Published online Dec 26, 2015. doi: 10.4330/wjc.v7.i12.931
Peer-review started: February 2, 2015
First decision: June 3, 2015
Revised: July 14, 2015
Accepted: September 29, 2015
Article in press: September 30, 2015
Published online: December 26, 2015
AIM: To study trends in the epidemiology, clinical presentation, microbiology and prognosis of non-typhoid Salmonella (NTS) myocarditis.
METHODS: We performed a systematic literature search for all reported NTS cases. The search yielded 838 publications. A total of 21 papers were deemed eligible. No language restrictions were enforced. Articles that were not written in English were translated. Pre-specified data such as clinical presentation, electrocardiogram (ECG) changes, transthoracic echocardiographic findings, cardiac magnetic resonance findings, microbiology cultures, Salmonella species, inflammatory markers (erythrocyte sedimentation rate and C-reactive protein), cardiac biomarkers and severity of illness were collected using data extraction sheets. Cases were classified by age into 2 groups; pediatric cases (defined as < 18 years old) and adult cases (defined ≥ 18 years old). The mean age of patients and standard deviations were calculated. The data was analyzed with IBM SPSS Statistics (Windows, Version 20.0. Armonk, NY: IBM Corp.) for demographic characteristics, presenting symptoms, microbiology, diagnostic methods, treatment modalities and outcome.
RESULTS: From the selected articles, we identified a total of 24 individual cases with verifiable data. There were 20 males with a male to female ratio of 5:1. The mean age at presentation was 30.8 years (range 1 mo-67 years), 16% of cases were children aged < 18 years. Most patients presented with chest pain, fever, and abdominal pain. The most common ECG finding was ST elevation. Cardiac biomarkers were elevated in around 70% of cases. Salmonella Enteritidis was the most common NTS isolated. Definitive diagnosis was established by blood and stool cultures in most of the cases. The pediatric and adults cases had similar incidence of bacteremia (40% vs 36.8%) while the pediatric group had more stool cultures positive compared to the adult group (100% vs 63.1%). Eighty-three percent of patients received antibiotics and 58% were successfully treated through conservative management. The overall mortality was 24% and 42% of patients required intensive care.
CONCLUSION: This systematic review of published cases shows that NTS myocarditis occurs predominantly in young adults and carries a poor prognosis.
Core tip: Myocarditis is a rare extra-intestinal manifestation of non-typhoid Salmonella infection. In our review, the most common presenting symptoms were fever, abdominal pain, and chest pain and the most frequent electrocardiogram finding was ST segment elevation. Around 70% of patients had positive cardiac biomarkers (creatine kinase and/or troponin). Salmonella Enteritidis was the most common pathogen identified. Mortality appears to be high as is seen with all bacterial myocarditis, and intensive care unit admission is warranted in a large number of cases.
- Citation: Villablanca P, Mohananey D, Meier G, Yap JE, Chouksey S, Abegunde AT. Salmonella Berta myocarditis: Case report and systematic review of non-typhoid Salmonella myocarditis. World J Cardiol 2015; 7(12): 931-937
- URL: https://www.wjgnet.com/1949-8462/full/v7/i12/931.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i12.931
Salmonella species are gram-negative bacilli that are responsible for significant morbidity and mortality in both developing and developed nations. They are responsible for a wide spectrum of disease including enteric fever or typhoid fever [Salmonella Typhi (S. Typhi) and (S. Paratyphi)], as well as a range of clinical syndromes including diarrheal illness caused by a group of bacteria known as non-typhoid Salmonella (NTS)[1], Salmonella associated myocarditis is a rare entity described in case reports. Most case reports have described myocarditis associated with S. Typhi and Para-typhi infections. However, myocarditis associated with NTS, the species most commonly found in the western hemisphere, has been infrequently reported. Also, there is no existing structured analysis on this subject. We report an illustrative case and carefully analyze the available literature on NTS myocarditis in order to describe the epidemiological distribution, diagnostic trends and prognosis.
