dilated cardiomyopathy. At univariate analysis, QTe-slope, LVEF, non-sustained VT, and standard deviation of RR intervals (SDNN) were the variables significantly related to major arrhythmic events. At multivariate analysis only the QTe-slope, LVEF and non-sustained VT remained significantly associated with these events, independently of SDNN, a QRS duration > 120 ms or beta-blocker therapy. Combining LVEF (< 35% vs > 35%), non-sustained VT and QTe-slope (> 0.19 vs < 0.19), patients with non-sustained VT and LVEF < 35% and patients with LVEF < 35% and a steeper QTe slope presented a greater risk of arrhythmic events than patients with a higher LVEF and non-sustained VT or steeper QTe slope. Considering these variables together, the population of patients with low LVEF and presence of non-sustained VT and a QTe slope > 0.19 represented the subgroup with the highest probability of arrhythmic events[7]. Chevalier et al[141] showed the association between a steeper QTe slope and a greater arrhythmic risk in a population of patients with ischemic cardiomiopathy. They found that QTe slope was a more powerful independent predictor of sudden death than LVEF, HR variability and late potentials in these patients. Another parameter of QT dynamicity, QT/RR variability ratio has been shown by Jensen et al[137] to be related to the sudden arrhythmic death risk in a population of post-acute myocardial infarction patients. Moreover, QTe slope was shown to be important in arrhythmic risk stratification also in patients with HF, regardless of etiology, both with relatively preserved LVEF (> 35%)[142] and with reduced LVEF[143]. Recently Quinteiro et al[144] evaluated the role of QTe slope in arrhythmic risk stratification in a small population of patients with HCM. They found that these patients presented an impaired QT dynamicity and QT slope was helpful in order to identify high risk patients. Several studies have also demonstrated the prognostic role of “beat to beat” QT variability. Berger et al[9] showed that there was an increased repolarization variability in a group of patients with ischemic and non-ischemic dilated cardiomyopathy, compared with control subjects, regardless of HR. Subsequent studies confirmed the increased repolarization variability in ischemic and non-ischemic cardiomyopathy and in different clinical settings, including acute myocardial ischemia, left ventricular hypertrophy, HCM, left ventricular dysfunction and LQTS[145]. Atiga et al[146] demonstrated the relation between an increased QTVI and arrhythmic risk in a population of patients presenting for electrophysiologic study. The QTVI had greater values in patients with ischemic or non- ischemic heart disease than in healthy subjects, and among patients with cardiac disease those with SCD had the highest values. A QTVI ≥ 0.1 was a predictor of a higher risk of arrhythmias. Therefore the authors concluded that this index identified patients with SCD, and predicted arrhythmia-free survival. The same group also investigated the prognostic role of QT variability in a population of patients with HCM, concluding that patients with HCM presented the increased repolarization variability and had a greater risk of SCD. The highest QTVI values were found in patients with b-MHC gene mutation, a mutation associated with a worse prognosis[147]. In a substudy of the MADIT trial, Haigney et al[6] have shown that, in a wide population of postinfarction patients with a low LVEF, increased QT variability was associated to the occurrence of malignant ventricular arrhythmias (VT/ventricular fibrillation). Piccirillo et al[148] also evaluated the role of QT variability in a population of patients with ischemic cardiomyopathy but with a LVEF between 35 % and 40% and in NHYA class I. A QTVI greater than or equal to the 80th percentile identified a high risk of SCD, therefore this parameter might be useful to stratify the risk of sudden death in this population of patients who do not currently meet the criteria for ICD implantation for primary prevention. Tereshchenko et al[149] investigated the role of QT variability in a population of patients with cardiac structural disease who had undergone ICD implantation for primary or secondary prevention of SCD. This study confirmed the prognostic utility of the increased repolarization variability, able to predict the malignant arrhythmic events risk in this population of patients. Nevertheless, the same group more recently investigated the prognostic role of QT variability in a population of patients with chronic HF (NYHA class II-III), both with preserved and reduced LVEF and found that an increased QTVI was a predictor of cardiovascular mortality, but not of SCD, regardless of LVEF. The hypothesis of the authors was that in these patients the elevated QTVI was due to depressed HR variability, a predictor of cardiovascular mortality, and not to an increased QT beat to beat variability[150]. In a heterogeneous population of patients with mild to moderate HF, the role of QTVI in predicting increased cardiovascular mortality has been investigated. The authors observed that QTVI, as an expression of increased repolarization liability, is a marker of increased risk of cardiovascular mortality[151]. Finally, a very recent study on a population of patients with ischemic dilated cardiomyopathy, both with reduced and with preserved LVEF, confirmed the utility of QT variability in order to identify the patients with a higher risk of SCD[152]. Therefore increased QT/RR slopes and an increased QT variability can reflect a greater vulnerability of the myocardium and predispose the development of malignant arrhythmias. Thus the dynamic analysis of VR represents an interesting tool to improve the accuracy in stratifying the risk of arrhythmic events.
Electrical alternans is defined as a change of the amplitude of the waves of the ECG which manifests itself at alternate heartbeats. It was described for the first time by Hering[153] in 1908. Much of the interest in the alternans phenomenon has focused on alternans occurring during the repolarization phase of the cardiac APs, also known as repolarization alternans and in particular in microvolt level beat-to-beat alternation in T-wave morphology: The microvolt T wave alternans (MTWA). That has been recognized as being a strong predictor of ventricular arrhythmias, as assessed in a variety of clinical and experimental studies[154-157]. There are two main theories that explain this mechanism. The first refers to a spatial dispersion of refractoriness which results in changes in the impulse propagation and repolarization. In this case the alternation of repolarization would be secondary to the alternation in the propagation of the impulse. This would occur when the refractory period of the cell is shorter than the time between two successive activations. It would block one-way and re-entry. This hypothesis was supported by an experimental study.. According to the second hypothesis MTWA would be caused by an alternation in repolarization of the APs resulting in a secondary propagation of alternans APs[158-160].