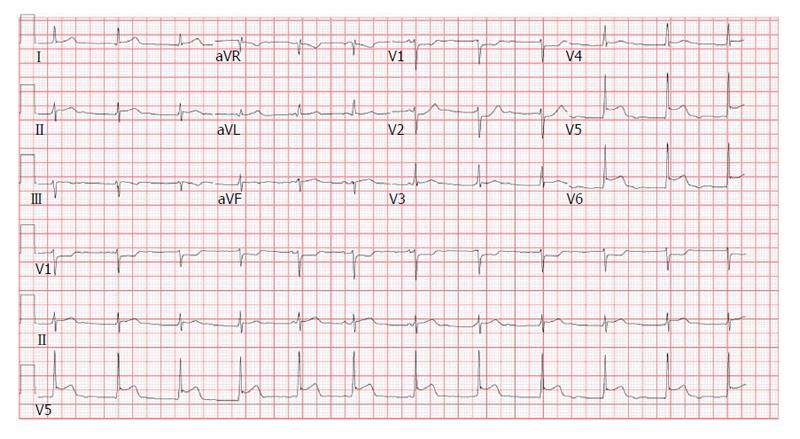
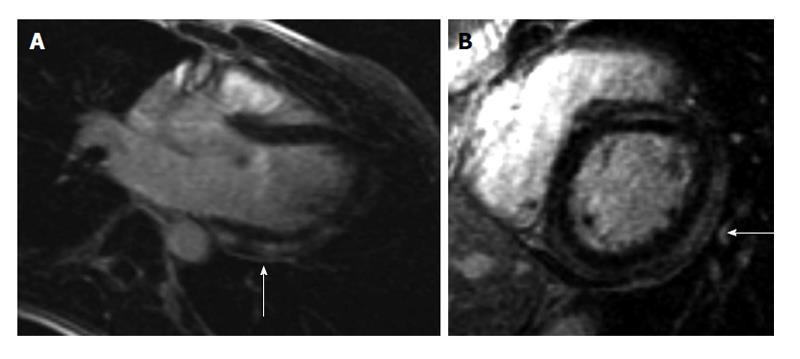
A 19-year-old male with no significant past medical history presented to the emergency department (ED) with a 2-d history of watery non-bloody diarrhea associated with diffuse abdominal cramping, fever, nighttime chills and sweats. He recalled eating jerk chicken from a local restaurant and a sausage-egg biscuit prior to onset of diarrhea. He ate alone and denied any sick contacts or recent travel. On admission his vitals were as follows: Temperature 38.8 °C, heart rate (HR) 103/min and blood pressure (BP) 122/94 mmHg. Physical exam was unremarkable except for mild abdominal tenderness and dehydration. He received intravenous fluids and was discharged with a diagnosis of possible viral gastroenteritis. Forty-eight hours after discharge, he developed acute-onset chest pain (CP) and shortness of breath (SOB). The patient described the CP, as a retrosternal “squeezing” pain, 8/10 in severity, not radiating and associated with SOB at rest along with intermittent palpitations. He did not recognize any aggravating or relieving factors. When the symptoms persisted for 24 h, the patient came to the ED again and this time his vitals were as follows: BP 112/65 mmHg, HR 81/min, temperature 37.3 °C and respiratory rate 18 breaths/min with oxygen saturation of 97% on room air. Physical examination was unrevealing. Initial electrocardiogram (ECG) was significant for ST segment depression in leads V1 and V2 with ST segment elevations in leads V5 and V6 (Figure 1). Chest X-ray (CXR) did not show any cardiopulmonary process. Initial troponin I was 6.23 ng/mL (0.000-0.034 ng/mL) and a repeat assay 6 h later was 13.2 ng/mL. CBC showed hemoglobin of 12.5 g/dL, white blood cell count (WBC) of 6.9 × 109/L (4.4 × 109-10.6 × 109/L) with 26% bands. Kidney function and electrolytes were within normal limits. Liver enzymes showed alkaline phosphatase of 33 IU/L (50-120 U/L), aspartate aminotransferase of 42 IU/L (0-40 IU/L) and lactate dehydrogenase of 325 IU/L (85-210 IU/L). Table 1 summarizes the laboratory and imaging investigations for this patient. Within a few hours of admission, the patient became hypotensive with BP of 90/49 mmHg and the troponin went up to 18.9 ng/mL. He was subsequently transferred to the cardiac intensive care unit (ICU). Transthoracic echocardiogram (TTE) showed an ejection fraction (EF) of 40% with no regional wall motion abnormalities or pericardial fluid. Due to suspicion for myocarditis, a cardiac magnetic resonance imaging (CMRI) was done which showed multiple areas of abnormal sub-epicardial and mid-myocardial contrast hyper enhancement involving the