The analysis is based on the alignment of ECG cycles to the QRS complex and on the measurement of T-wave amplitude. The beat-to-beat fluctuations of the T-wave are then analyzed using fast Fourier transformation and MTWA is represented by the pronounced peak visible in the spectrum at 0.5 cycles/beat. The MTWA is considered significant when the alternans voltage exceeds a threshold (usually 1.9 microV) and if the alternans ratio K is ≥ 3. In general, we can judge as positive an alternans lasting more than 1 min, at a HR ≤ 110 beats/min[161]. Because of the strong correlation between the phenomenon of MWTA and the occurrence of ventricular re-entrant arrhythmias, its identification was proposed as a test to stratify the risk of ventricular arrhythmias[162,163].
The phenomenon is dependent on the HR. The treadmill test, a non-invasive and inexpensive test, is generally used for recognition. However, there are discordant opinions on the fact that a negative MTWA test can really identify a group of patients at extremely low risk of SCD or cardiac arrest[164-167].
Hohnloser et al[168] found that MWTA is predictive of major ventricular arrhythmias with a very low event rate among patients with a negative MWTA. In another meta-analysis, a positive MTWA was associated with a 2.5-fold higher risk of cardiac death and severe arrhythmias in both ischemic and non-ischemic cardiomyopathy[169]. The Alternans Before Cardioverter Defibrillator (ABCD) trial demonstrated the role of MWTA in guiding the ICD implantation for primary prevention in patients with LVEF ≤ 40%, coronary artery disease and non-sustained VT[170]. The analysis of data from five studies involving 2883 patients without ICD demonstrated that in patients with a LVEF > 35%, a positive MWTA identified those more prone to experience major arrhythmic events and SCD[171].
Despite the evidence suggesting a possible role of MWTA in predicting the arrhythmic risk, discordant data are available in the literature. Gupta et al[172] demonstrated that spectrally derived MTWA testing has limitations in its feasibility and is not specific enough to evaluate the arrhythmic risk and to guide strategic clinical decisions. In a population of patients with HF, Kraaier et al[173] observed that MTWA testing could be carried out only in a half of the population, and often resulted indeterminate. The authors concluded that MTWA treadmill testing is not suitable to stratify the risk of SCD in patients with HF.
There is much evidence that underlines the role of VR alterations in predisposing to lethal arrhythmias and the analysis of VR has become an interesting tool to refine the risk stratification of arrhythmic events. Different cardiac structural and electrical alterations may cause abnormalities in APs and in refractory period leading to perturbations of repolarization that can favor the onset of severe ventricular arrhythmias. Several measures of ventricular repolarization have been suggested with the aim of stratifying the arrhythmic risk allowing identification of those patients more prone to experiencing major arrhythmic events.
The measure of QT interval has gained growing interest since the first description of congenital LQTS and SQTS. Moreover, several factors such as drugs, advanced age, female sex, personal medical history, metabolic and electrolyte disorders can cause QTc prolongations and predispose to arrhythmic events. However, despite the vast number of studies evaluating the role of prolonged QTc as a marker of arrhythmic risk, opinions on the utility of QTc measurement in daily clinical practice are not univocal.
In order to overcome some of the limitations of QTc assessment, other strategies evaluating VR have been proposed. The analysis of the QTd seems to more accurately represent the non-uniform prolongation of APs and heterogeneity of the duration of the refractory periods and the conduction velocities of adjacent myocardial areas, allowing a better analysis of VR. The role of QTd analysis as an arrhythmic risk predictor has been well-established, but this evaluation of VR has not always been effective in identifying patients at higher risk of arrhythmic events. Moreover there are no recommended cut-off values for QTd.
Therefore, dynamic measures of VR, such as QT dynamicity and variability, reflecting the dynamic behaviour of VR, were developed as prognostic markers of arrhythmic risk. In addition, MWTA, another ECG parameter expression of alternans during VR, has been shown to predict arrhythmic events, although there are limitations in its feasibility. However there are no recommendations for the routine use of these parameters.
In conclusion, several measure of VR have been proposed but, despite the first studies concerning VR being nearly outdated, their role as a predictor of ventricular arrhythmias is not always clear and definite recommendations on their use in different clinical settings and for each single patient are still lacking and unfocused.
More attention should be paid to collecting a complete medical history of the patient and detecting the presence of well-established risk factors, cardiovascular conditions and medications known to cause QTc prolongation.
In the absence of one-size-fits all approach to the risk stratification of arrhythmic events, it is desirable to combine different measures of VR with other predictors in each specific clinical setting.
Further robust and tailored studies are required to settle the existing issues and provide useful prognostic tools for clinician.
P- Reviewer: Caceres-Loriga F, Chang ST S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Yan GX, Lankipalli RS, Burke JF, Musco S, Kowey PR. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol. 2003;42:401-409. [PubMed] |
| 2. | Van damr D. The t wave and ventricular repolarization. Am J Cardiol. 1964;14:294-300. [PubMed] |
| 3. | Franz MR, Bargheer K, Rafflenbeul W, Haverich A, Lichtlen PR. Monophasic action potential mapping in human subjects with normal electrocardiograms: direct evidence for the genesis of the T wave. Circulation. 1987;75:379-386. [PubMed] |
| 4. | Zareba W, Moss AJ, le Cessie S. Dispersion of ventricular repolarization and arrhythmic cardiac death in coronary artery disease. Am J Cardiol. 1994;74:550-553. [PubMed] |
| 5. | Ikeda T, Sakata T, Takami M, Kondo N, Tezuka N, Nakae T, Noro M, Enjoji Y, Abe R, Sugi K. Combined assessment of T-wave alternans and late potentials used to predict arrhythmic events after myocardial infarction. A prospective study. J Am Coll Cardiol. 2000;35:722-730. [PubMed] |
| 6. | Haigney MC, Zareba W, Gentlesk PJ, Goldstein RE, Illovsky M, McNitt S, Andrews ML, Moss AJ. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2004;44:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Iacoviello M, Forleo C, Guida P, Romito R, Sorgente A, Sorrentino S, Catucci S, Mastropasqua F, Pitzalis M. Ventricular repolarization dynamicity provides independent prognostic information toward major arrhythmic events in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2007;50:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Iacoviello M, Monitillo F. Non-invasive evaluation of arrhythmic risk in dilated cardiomyopathy: From imaging to electrocardiographic measures. World J Cardiol. 2014;6:562-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96:1557-1565. [PubMed] |
| 10. | Turrini P, Corrado D, Basso C, Nava A, Bauce B, Thiene G. Dispersion of ventricular depolarization-repolarization: a noninvasive marker for risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2001;103:3075-3080. [PubMed] |
| 11. | Coudercj JP. Measurement and regulation of cardiac ventricular repolarization: from the QT interval to repolarization morphology. Philos Trans A Math Phys Eng Sci. 2009;367:1283-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Jardin CG, Putney D, Michaud S. Assessment of drug-induced torsade de pointes risk for hospitalized high-risk patients receiving QT-prolonging agents. Ann Pharmacother. 2014;48:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Trinkley KE, Page RL, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013;29:1719-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation. 1978;57:1074-1077. [PubMed] |
| 15. | Monahan BP, Ferguson CL, Killeavy ES, Lloyd BK, Troy J, Cantilena LR. Torsades de pointes occurring in association with terfenadine use. JAMA. 1990;264:2788-2790. [PubMed] |
| 16. | Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013-1022. [PubMed] |
| 17. | Grimm W, Christ M, Bach J, Müller HH, Maisch B. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation. 2003;108:2883-2891. [PubMed] |
| 18. | Zareba W, Bayes de Luna A. QT dynamics and variability. Ann Noninvasive Electrocardiol. 2005;10:256-262. [PubMed] |
| 19. | Willems JL, Arnaud P, van Bemmel JH, Degani R, Macfarlane PW, Zywietz C. Common standards for quantitative electrocardiography: goals and main results. CSE Working Party. Methods Inf Med. 1990;29:263-271. [PubMed] |
| 20. | Savelieva I, Yi G, Guo X, Hnatkova K, Malik M. Agreement and reproducibility of automatic versus manual measurement of QT interval and QT dispersion. Am J Cardiol. 1998;81:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Murray A, McLaughlin NB, Bourke JP, Doig JC, Furniss SS, Campbell RW. Errors in manual measurement of QT intervals. Br Heart J. 1994;71:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Sano M, Aizawa Y, Katsumata Y, Nishiyama N, Takatsuki S, Kamitsuji S, Kamatani N, Fukuda K. Evaluation of differences in automated QT/QTc measurements between Fukuda Denshi and Nihon Koden systems. PLoS One. 2014;9:e106947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Lepeschkin E, Surawicz B. The measurement of the Q-T interval of the electrocardiogram. Circulation. 1952;6:378-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 445] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 24. | Campbell RW, Gardiner P, Amos PA, Chadwick D, Jordan RS. Measurement of the QT interval. Eur Heart J. 1985;6 Suppl D:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Cowan JC, Yusoff K, Moore M, Amos PA, Gold AE, Bourke JP, Tansuphaswadikul S, Campbell RW. Importance of lead selection in QT interval measurement. Am J Cardiol. 1988;61:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Higham PD, Campbell RW. QT dispersion. Br Heart J. 1994;71:508-510. [PubMed] |
| 27. | Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA. 2003;289:2120-2127. [PubMed] |
| 28. | Morganroth J, Brozovich FV, McDonald JT, Jacobs RA. Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiol. 1991;67:774-776. [PubMed] |
| 29. | Viskin S, Rosovski U, Sands AJ, Chen E, Kistler PM, Kalman JM, Rodriguez Chavez L, Iturralde Torres P, Cruz F FE, Centurión OA. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Heart Rhythm. 2005;2:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 30. | Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, Kovacs RJ. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 31. | Anderson ME, Al-Khatib SM, Roden DM, Califf RM. Cardiac repolarization: current knowledge, critical gaps, and new approaches to drug development and patient management. Am Heart J. 2002;144:769-781. [PubMed] |
| 32. | Fridericia LS. The duration of systole in an electrocardiogram in normal humans and in patients with heart disease. 1920. Ann Noninvasive Electrocardiol. 2003;8:343-351. [PubMed] |
| 34. | Aytemir K, Maarouf N, Gallagher MM, Yap YG, Waktare JE, Malik M. Comparison of formulae for heart rate correction of QT interval in exercise electrocardiograms. Pacing Clin Electrophysiol. 1999;22:1397-1401. [PubMed] |