posterior, inferior and anterior walls of the left ventricle, the anterior wall of right ventricle and the inter-ventricular septum reflecting multifocal biventricular myocarditis (Figure 2). Stool culture came back positive for Salmonella Berta and therapy was initiated with sulfamethoxazole/trimethoprim. The patient gradually improved over the next 4 d and his CP resolved. He received 14 d of therapy during which symptoms resolved completely and EF normalized.
| Diagnostic test | Result |
| Hemoglobin | 12.5 g/dL |
| WBC | 6.9 × 109/L |
| Kidney function and electrolytes | Within normal limits |
| Alkaline phosphatase | 33 IU/L |
| Aspartate aminotransferase | 42 IU/L |
| Lactate dehydrogenase | 325 IU/L |
| Troponin I (at presentation) | 6.23 ng/mL |
| Troponin I (6 h later) | 13.2 ng/mL |
| ECG | ST depression in V1 and V2 with ST elevations in V5 and V6 |
| TTE | EF of 40% with no regional wall motion abnormalities or pericardial fluid |
| CMR | Multiple areas of abnormal sub-epicardial and mid-myocardial contrast hyper enhancement involving the posterior, inferior and anterior walls of the left ventricle, the anterior wall of right ventricle and the inter-ventricular septum reflecting multifocal biventricular myocarditis |
This systematic review was conducted according to the PRISMA guidelines[2]. A computer-assisted literature search of PubMed, EMBASE CENTRAL and Google search engine was conducted. We also performed manual searches of the reference lists of studies, reviews, editorials, and letters, as well as related conference proceedings. Search terms keywords included “Salmonella myocarditis”, “bacterial myocarditis”, “non-typhoidal Salmonella” as well as combinations of these terms. No language restrictions were enforced. Articles that were not written in English were translated.
Inclusion criteria for publications in this systematic review: (1) Articles reporting original data; (2) Articles including patients with at least 1 blood and/or stool culture or tissue finding confirming diagnosis of Salmonella; (3) Articles including patients with clinical, electrocardiographic, or imaging evidence suggesting myocardial involvement; and (4) Articles providing data on at least one of the following: Clinical presentation, ECG description or original ECG, serum cardiac markers, any radiologic images. Exclusion criteria are listed as follows: (1) Articles including patients with myocarditis and infection with Salmonella typhi or para-typhi; (2) Articles reporting conditions that might present with clinical and imaging abnormalities similar to Salmonella myocarditis; (3) Articles on Salmonella infection affecting other organs; and (4) Review articles.
The following data was extracted: age, gender, presenting complaints (CP SOB, diarrhea) fever > 37.5 °C, white blood cell count, serum cardiac markers [creatine kinase (CK) and Troponin], ECG characteristics, microbiology cultures, inflammatory markers [erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)], TTE findings, CMRI findings, CXR findings, hemodynamics (BP and HR), Salmonella species and possible source of infection, need for ICU admission and outcomes (dead or alive). There was no age restriction for inclusion of cases in the study. Cases were classified by age into 2 groups; pediatric cases (defined as < 18 years old) and adult cases (defined ≥ 18 years old). The mean age of patients and standard deviations were calculated. All available ECG descriptions for each case were obtained and original data was analyzed. The ECG data was grouped into the following categories: ST segment elevation, ST segment depression, affected walls, T wave inversion, and others findings that included specific abnormalities such as prolonged QT, atrioventricular blocks (AVB), premature ventricular complex (PVC) were also recorded. Serum cardiac markers (CK and/or troponin) inflammatory markers (CRP and/or ESR) were classified as normal or elevated. Fever was defined as temperature > 37.5 °C, leukocytosis as WBC count > 10000/mm3, reduced EF as < 50%, hypotension as systolic BP < 90 mmHg and diastolic BP < 60 mmHg, tachycardia as HR > 100/min. If data was reported without quantification but the description matched the criteria it was considered a positive finding. CXR and CMRI findings were obtained from the report or direct data analysis if there was an available image. The prevalence of the different measured variables was calculated from the extracted data. The “not available” data cases were not considered in the calculation. Data was analyzed with IBM SPSS Statistics (Windows, Version 20.0. Armonk, NY: IBM Corp) for demographic characteristics, presenting symptoms, microbiology, diagnostic methods, treatment modalities and outcome. The analysis was reviewed by Villablanca P (MD MS-Clinical Research).
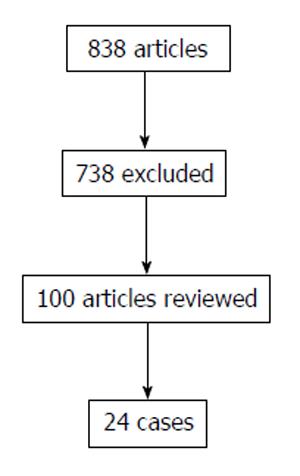
The literature search yielded a total of 838 publications. Following the exclusion criteria, 816 citations were excluded after examining titles and abstracts, leaving twenty-one articles with 24 patients for detailed evaluation (Figure 3)[3-23].
There were 19 adult and 5 pediatric cases, 20 males with a male-to-female ratio of 5:1. Female prevalence was higher in pediatric population (60%) as compared to adults (5.2%). The age of the patients ranged from 1 mo to 67 years. The mean age at presentation was 30.8 years. The mean age for adults and pediatrics cases was 36.6 years (range: 18-67 years) 9.6 years (range: 0.1-16 years) respectively.
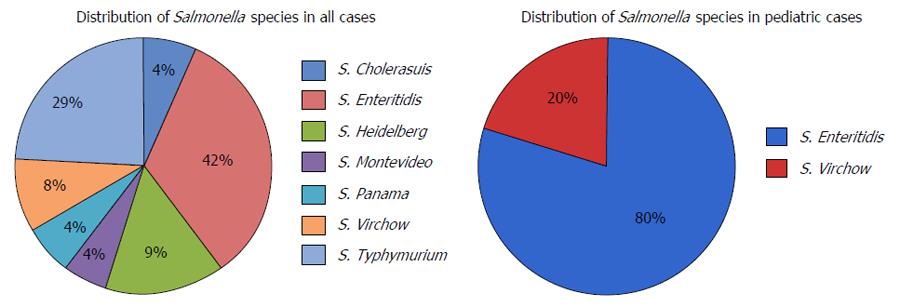
NTS species were identified either by blood cultures or stool cultures; only one patient had both cultures positive. One pediatric case was confirmed with myocardial biopsy after the patient died (Table 2). The pediatric and adults cases had similar incidence of bacteremia (40% vs 36.8%) while the pediatric group had more stool cultures positive compared to the adult group (100% vs 63.1%). S. Enteritidis was the most common pathogen found among all the reported cases, with a total of 10 cases (41.6%). Salmonella typhymurium was the most frequently reported pathogen in the adult group (36.8% of cases) and S. Enteritidis was the most frequently reported pathogen in the pediatric group (80% of cases) (Figure 4).