| 35. | Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001;12:411-420. [PubMed] |
| 36. | Boyle NG, Weiss JN. Making QT correction simple is complicated. J Cardiovasc Electrophysiol. 2001;12:421-423. [PubMed] |
| 37. | Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 611] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 38. | Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 39. | Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888-1894. [PubMed] |
| 40. | Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Jui J. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 41. | Johnson JN, Grifoni C, Bos JM, Saber-Ayad M, Ommen SR, Nistri S, Cecchi F, Olivotto I, Ackerman MJ. Prevalence and clinical correlates of QT prolongation in patients with hypertrophic cardiomyopathy. Eur Heart J. 2011;32:1114-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Wu MH. Left ventricular-to-right atrial shunt in perimembranous trabecular ventricular septal defect with aneurysmal transformation. Am J Cardiol. 1990;65:1049-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Robinson JL, Priori SG, Benhorin J, Locati EH, Towbin JA, Keating MT. Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960-965. [PubMed] |
| 44. | De Bruin ML, Langendijk PN, Koopmans RP, Wilde AA, Leufkens HG, Hoes AW. In-hospital cardiac arrest is associated with use of non-antiarrhythmic QTc-prolonging drugs. Br J Clin Pharmacol. 2007;63:216-223. [PubMed] |
| 45. | Jervell A, Lange-nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J. 1957;54:59-68. [PubMed] |
| 46. | Campuzano O, Sarquella-Brugada G, Mademont-Soler I, Allegue C, Cesar S, Ferrer-Costa C, Coll M, Mates J, Iglesias A, Brugada J. Identification of Genetic Alterations, as Causative Genetic Defects in Long QT Syndrome, Using Next Generation Sequencing Technology. PLoS One. 2014;9:e114894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Goldenberg I, Zareba W, Moss AJ. Long QT Syndrome. Curr Probl Cardiol. 2008;33:629-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89-95. [PubMed] |
| 49. | Mizusawa Y, Horie M, Wilde AA. Genetic and clinical advances in congenital long QT syndrome. Circ J. 2014;78:2827-2833. [PubMed] |
| 50. | Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace. 2013;15:1389-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 422] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 51. | Kapplinger JD, Giudicessi JR, Ye D, Tester DJ, Callis TE, Valdivia CR, Makielski JC, Wilde AA, Ackerman MJ. Enhanced Classification of Brugada Syndrome-Associated and Long-QT Syndrome-Associated Genetic Variants in the SCN5A-Encoded Na(v)1.5 Cardiac Sodium Channel. Circ Cardiovasc Genet. 2015;8:582-595. [PubMed] |
| 52. | Ma D, Wei H, Lu J, Huang D, Liu Z, Loh LJ, Islam O, Liew R, Shim W, Cook SA. Characterization of a novel KCNQ1 mutation for type 1 long QT syndrome and assessment of the therapeutic potential of a novel IKs activator using patient-specific induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther. 2015;6:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 53. | Kolder IC, Tanck MW, Postema PG, Barc J, Sinner MF, Zumhagen S, Husemann A, Stallmeyer B, Koopmann TT, Hofman N. Analysis for Genetic Modifiers of Disease Severity in Patients With Long-QT Syndrome Type 2. Circ Cardiovasc Genet. 2015;8:447-456. [PubMed] |
| 54. | Schwartz PJ, Crotti L. QTc behavior during exercise and genetic testing for the long-QT syndrome. Circulation. 2011;124:2181-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 55. | Gussak I, Brugada P, Brugada J, Wright RS, Kopecky SL, Chaitman BR, Bjerregaard P. Idiopathic short QT interval: a new clinical syndrome? Cardiology. 2000;94:99-102. [PubMed] |
| 56. | Gaita F, Giustetto C, Bianchi F, Wolpert C, Schimpf R, Riccardi R, Grossi S, Richiardi E, Borggrefe M. Short QT Syndrome: a familial cause of sudden death. Circulation. 2003;108:965-970. [PubMed] |
| 57. | Frea S, Giustetto C, Capriolo M, Scrocco C, Fornengo C, Benedetto S, Bianchi F, Pidello S, Morello M, Gaita F. New echocardiographic insights in short QT syndrome: More than a channelopathy? Heart Rhythm. 2015;12:2096-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, Menendez TM, Brugada J, Pollevick GD, Wolpert C. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109:30-35. [PubMed] |
| 59. | Guss SB, Kastor JA, Josephson ME, Schare DL. Human ventricular refractoriness. Effects of cycle length, pacing site and atropine. Circulation. 1976;53:450-455. [PubMed] |
| 60. | Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795-803. [PubMed] |
| 61. | Roden DM, Viswanathan PC. Genetics of acquired long QT syndrome. J Clin Invest. 2005;115:2025-2032. [PubMed] |
| 62. | Nachimuthu S, Assar MD, Schussler JM. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3:241-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 63. | Rajamani S, Eckhardt LL, Valdivia CR, Klemens CA, Gillman BM, Anderson CL, Holzem KM, Delisle BP, Anson BD, Makielski JC. Drug-induced long QT syndrome: hERG K+ channel block and disruption of protein trafficking by fluoxetine and norfluoxetine. Br J Pharmacol. 2006;149:481-489. [PubMed] |
| 64. | Kuryshev YA, Wang L, Wible BA, Wan X, Ficker E. Antimony-based antileishmanial compounds prolong the cardiac action potential by an increase in cardiac calcium currents. Mol Pharmacol. 2006;69:1216-1225. [PubMed] |
| 65. | Arking DE, Pulit SL, Crotti L, van der Harst P, Munroe PB, Koopmann TT, Sotoodehnia N, Rossin EJ, Morley M, Wang X. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat Genet. 2014;46:826-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 66. | Makita N, Horie M, Nakamura T, Ai T, Sasaki K, Yokoi H, Sakurai M, Sakuma I, Otani H, Sawa H. Drug-induced long-QT syndrome associated with a subclinical SCN5A mutation. Circulation. 2002;106:1269-1274. [PubMed] |
| 67. | Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore). 2003;82:282-290. [PubMed] |
| 68. | Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590-2597. [PubMed] |
| 69. | Vieweg WV, Wood MA, Fernandez A, Beatty-Brooks M, Hasnain M, Pandurangi AK. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26:997-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Brown DW, Giles WH, Greenlund KJ, Valdez R, Croft JB. Impaired fasting glucose, diabetes mellitus, and cardiovascular disease risk factors are associated with prolonged QTc duration. Results from the Third National Health and Nutrition Examination Survey. J Cardiovasc Risk. 2001;8:227-233. [PubMed] |
| 71. | Yang T, Roden DM. Extracellular potassium modulation of drug block of IKr. Implications for torsade de pointes and reverse use-dependence. Circulation. 1996;93:407-411. [PubMed] |
| 72. | Mozos I. Arrhythmia risk in liver cirrhosis. World J Hepatol. 2015;7:662-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 73. | Bakiner O, Ertorer ME, Haydardedeoglu FE, Bozkirli E, Tutuncu NB, Demirag NG. Subclinical hypothyroidism is characterized by increased QT interval dispersion among women. Med Princ Pract. 2008;17:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Girardin FR, Gex-Fabry M, Berney P, Shah D, Gaspoz JM, Dayer P. Drug-induced long QT in adult psychiatric inpatients: the 5-year cross-sectional ECG Screening Outcome in Psychiatry study. Am J Psychiatry. 2013;170:1468-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 75. | Sala M, Vicentini A, Brambilla P, Montomoli C, Jogia JR, Caverzasi E, Bonzano A, Piccinelli M, Barale F, De Ferrari GM. QT interval prolongation related to psychoactive drug treatment: a comparison of monotherapy versus polytherapy. Ann Gen Psychiatry. 2005;4:1. [PubMed] |
| 76. | Tisdale JE, Wroblewski HA, Overholser BR, Kingery JR, Trujillo TN, Kovacs RJ. Prevalence of QT interval prolongation in patients admitted to cardiac care units and frequency of subsequent administration of QT interval-prolonging drugs: a prospective, observational study in a large urban academic medical center in the US. Drug Saf. 2012;35:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Kelly HG, Fay JE, Laverty SG. Thioridazine hydrochloride (mellaril): its effect on the electrocardiogram and a report of two fatalities with electrocardiographic abnormalities. Can Med Assoc J. 1963;89:546-554. [PubMed] |
| 78. | Mehta D, Mehta S, Petit J, Shriner W. Cardiac arrhythmia and haloperidol. Am J Psychiatry. 1979;136:1468-1469. [PubMed] |
| 79. | Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation, torsade de pointes and sudden death. Drugs. 2002;62:1649-1671. [PubMed] |
| 80. | Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH. Thioridazine and sudden unexplained death in psychiatric in-patients. Br J Psychiatry. 2002;180:515-522. [PubMed] |
| 81. | Freeman I, Grunwald AM, Robin B, Rao PS, Bodenheimer MM. Effect of early reperfusion on use of triphenyltetrazolium chloride to differentiate viable from non-viable myocardium in area of risk. Cardiovasc Res. 1990;24:109-114. [PubMed] |
| 82. | Hennessy S, Bilker WB, Knauss JS, Kimmel SE, Margolis DJ, Morrison MF, Reynolds RF, Glasser DB, Strom BL. Comparative cardiac safety of low-dose thioridazine and low-dose haloperidol. Br J Clin Pharmacol. 2004;58:81-87. [PubMed] |
| 83. | Hoehns JD, Stanford RH, Geraets DR, Skelly KS, Lee HC, Gaul BL. Torsades de pointes associated with chlorpromazine: case report and review of associated ventricular arrhythmias. Pharmacotherapy. 2001;21:871-883. [PubMed] |
| 84. | Leonard CE, Freeman CP, Newcomb CW, Bilker WB, Kimmel SE, Strom BL, Hennessy S. Antipsychotics and the Risks of Sudden Cardiac Death and All-Cause Death: Cohort Studies in Medicaid and Dually-Eligible Medicaid-Medicare Beneficiaries of Five States. J Clin Exp Cardiolog. 2013;Suppl 10:1-9. [PubMed] |
| 85. | Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1048-1052. [PubMed] |
| 86. | Liperoti R, Gambassi G, Lapane KL, Chiang C, Pedone C, Mor V, Bernabei R. Conventional and atypical antipsychotics and the risk of hospitalization for ventricular arrhythmias or cardiac arrest. Arch Intern Med. 2005;165:696-701. [PubMed] |
| 87. | Murray-Thomas T, Jones ME, Patel D, Brunner E, Shatapathy CC, Motsko S, Van Staa TP. Risk of mortality (including sudden cardiac death) and major cardiovascular events in atypical and typical antipsychotic users: a study with the general practice research database. Cardiovasc Psychiatry Neurol. 2013;2013:247486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 818] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 89. | Chung AK, Chua SE. Effects on prolongation of Bazett’s corrected QT interval of seven second-generation antipsychotics in the treatment of schizophrenia: a meta-analysis. J Psychopharmacol. 2011;25:646-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 90. | Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65:189-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 91. | Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 92. | Koponen H, Alaräisänen A, Saari K, Pelkonen O, Huikuri H, Raatikainen MJ, Savolainen M, Isohanni M. Schizophrenia and sudden cardiac death: a review. Nord J Psychiatry. 2008;62:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Fujii K, Ozeki Y, Okayasu H, Takano Y, Shinozaki T, Hori H, Orui M, Horie M, Kunugi H, Shimoda K. QT is longer in drug-free patients with schizophrenia compared with age-matched healthy subjects. PLoS One. 2014;9:e98555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Shulman M, Miller A, Misher J, Tentler A. Managing cardiovascular disease risk in patients treated with antipsychotics: a multidisciplinary approach. J Multidiscip Healthc. 2014;7:489-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Schneeweiss S, Avorn J. Antipsychotic agents and sudden cardiac death--how should we manage the risk? N Engl J Med. 2009;360:294-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Wu CS, Tsai YT, Tsai HJ. Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: a nation-wide case-crossover study. J Am Heart Assoc. 2015;4:pii: e001568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 97. | De Hert M, Vancampfort D, Correll CU, Mercken V, Peuskens J, Sweers K, van Winkel R, Mitchell AJ. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 98. | Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161:1-56. [PubMed] |