| n (%) | n1 | |
| Culture | ||
| Blood | 7 (29.1) | 24 |
| Stool | 16 (66.6) | 24 |
| Myocardial biopsy | 1 (4.1) | 24 |
| Presenting sign or symptom | ||
| Dyspnea | 6 (25) | 24 |
| Chest pain | 15 (62.5) | 24 |
| Fever | 16 (66.6) | 24 |
| Diarrhea | 5 (22.7) | 22 |
| Abdominal pain | 15 (71.4) | 21 |
| Hypotension | 5 (21.7) | 23 |
| Tachycardia | 13 (56.5) | 23 |
| ECG abnormalities | ||
| ST elevation | 12 (52.1) | 23 |
| ST depression | 4 (17.3) | 23 |
| T-wave inversion | 6 (26) | 23 |
| Infero-lateral | 12 (52.1) | 23 |
| Antero-lateral | 6 (26) | 23 |
| Inflammatory markers | ||
| WBC > 10000/mm3 | 8 (40) | 20 |
| Elevated troponin | 9 (69.2) | 13 |
| Elevated CK | 10 (66.6) | 15 |
| Elevated ESR or CRP | 9 (100) | 9 |
| TTE | ||
| Reduced ejection fraction | 4 (36.4) | 11 |
| Regional wall motion abnormality | 5 (45.5) | 11 |
| Chest X-ray | ||
| Cardiomegaly | 3 (27.7) | 11 |
| Pulmonary edema | 3 (27.7) | 11 |
| CMR | 2 (100) | 2 |
Fever, abdominal pain, and CP were the most common reported symptoms. Fever was present in 66.6% of the cases. SOB, chills and sweating were less prevalent. Of note, less than 25% of the cases had associated diarrhea. More than half of the cases of NTS presented with tachycardia and around 20% with hypotension. There was history of recent travel in 8 cases with a wide distribution around the world including Pakistan, Bali, Dominican Republic, Spain and Eastern Europe. Average prodrome was 4.6 d. Duration of prodromal symptoms was slightly longer in the pediatric cases (8.1 d). Four cases recalled a possible source of infection, which included eating chicken, rice-eggs, sausages with spoiled meat and dumplings. Table 2 illustrates the frequency of presenting signs and symptoms.
The most common finding on ECG was the presence of ST segment elevation (52.1%). ST segment depression was seen in a small number of the cases with only one pediatric case. Adults had a higher prevalence of more serious ECG findings including 3rd degree AVB (10.5%, n = 2) and ventricular fibrillation (5.2%, n = 1). More benign findings like 1st degree AVB, prolonged QT, PVC, right bundle branch block and low voltage were present in both groups. Table 2 shows the frequency of different ECG abnormalities along with area of involvement.
Variability in the choice of biomarkers was seen in the cases reviewed. Older cases used CK since troponin was not available. The prevalence of elevated CK and/or troponin in patients presenting with Salmonella myocarditis was near 70%. Prevalence of reported elevated troponin was high, in both, adults (71.4%, n = 10) and pediatric cases (40%, n = 2). However, troponin assay was not available at that time when most of the pediatric cases were reported. Most of the cases measured troponin I (55%, n = 5), followed by troponin T (33%, n = 3), and one case did not specify the kind of troponin used. Among the cases reviewed, the prevalence of elevated white count > 10000/mm3 was 40% of which 25% had a left shift. Where it was measured, elevation in ESR or CRP was noted in 100% of cases of Salmonella myocarditis. Frequency of abnormalities in inflammatory and cardiac biomarkers is illustrated in Table 2.
Almost 30% patients had evidence of pulmonary edema on CXR and less than 1/3 had cardiomegaly. On TTE, 36.4% reported reduced EF (less than 50%). More than 50% of the adults had regional wall motion abnormalities. Pericardial effusion was seen exclusively in children (3 cases). Out of the 24 cases, only 2 received CMRI scans, both of which revealed delayed gadolinium enhancements. Frequency of abnormal imaging studies is shown in Table 2.
Myocarditis is an inflammatory condition of the myocardium with both infectious and non-infectious etiologies. It most commonly presents as non-ischemic cardiomyopathy but manifestations can range from sudden death, new onset atrial or ventricular arrhythmias, complete heart block or an acute myocardial infarction[24]. Viral infections are the most common cause of myocarditis with an epidemiological change from Coxsackie B virus and adenovirus being identified in the past to parvovirus B19 being the most common etiological agent currently[24]. Bacterial myocarditis is uncommon with a prevalence ranging from 0.2% to 1.5%[25]. However, it should always be considered in patients with sepsis and ventricular dysfunction[26].
Salmonella species are gram-negative bacilli that are responsible for significant morbidity and mortality in both developing and developed nations. Both typhoidal and NTS can have extra-intestinal manifestations however myocarditis is an uncommon extra-intestinal manifestation[27]. NTS are food-borne pathogens that are responsible for diarrheal illness. There is about a 5% incidence of invasion beyond the gastrointestinal tract present most commonly in immunocompromised hosts[28]. In our review, the major presenting symptoms were fever and CP with only around 20% having diarrhea. A large study of over 7000 human Salmonella infections noted cardiac involvement in the form of endocarditis in 20 patients all of whom had a rapidly fatal outcome[29]. It can be postulated that myocardial damage occurs secondary to involvement of endocardium or due to direct bacterial invasion from bacteremia. In addition to this, sepsis induced myocardial depression and subsequent remodeling may also play a part as it does in other bacterial myocarditis[26].
ECG analysis in myocarditis usually shows sinus tachycardia with non-specific ST segment changes and T wave abnormalities[24]. In our review, the most common ECG abnormality was ST elevation. Troponins have been shown to have high specificity (89%) and low sensitivity (34%) for diagnosis of myocarditis[30]. In our review troponins were elevated in majority of the patients with NTS myocarditis. Even though the gold standard for diagnosis remains endomyocardial biopsy, CMRI is slowly replacing the need for more invasive procedures[31]. In a recent study of 82 patients with troponin elevation without significant coronary artery disease, late gadolinium enhancement CMRI established a diagnosis of myocarditis in 80% of the patients in comparison to 88% diagnosed with endomyocardial biopsy[31]. In our review, only 2 patients received CMRI with both cases showing evidence of myocarditis. TTE showed a reduced EF in around 36% of the cases with around 50% of adult cases showing regional wall motion abnormalities. Interestingly, all of the pediatric cases showed pericardial fluid suggestive of increased incidence of pericardial disease in pediatric population affected by Salmonella.
The most common etiological agent overall was S. Enteritidis (40%). This is in concordance with a Malaysian retrospective analysis of 55 patients with NTS bacteremia, which showed that S. Enteritidis had the maximum blood invasiveness[28]. The overall mortality in our patients was around 20% with 40% of patients requiring ICU stay. Antibiotic therapy is not recommended for NTS gastrointestinal infections. However, it should be considered if patients are at risk for invasive disease (age > 50 or neonates, immunosuppressed, sickle cell patients and those with vascular abnormalities)[27]. Treatment of myocarditis caused by NTS has not been detailed in any study, but in essence it can be treated as NTS bacteremia or as a life threatening infection. In those cases affected by life threatening infections, treatment should be started with both, a third-generation cephalosporin and a fluoroquinolone until the susceptibility is known[32]. Ninety percent of the patients included in this review received antibiotic treatment.
Results of this systematic review of published cases shows that NTS myocarditis occurs predominantly in young adults and carries a poor prognosis. The initial diagnostic approach is similar to myocarditis due to other etiologies and includes ECG, TTE and CXR. Upon diagnosis, patients should receive supportive therapy for myocarditis in addition to antibiotics. Mortality appears to be high as with all bacterial myocarditis and ICU admission is warranted in a large number of cases. Salmonella infections are a rare cause of myocarditis but should always be considered in cases presenting with features of myocarditis and evidence of Salmonella infection in the absence of viral etiology.
Salmonella species are bacteria that are responsible for significant morbidity and mortality in both developing and developed nations. They are responsible for a wide spectrum of disease including enteric fever or typhoid fever (S. Typhi and S. Paratyphi) and a range of clinical syndromes including diarrheal illness caused by a group of bacteria known as non-typhoid Salmonella (NTS). In rare circumstances Salmonella can cause inflammation of the myocardium (myocarditis).
To the best of our knowledge, no systematic review of NTS myocarditis has previously been published. The authors carefully analyze the available literature on NTS myocarditis in order to describe the epidemiological distribution, diagnostic trends and prognosis of this condition.
Salmonella Enteritidis was the most common pathogen identified in these cases. Around 30% of patients had bacteremia and 100% of pediatric patients had either stool or blood culture positive for Salmonella. Fever, abdominal pain and chest pain were the most common presenting symptoms and ST segment elevation was the most frequent electrocardiogram finding. Around 70% of patients had positive cardiac biomarkers (creatine kinase and/or Troponin). Mortality appears to be high as with all bacterial myocarditis and intensive care unit admission is warranted in a large number of cases.
Salmonella infections are a rare cause of myocarditis but should always be considered in cases presenting with features of myocarditis and evidence of Salmonella infection in the absence of viral etiology.
Salmonella species are gram-negative bacteria that cause a wide range of diseases. S. Berta, S. Typhi, S. Paratyphi, S. Enteritidis, etc., are all serotypes of the Genus Salmonella. However, based on clinical syndromes, i.e., causation of enteric fever, salmonella can be divided into typhoid salmonella and NTS. Myocarditis is an inflammatory condition of the muscular wall of the heart.
NTS infection involving myocarditis is rare, and the current case presentation and systemic review of the disease is therefore unique.
P- Reviewer: Merkely B, Takano M S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Gordon MA. Salmonella infections in immunocompromised adults. J Infect. 2008;56:413-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 2. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-W94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3566] [Cited by in F6Publishing: 3864] [Article Influence: 257.6] [Reference Citation Analysis (0)] |
| 3. | Sanders V, Misanik LF. Salmonella myocarditis. report of a case with ventricular rupture. Am Heart J. 1964;68:682-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Shilkin KB. Salmonella typhimurium pancarditis. Postgrad Med J. 1969;45:40-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Scarabicchi S, Ribaldone D, Giambartolomei G. [Myocarditis caused by Salmonella. Personal cases]. Minerva Cardioangiol. 1976;24:563-570. [PubMed] [Cited in This Article: ] |
| 6. | Simonsen J, Falk E. A case of sudden cardiac death in connection with Salmonella typhimurium infection. Forensic Sci Int. 1980;16:283-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Theler-Ballmer D, Noseda G, Reiner M, Keller H. [Pericarditis and myocarditis in salmonellosis]. Schweiz Med Wochenschr. 1980;110:1394-1401. [PubMed] [Cited in This Article: ] |
| 8. | Burt CR, Proudfoot JC, Roberts M, Horowitz RH. Fatal myocarditis secondary to Salmonella septicemia in a young adult. J Emerg Med. 1990;8:295-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Götz M, Juchems R. [Myocarditis caused by Salmonella typhimurium]. Klin Wochenschr. 1983;61:1153-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Huppertz HI, Sandhage K. Salmonella enteritidis in reactive carditis. Lancet. 1993;342:1488-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Oziol E, Bonal J, Chauveau E, Talard P, Carli P, Chagnon A. [Acute myocarditis in non-typhoid Salmonella infection]. Arch Mal Coeur Vaiss. 1995;88:99-101. [PubMed] [Cited in This Article: ] |
| 12. | Neuwirth C, Francois C, Laurent N, Pechinot A. Myocarditis due to Salmonella virchow and sudden infant death. Lancet. 1999;354:1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | O’Connor K. Acute myocarditis precipitated by Salmonella Montevideo infection: a case report. Ir Med J. 2000;93:21-22. [PubMed] [Cited in This Article: ] |
| 14. | Wanby P, Olsen B. Myocarditis in a patient with salmonella and campylobacter enteritis. Scand J Infect Dis. 2001;33:860-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Guerrero Ortiz M, Manrique Legaz A, Díaz Izquierdo L. [Enteric salmonella pancarditis. Diagnosis of site by examination with 67Gallium]. Rev Esp Med Nucl. 2003;22:106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 16. | Williams P, Lainchbury J. Enteritis-associated myocarditis. Heart Lung Circ. 2004;13:106-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Franczuk P, Rewiuk K, Grodzicki T. Myocarditis related to Salmonella enteritidis infection. Cardiol J. 2007;14:589-591. [PubMed] [Cited in This Article: ] |
| 18. | Hammerer M, Altenberger J, Pichler M. [Sudden cardiac death 43 months after fulminant myocarditis and two-peaked myositis in non-typhoid salmonellosis]. Wien Med Wochenschr. 2008;158:509-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Rossetti B, Nguisseu G, Buracci A, Migliorini L, Zanelli G. Myocarditis mimicking an acute coronary syndrome: a case related to Salmonella enteritis. Gastroenterol Res Pract. 2009;2009:931853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Hibbert B, Costiniuk C, Hibbert R, Joseph P, Alanazi H, Simard T, Dennie C, Angel JB, O’Brien ER. Cardiovascular complications of Salmonella enteritidis infection. Can J Cardiol. 2010;26:323-325. [PubMed] [Cited in This Article: ] |
| 21. | Papamichalis P, Argyraki K, Papamichalis M, Loukopoulos A, Dalekos GN, Rigopoulou EI. Salmonella enteritidis Infection Complicated by Acute Myocarditis: A Case Report and Review of the Literature. Cardiol Res Pract. 2011;2011:574230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Childs L, Gupta S. Salmonella enteritidis induced myocarditis in a 16-year-old girl. BMJ Case Rep. 2012;2012:pii: bcr2012007628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Brice J, Baumard S, Loeb F, Brasme L, Jaussaud R, N’Guyen Y. [Salmonella enteritidis infection complicated by acute myocarditis]. Med Mal Infect. 2013;43:248-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Cooper LT. Myocarditis. N Engl J Med. 2009;360:1526-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1022] [Cited by in F6Publishing: 910] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 25. | Wasi F, Shuter J. Primary bacterial infection of the myocardium. Front Biosci. 2003;8:s228-s231. [PubMed] [Cited in This Article: ] |
| 26. | Haddad F, Berry G, Doyle RL, Martineau P, Leung TK, Racine N. Active bacterial myocarditis: a case report and review of the literature. J Heart Lung Transplant. 2007;26:745-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Sánchez-Vargas FM, Abu-El-Haija MA, Gómez-Duarte OG. Salmonella infections: an update on epidemiology, management, and prevention. Travel Med Infect Dis. 2011;9:263-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Dhanoa A, Fatt QK. Non-typhoidal Salmonella bacteraemia: epidemiology, clinical characteristics and its’ association with severe immunosuppression. Ann Clin Microbiol Antimicrob. 2009;8:15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Saphra I, Winter JW. Clinical manifestations of salmonellosis in man; an evaluation of 7779 human infections identified at the New York Salmonella Center. N Engl J Med. 1957;256:1128-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 334] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Schultz JC, Hilliard AA, Cooper LT, Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clin Proc. 2009;84:1001-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 31. | Baccouche H, Mahrholdt H, Meinhardt G, Merher R, Voehringer M, Hill S, Klingel K, Kandolf R, Sechtem U, Yilmaz A. Diagnostic synergy of non-invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur Heart J. 2009;30:2869-2879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 32. | Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32:263-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 672] [Article Influence: 29.2] [Reference Citation Analysis (0)] |