| 99. | Lieberman JA, Merrill D, Parameswaran S. APA Guidance on the Use of Antipsychotic Drugs and Cardiac Sudden Death. [accessed 2009]. Available from: https://www.omh.ny.gov/omhweb/advisories/adult_antipsychotic_use_attachement.html. |
| 100. | Shah AA, Aftab A, Coverdale J. QTc prolongation with antipsychotics: is routine ECG monitoring recommended? J Psychiatr Pract. 2014;20:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 101. | Elming H, Brendorp B, Køber L, Sahebzadah N, Torp-Petersen C. QTc interval in the assessment of cardiac risk. Card Electrophysiol Rev. 2002;6:289-294. [PubMed] |
| 102. | Kenigsberg DN, Khanal S, Kowalski M, Krishnan SC. Prolongation of the QTc interval is seen uniformly during early transmural ischemia. J Am Coll Cardiol. 2007;49:1299-1305. [PubMed] |
| 103. | Ahnve S. QT interval prolongation in acute myocardial infarction. Eur Heart J. 1985;6 Suppl D:85-95. [PubMed] |
| 104. | Locati E, Schwartz PJ. Prognostic value of QT interval prolongation in post myocardial infarction patients. Eur Heart J. 1987;8 Suppl A:121-126. [PubMed] |
| 105. | Kannan KK, Petef M, Fridborg K, Cid-Dresdner H, Lövgren S. Structure and function of carbonic anhydrases. Imidazole binding to human carbonic anhydrase B and the mechanism of action of carbonic anhydrases. FEBS Lett. 1977;73:115-119. [PubMed] |
| 106. | Nielsen JB, Graff C, Rasmussen PV, Pietersen A, Lind B, Olesen MS, Struijk JJ, Haunsø S, Svendsen JH, Køber L. Risk prediction of cardiovascular death based on the QTc interval: evaluating age and gender differences in a large primary care population. Eur Heart J. 2014;35:1335-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 107. | Pohjola-Sintonen S, Siltanen P, Haapakoski J. Usefulness of QTc interval on the discharge electrocardiogram for predicting survival after acute myocardial infarction. Am J Cardiol. 1986;57:1066-1068. [PubMed] |
| 108. | Wheelan K, Mukharji J, Rude RE, Poole WK, Gustafson N, Thomas LJ, Strauss HW, Jaffe AS, Muller JE, Roberts R. Sudden death and its relation to QT-interval prolongation after acute myocardial infarction: two-year follow-up. Am J Cardiol. 1986;57:745-750. [PubMed] |
| 109. | Brendorp B, Elming H, Jun L, Køber L, Malik M, Jensen GB, Torp-Pedersen C. Qtc interval as a guide to select those patients with congestive heart failure and reduced left ventricular systolic function who will benefit from antiarrhythmic treatment with dofetilide. Circulation. 2001;103:1422-1427. [PubMed] |
| 110. | Vrtovec B, Delgado R, Zewail A, Thomas CD, Richartz BM, Radovancevic B. Prolonged QTc interval and high B-type natriuretic peptide levels together predict mortality in patients with advanced heart failure. Circulation. 2003;107:1764-1769. [PubMed] |
| 111. | Breidthardt T, Christ M, Matti M, Schrafl D, Laule K, Noveanu M, Boldanova T, Klima T, Hochholzer W, Perruchoud AP. QRS and QTc interval prolongation in the prediction of long-term mortality of patients with acute destabilised heart failure. Heart. 2007;93:1093-1097. [PubMed] |
| 112. | Ahmad K, Dorian P. Drug-induced QT prolongation and proarrhythmia: an inevitable link? Europace. 2007;9 Suppl 4:iv16-iv22. |
| 113. | Jacobson I, Carlsson L, Duker G. Beat-by-beat QT interval variability, but not QT prolongation per se, predicts drug-induced torsades de pointes in the anaesthetised methoxamine-sensitized rabbit. J Pharmacol Toxicol Methods. 2001;63:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 114. | Han J, Moe GK. Nonuniform recovery of excitability in ventricular muscle. Circ Res. 1964;14:44-60. [PubMed] |
| 115. | Merx W, Yoon MS, Han J. The role of local disparity in conduction and recovery time on ventricular vulnerability to fibrillation. Am Heart J. 1977;94:603-610. [PubMed] |
| 116. | Kuo CS, Munakata K, Reddy CP, Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983;67:1356-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 524] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 117. | Day CP, McComb JM, Campbell RW. QT dispersion: an indication of arrhythmia risk in patients with long QT intervals. Br Heart J. 1990;63:342-344. [PubMed] |
| 118. | Day CP, McComb JM, Campbell RW. QT dispersion in sinus beats and ventricular extrasystoles in normal hearts. Br Heart J. 1992;67:39-41. [PubMed] |
| 119. | Kautzner J, Gang Y, Kishore AGR, Copie X, Janota T, Nagayoshi H, Camm AJ, Malik M. Interobserver reproducibility of QT interval and QT dispersion in patients after acute myocardial infarction. Ann Noninv Electrocardiol. 1996;1:363-374. |
| 120. | Couderc JP, Xiaojuan X, Zareba W, Moss AJ. Assessment of the stability of the individual-based correction of QT interval for heart rate. Ann Noninvasive Electrocardiol. 2005;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 121. | Zaidi M, Robert A, Fesler R, Derwael C, Brohet C. Dispersion of ventricular repolarisation: a marker of ventricular arrhythmias in patients with previous myocardial infarction. Heart. 1997;78:371-375. [PubMed] |
| 122. | Perkiömäki JS, Huikuri HV, Koistinen JM, Mäkikallio T, Castellanos A, Myerburg RJ. Heart rate variability and dispersion of QT interval in patients with vulnerability to ventricular tachycardia and ventricular fibrillation after previous myocardial infarction. J Am Coll Cardiol. 1997;30:1331-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 123. | Josephson ME, Horowitz LN, Farshidi A, Kastor JA. Recurrent sustained ventricular tachycardia. 1. Mechanisms. Circulation. 1978;57:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 480] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 124. | Josephson ME, Horowitz LN, Farshidi A, Spear JF, Kastor JA, Moore EN. Recurrent sustained ventricular tachycardia. 2. Endocardial mapping. Circulation. 1978;57:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 276] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 125. | de Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE, Dingemans KP, van Hemel NM, Hauer RN. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77:589-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 550] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 126. | Kuo CS, Reddy CP, Munakata K, Surawicz B. Mechanism of ventricular arrhythmias caused by increased dispersion of repolarization. Eur Heart J. 1985;6 Suppl D:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 127. | Vaitkus PT, Kindwall KE, Marchlinski FE, Miller JM, Buxton AE, Josephson ME. Differences in electrophysiological substrate in patients with coronary artery disease and cardiac arrest or ventricular tachycardia. Insights from endocardial mapping and signal-averaged electrocardiography. Circulation. 1991;84:672-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 128. | Kuo CS, Atarashi H, Reddy CP, Surawicz B. Dispersion of ventricular repolarization and arrhythmia: study of two consecutive ventricular premature complexes. Circulation. 1985;72:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 129. | Downar E, Harris L, Mickleborough LL, Shaikh N, Parson ID. Endocardial mapping of ventricular tachycardia in the intact human ventricle: evidence for reentrant mechanisms. J Am Coll Cardiol. 1988;11:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 130. | Zimarino M, Corazzini A, Tatasciore A, Marazia S, Torge G, Di Iorio C, De Caterina R. Defective recovery of QT dispersion predicts late cardiac mortality after percutaneous coronary intervention. Heart. 2011;97:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 131. | Coumel P, Maison-Blanche P, Badilini F. Dispersion of ventricular repolarization: reality? Illusion? Significance? Circulation. 1998;97:2491-2493. [PubMed] |
| 132. | Zareba W. QT-RR slope: dynamics of repolarization in the risk stratification. J Cardiovasc Electrophysiol. 2003;14:234-235. [PubMed] |
| 133. | Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 656] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 134. | Porta A, Tobaldini E, Gnecchi-Ruscone T, Montano N. RT variability unrelated to heart period and respiration progressively increases during graded head-up tilt. Am J Physiol Heart Circ Physiol. 2010;298:H1406-H1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 135. | Copie X, Alonso C, Lavergne T, Iliou MC, Guize L, Le Heuzey JY. Reproducibility of QT-interval measurements obtained from 24-hour digitized ambulatory three-lead electrocardiograms in patients with acute myocardial infarction and healty volunteers. ANE. 1998;3:38-45. |
| 136. | Extramiana F, Maison-Blanche P, Badilini F, Pinoteau J, Deseo T, Coumel P. Circadian modulation of QT rate dependence in healthy volunteers: gender and age differences. J Electrocardiol. 1999;32:33-43. [PubMed] |
| 137. | Jensen BT, Abildstrom SZ, Larroude CE, Agner E, Torp-Pedersen C, Nyvad O, Ottesen M, Wachtell K, Kanters JK. QT dynamics in risk stratification after myocardial infarction. Heart Rhythm. 2005;2:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 138. | Burattini L, Zareba W. Time-domain analysis of beat-to-beat variability of repolarization morphology in patients with ischemic cardiomyopathy. J Electrocardiol. 1999;32 Suppl:166-172. [PubMed] |
| 139. | Starc V, Schlegel TT. Real-time multichannel system for beat-to-beat QT interval variability. J Electrocardiol. 2006;39:358-367. [PubMed] |
| 140. | Baumert M, Starc V, Porta A. Conventional QT variability measurement vs. template matching techniques: comparison of performance using simulated and real ECG. PLoS One. 2012;7:e41920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 141. | Chevalier P, Burri H, Adeleine P, Kirkorian G, Lopez M, Leizorovicz A, André-Fouët X, Chapon P, Rubel P, Touboul P. QT dynamicity and sudden death after myocardial infarction: results of a long-term follow-up study. J Cardiovasc Electrophysiol. 2003;14:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 142. | Cygankiewicz I, Zareba W, Vazquez R, Bayes-Genis A, Pascual D, Macaya C, Almendral J, Fiol M, Bardaji A, Gonzalez-Juanatey JR. Risk stratification of mortality in patients with heart failure and left ventricular ejection fraction & gt; 35%. Am J Cardiol. 2009;103:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 143. | Pathak A, Curnier D, Fourcade J, Roncalli J, Stein PK, Hermant P, Bousquet M, Massabuau P, Sénard JM, Montastruc JL. QT dynamicity: a prognostic factor for sudden cardiac death in chronic heart failure. Eur J Heart Fail. 2005;7:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 144. | Quinteiro RA, Biagetti MO, Fernandez A, Borzone FR, Gargano A, Casabe HJ. Can QT/RR relationship differentiate between low- and high-risk patients with hypertrophic cardiomyopathy? Ann Noninvasive Electrocardiol. 2015;20:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 145. | Tereshchenko LG, Berger RD. Towards a better understanding of QT interval variability. Ther Adv Drug Saf. 2011;2:245-251. [PubMed] |
| 146. | Atiga WL, Calkins H, Lawrence JH, Tomaselli GF, Smith JM, Berger RD. Beat-to-beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol. 1998;9:899-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 205] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 147. | Atiga WL, Fananapazir L, McAreavey D, Calkins H, Berger RD. Temporal repolarization lability in hypertrophic cardiomyopathy caused by beta-myosin heavy-chain gene mutations. Circulation. 2000;101:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 148. | Piccirillo G, Magrì D, Matera S, Magnanti M, Torrini A, Pasquazzi E, Schifano E, Velitti S, Marigliano V, Quaglione R. QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: a prospective study. Eur Heart J. 2007;28:1344-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 149. | Tereshchenko LG, Fetics BJ, Domitrovich PP, Lindsay BD, Berger RD. Prediction of ventricular tachyarrhythmias by intracardiac repolarization variability analysis. Circ Arrhythm Electrophysiol. 2009;2:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 150. | Tereshchenko LG, Cygankiewicz I, McNitt S, Vazquez R, Bayes-Genis A, Han L, Sur S, Couderc JP, Berger RD, de Luna AB. Predictive value of beat-to-beat QT variability index across the continuum of left ventricular dysfunction: competing risks of noncardiac or cardiovascular death and sudden or nonsudden cardiac death. Circ Arrhythm Electrophysiol. 2012;5:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 151. | Dobson CP, La Rovere MT, Pinna GD, Goldstein R, Olsen C, Bernardinangeli M, Veniani M, Midi P, Tavazzi L, Haigney M. QT variability index on 24-hour Holter independently predicts mortality in patients with heart failure: analysis of Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca (GISSI-HF) trial. Heart Rhythm. 2011;8:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 152. | Fischer C, Seeck A, Schroeder R, Goernig M, Schirdewan A, Figulla HR, Baumert M, Voss A. QT variability improves risk stratification in patients with dilated cardiomyopathy. Physiol Meas. 2015;36:699-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 153. | Hering HE. Das Wesen des Herzalternans. Munchen Med Wchenshr. 1908;4:1417-1421. |
| 154. | Salerno JA, Previtali M, Panciroli C, Klersy C, Chimienti M, Regazzi Bonora M, Marangoni E, Falcone C, Guasti L, Campana C. Ventricular arrhythmias during acute myocardial ischaemia in man. The role and significance of R-ST-T alternans and the prevention of ischaemic sudden death by medical treatment. Eur Heart J. 1986;7 Suppl A:63-75. [PubMed] |
| 155. | Schwartz PJ, Malliani A. Electrical alternation of the T-wave: clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long Q-T syndrome. Am Heart J. 1975;89:45-50. [PubMed] |
| 156. | Smith JM, Clancy EA, Valeri CR, Ruskin JN, Cohen RJ. Electrical alternans and cardiac electrical instability. Circulation. 1988;77:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 278] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 157. | Kurz RW, Mohabir R, Ren XL, Franz MR. Ischaemia induced alternans of action potential duration in the intact-heart: dependence on coronary flow, preload and cycle length. Eur Heart J. 1993;14:1410-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 158. | Dilly SG, Lab MJ. Electrophysiological alternans and restitution during acute regional ischaemia in myocardium of anaesthetized pig. J Physiol. 1988;402:315-333. [PubMed] |
| 159. | Murphy CF, Lab MJ, Horner SM, Dick DJ, Harrison FG. Regional electromechanical alternans in anesthetized pig hearts: modulation by mechanoelectric feedback. Am J Physiol. 1994;267:H1726-H1735. [PubMed] |
| 160. | Rubenstein DS, Lipsius SL. Premature beats elicit a phase reversal of mechanoelectrical alternans in cat ventricular myocytes. A possible mechanism for reentrant arrhythmias. Circulation. 1995;91:201-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 161. | Klingenheben T, Hohnloser SH. Clinical value of T-wave alternans assessment. Card Electrophysiol Rev. 2002;6:323-328. [PubMed] |
| 162. | Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 565] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 163. | Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 832] [Cited by in RCA: 715] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 164. | Cohen RJ. Enhancing specificity without sacrificing sensitivity: potential benefits of using microvolt T-wave alternans testing to risk stratify the MADIT-II population. Card Electrophysiol Rev. 2003;7:438-442. [PubMed] |
| 165. | Myles RC, Jackson CE, Tsorlalis I, Petrie MC, McMurray JJ, Cobbe SM. Is microvolt T-wave alternans the answer to risk stratification in heart failure? Circulation. 2007;116:2984-2991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 166. | Verrier RL, Kumar K, Josephson ME. The frustrating search for arrhythmia risk stratifiers in heart failure due to nonischemic cardiomyopathy: does T-wave alternans testing help? J Am Coll Cardiol. 2007;50:1905-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 167. | Cantillon DJ, Stein KM, Markowitz SM, Mittal S, Shah BK, Morin DP, Zacks ES, Janik M, Ageno S, Mauer AC. Predictive value of microvolt T-wave alternans in patients with left ventricular dysfunction. J Am Coll Cardiol. 2007;50:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 168. | Hohnloser SH, Ikeda T, Cohen RJ. Evidence regarding clinical use of microvolt T-wave alternans. Heart Rhythm. 2009;6:S36-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 169. | Calò L, De Santo T, Nuccio F, Sciarra L, De Luca L, Stefano LM, Piroli E, Zuccaro L, Rebecchi M, de Ruvo E. Predictive value of microvolt T-wave alternans for cardiac death or ventricular tachyarrhythmic events in ischemic and nonischemic cardiomyopathy patients: a meta-analysis. Ann Noninvasive Electrocardiol. 2011;16:388-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 170. | Costantini O, Hohnloser SH, Kirk MM, Lerman BB, Baker JH, Sethuraman B, Dettmer MM, Rosenbaum DS. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol. 2009;53:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 171. | Merchant FM, Ikeda T, Pedretti RF, Salerno-Uriarte JA, Chow T, Chan PS, Bartone C, Hohnloser SH, Cohen RJ, Armoundas AA. Clinical utility of microvolt T-wave alternans testing in identifying patients at high or low risk of sudden cardiac death. Heart Rhythm. 2012;9:1256-1264.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 172. | Gupta A, Hoang DD, Karliner L, Tice JA, Heidenreich P, Wang PJ, Turakhia MP. Ability of microvolt T-wave alternans to modify risk assessment of ventricular tachyarrhythmic events: a meta-analysis. Am Heart J. 2012;163:354-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 173. | Kraaier K, McCracken T, van der Palen J, Wilde AA, Scholten MF. Is T-wave alternans testing feasible in candidates for prophylactic implantable defibrillators? Neth Heart J. 2011;19:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |