Published online May 26, 2014. doi: 10.4330/wjc.v6.i5.227
Revised: February 20, 2014
Accepted: April 16, 2014
Published online: May 26, 2014
Processing time: 173 Days and 9.3 Hours
Hypertension causes significant morbidity and mortality worldwide, owing to its deleterious effects on the cardiovascular and renal systems. Primary hyperaldosteronism (PA) is the most common cause of reversible hypertension, affecting 5%-18% of adults with hypertension. PA is estimated to result from bilateral adrenal hyperplasia in two-thirds of patients, and from unilateral aldosterone-secreting adenoma in approximately one-third. Suspected cases are initially screened by measurement of the plasma aldosterone-renin-ratio, and may be confirmed by additional noninvasive tests. Localization of aldostosterone hypersecretion is then determined by computed tomography imaging, and in selective cases with adrenal vein sampling. Solitary adenomas are managed by laparoscopic or robotic resection, while bilateral hyperplasia is treated with mineralocorticoid antagonists. Biochemical cure following adrenalectomy occurs in 99% of patients, and hemodynamic improvement is seen in over 90%, prompting a reduction in quantity of anti-hypertensive medications in most patients. End-organ damage secondary to hypertension and excess aldosterone is significantly improved by both surgical and medical treatment, as manifested by decreased left ventricular hypertrophy, arterial stiffness, and proteinuria, highlighting the importance of proper diagnosis and treatment of primary hyperaldosteronism. Although numerous independent predictors of resolution of hypertension after adrenalectomy for unilateral adenomas have been described, the Aldosteronoma Resolution Score is a validated multifactorial model convenient for use in daily clinical practice.
Core tip: Primary hyperaldosteronism is the most common reversible form of secondary hypertension. After appropriate diagnosis and localization studies, adrenalectomy is the procedure of choice for unilateral aldosterone-secreting adenomas, while medical therapy is best for bilateral adrenal hyperplasia. Surgical resection improves or cures biochemical and hemodynamic perturbations in most patients, and halts or reverses many of the deleterious effects of hyperaldosteronism. Predicting which patients will benefit most from adrenalectomy is aided by the Aldosteronoma Resolution Score.
- Citation: Aronova A, III TJF, Zarnegar R. Management of hypertension in primary aldosteronism. World J Cardiol 2014; 6(5): 227-233
- URL: https://www.wjgnet.com/1949-8462/full/v6/i5/227.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i5.227
Hypertension is one of the most prominent risk factors for morbidity and mortality worldwide, accounting for 45% of deaths due to heart disease and 51% due to stroke[1,2]. In the United States alone, 69 million adults (29%) have hypertension, in whom it is significantly associated with myocardial infarction, cerebrovascular accidents, heart failure and renal disease[3,4]. Given the large impact on global health, controlling hypertension is of utmost importance. Significant efforts have been made to characterize potentially curable, or secondary, types of hypertension such as renovascular hypertension, pheochromocytoma, Cushing’s syndrome and primary hyperaldosteronism.
Primary hyperaldosteronism (PA) is the leading cause of secondary hypertension, and can be identified in 5% to 18% of hypertensive patients[5,6]. First described by Conn in 1955 in a patient presenting with resistant hypertension and hypokalemia who was found to have an aldosterone-secreting adrenal adenoma[7], PA can present in a myriad of clinical scenarios. Most recent epidemiologic studies have shown that approximately 60% of patients are found to have bilateral idiopathic hyperplasia, also known as idiopathic hyperaldosteronism (IHA), while 30% present with unilateral aldosterone-producing adenomas (APA)[8]. One to two percent of patients present with primary or unilateral adrenal hyperplasia (UAH), 1% with aldosterone-secreting adrenocortical carcinoma, 1% with familial hyperaldosteronism, and 1% with ectopic aldosterone-producing adenoma or carcinoma[6,9,10].
Classically, excessive aldosterone secretion not only results in difficult to manage hypertension in the majority of patients, but also produces biochemical effects of hypokalemia in 10%-30% of patients[11]. More recent data, however, suggest that most patients with PA are actually normokalemic[6,11,12]. In addition, aldosterone hypersecretion has been linked to significant and potentially reversible end-organ damage, particularly in the cardiovascular and renal systems[13]. For instance, Tanabe et al[14] demonstrated that patients with PA have more pronounced cardiac hypertrophy compared to patients with essential or other secondary causes of hypertension. Fortunately, timely correction of aldosterone levels can prevent or reverse some of these effects[15]. This review will describe the current methods of diagnosis and management of primary hyperaldosteronism, with a particular focus on the systemic effects of adrenalectomy as well as the predictors of resolution of hypertension after surgery.
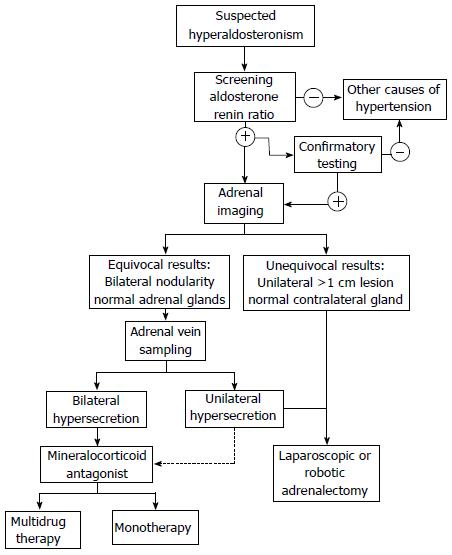
Patients with hypertension and hypokalemia, regardless of suspected cause (diuretics, incidentaloma), and patients with medically-resistant hypertension, should be considered for screening for primary hyperaldosteronism[16]. Initial evaluation of patients involves biochemical testing with plasma aldosterone (ng/dL) to renin (ng/mL per hour) ratio (ARR). This test identifies excessive aldosterone secretion with simultaneous suppression of plasma renin activity. Although ARR is regarded as the ideal screening tool for PA, there exists some controversy regarding the clinical conditions under which the ARR is obtained, as well as the test’s diagnostic accuracy. Certain drugs, including beta-blockers, angiotensin-converting enzyme inhibitors (ACE-I), selective-serotonin reuptake inhibitors and oral contraceptives, have been shown to affect the results of the test[17,18]. Ideal testing conditions involve discontinuation of such medications two weeks prior [10,17,18]. However, in a recent study, Fisher et al[19] showed that doing so is impractical, and most patients are unable to be taken off their anti-hypertensive medications without the need for substitution by other agents to adequately control blood pressure or serious side effects such as hospitalization. Others suggest that only use of spironolactone will absolutely interfere with the interpretation of this ratio[16]. In addition, there is some disagreement regarding the requirement of a minimum plasma aldosterone level and the critical ARR cutoff for diagnosis. Most authors recommend an ARR of 20-40, and researchers found that ARR of at least 35 has 100% sensitivity and 92.3% specificity in diagnosing primary hyperaldosteronism[17,20,21]. Furthermore, biochemical testing should be done in the morning, in a seated position after an initial two-hour ambulatory period[18]. False negative and positive results can occur, as affected by age, smoking, medications, posture, and renal function, so it is generally advisable to repeat biochemical testing in patients with high pretest probability of PA, typically four weeks later[18].
Patients with suspected primary aldosteronism identified by screening ARR may undergo confirmatory testing or go on to localization studies. Confirmatory testing includes: the oral sodium loading test, the saline infusion test, the fludrocortisone suppression test, and the captopril challenge test[22]. Time, cost, patient compliance, and certain physiologic parameters need to be considered in choosing the specific confirmatory test. For instance, in patients with severe hypertension, cardiac or renal insufficiency, clinicians should avoid the oral sodium loading test and the saline infusion tests. In general, such additional testing often proves burdensome and in 30%-50% of cases does not prove to be abnormal in patients with high ARR suggestive of PA[10,22,23]. Currently, there is lack of evidence encouraging the use of any one of these tests as a gold standard and many physicians, including those in our own practice, no longer recommend confirmatory testing.
The etiology of aldosterone hypersecrecretion is established by imaging and adrenal vein sampling (AVS). The distinction between unilateral APA from bilateral hyperplasia is a key factor in determining the appropriate management. APAs are best managed by surgical resection, whereas the treatment for IHA is medical therapy. Current high-resolution computed tomography (CT) imaging has enhanced the classification of subtypes of hyperaldosteronism and the ability to identify APAs. The sensitivity and specificity of adrenal imaging with 1.25-3 mm cuts for APA is 78% and 75%, respectively[22,24]. Findings on adrenal CT include normal-appearing adrenals, unilateral macroadenomas (greater than 1 cm), unilateral microadenomas (less than 1 cm), bilateral micro- or macroadenomas, and minimal unilateral adrenal limb thickening[22]. Imaging in IHA can reveal normal-appearing adrenal glands or show nodular changes. As a result, radiologists can misread APAs as IHA, whereas microadenomas can be incorrectly labeled as areas of hyperplasia[22]. Several studies have shown that CT alone may lead to misdiagnosis in PA. In a systematic review, Kempers et al[25] found that 37.8% of patients who showed lateralization on CT/magnetic resonance imaging (MRI) had conflicting results on AVS. If imaging alone was used for localization, 14.6% of patients would have undergone inappropriate adrenalectomy, while 19.1% would have been inappropriately excluded from surgery. Furthermore, in 3.9% of patients, CT/MRI lateralized to the opposite side. These considerations have prompted many to regard AVS as a gold standard for lateralization. However, mandatory use remains a contentious topic. The United States Endocrine Society and Japan Endocrine Society guidelines recommend that AVS be performed in all patients who have diagnosed PA and are considering surgical resection[22,25,26]. However, the Adrenal Vein Sampling International Study showed that AVS is utilized routinely in only a few centers worldwide[27]. AVS requires highly skilled radiologists for successful cannulation of both adrenal veins and the procedure is not without complications. AVS is unsuccessful in up to 20% due to failure to cannulate the right adrenal vein, and even in experienced centers, the complication rate averages 0.5%-2.5%[24,25,28,29].
Despite recommendations from the endocrine societies, several groups continue to advocate for selective use. Zarnegar et al[30] and Tan et al[31] both demonstrated the effectiveness of AVS in cases of equivocal findings on initial imaging studies. Specifically, Zarnegar et al[30] compared outcomes after adrenalectomy for patients with > 1 cm adenomas with normal contralateral adrenal glands on CT to those who required AVS and CT (< 1 cm). They found similar outcomes in both groups as measured by biochemical and hemodynamic resolution, advocating for selective use of AVS for patients with smaller tumors or indeterminate imaging findings. A recently-issued consensus statement recommends certain patients with PA do not necessarily require AVS, including: patients who are < 40 years old with marked PA and clear unilateral adrenal adenoma and normal contralateral gland on imaging; patients who are not surgical candidates due to unacceptably high operative risk; patients with suspected adrenocortical carcinoma; or patients who have proven familial hyperaldosteronism[32].
Treatment of PA is aimed at prevention of morbidity and mortality associated with hypertension, hypokalemia and direct aldosterone-associated organ damage. Once the cause of hyperaldosteronism is established, the proper management strategy can be instituted (Figure 1). Adrenalectomy is the procedure of choice for documented unilateral secretion of aldosterone (APA or UAH), while medical therapy is warranted for bilateral aldosterone hypersecretion as with IHA and bilateral APA, or for patients who refuse surgery or are poor surgical candidates.
Medical management involves antagonism of the mineralocorticoid (MR) receptor with spironolactone or eplerenone. Spironolactone has been utilized for over four decades as a first-line agent at doses ranging 25-400 mg/d[22,33]. Hypokalemia typically resolves immediately, but blood pressure reduction may take several months to occur[6]. Anti-androgen side effects such as gynecomastia and dysmenorrhea can result from spironolactone due to cross-antagonism of the sex-steroid receptors, usually in a dose-dependent fashion[34,35]. Eplerenone is more specific for the aldosterone receptor and therefore causes fewer undesired side effects. It is, however, less potent[36]. A recent randomized trial comparing the two therapies showed that spironolactone from 75 to 225 mg/d was more efficacious than eplerenone at 100-300 mg/d for hypertension control[36]. In addition, since spironolactone is cheaper and more widely available, clinicians should weigh these factors when recommending the appropriate agent for medical management of PA[10,36]. It is noteworthy that hypervolemia can be prohibitive in using MR antagonists as sole agents for PA, and in approximately 50% of patients, a second agent such as a low-dose thiazide diuretic can help achieve adequate blood pressure control[37]. Other agents including sodium channel blockers (amiloride, triamterene), calcium channel blockers, ACE-I, and angiotensin-receptor blockers (ARB) have also been employed as secondary agents in PA, with variable effects on blood pressure and plasma aldosterone levels[37,38].
Adrenalectomy is the preferred treatment strategy for patients with demonstrable unilateral hypersecretion of aldosterone. The standard approach employed by most centers is lateral transperitoneal laparoscopic adrenalectomy as first described in 1992 by Gagner et al[39]. However, some surgeons prefer a posterior retroperitoneoscopic approach or robotic-assisted surgery. Proponents of the retroperitoneoscopic approach recommend this technique for smaller tumors (< 6 cm), prior abdominal surgery and lower body mass index (BMI)[40-42]. Several recent meta-analyses comparing transabdominal to retroperitoneal laparoscopic adrenalectomy found no significant differences between the two approaches[43,44]. Additionally, Brandao et al[45] systematically reviewed robotic-assisted adrenalectomy and found that it is equally safe and may even result in less blood loss and shorter hospital stay, compared to laparoscopic approaches.
Aldosterone hypersecretion causes hypertension and biochemical abnormalities with potassium hemostasis by activation of the renin-angiotensin-aldosterone-system (RAAS). It has been shown that abnormal activation of the RAAS correlates directly with end-organ damage in the cardiovascular and renal systems and it is well-documented that blockade of the angiotensin-II arm by ACE-I or ARB provides significant cardiovascular protection[13]. Pathophysiologically, aldosterone works to increase sodium absorption in the kidneys, leading to increased intravascular volume and thereby increased blood pressure. Cardiovascular damage occurs from increased left ventricular mass and hypertrophy as well as aldosterone-driven fibrosis and collagen production in the interventricular septum. Furthermore, perivascular inflammation, vascular remodeling in the heart and kidney, and direct damage to the nephron anatomy and physiology, are thought to contribute to sustained deleterious end-organ effects from aldosterone excess that may occur independent of hypertension[46-48]. In fact, compared to patients with essential hypertension, patients with primary hyperaldosteronism are at increased risk for these adverse effects, which are significantly reduced by surgical or medical management[49-51]. Milliez et al[52] demonstrated in a retrospective study a markedly increased incidence of stroke (12.9% vs 3.4%), non-fatal MI (4.0% vs 0.6%), and atrial fibrillation (7.3% vs 0.6%) in patients with PA compared to those with essential hypertension. There was no difference in the PA subtype. Additionally, Ribstein et al[53] reported significant decrease in proteinuria in patients with PA with treatment of aldosterone excess by adrenalectomy or spironolactone compared to control essential hypertension patients.
The treatment of aldosterone hypersecretion either by medical or surgical means is very effective. Nearly 100% of patients will experience a biochemical cure with normalization of hypokalemia and aldosterone levels[54,55]. These effects follow surgery relatively quickly. It is recommended that potassium supplements and MR antagonists should be discontinued on post-operative day 1, and antihypertensive medications reduced simultaneously. Patients are also instructed to eat a diet generous in salt for the first month after surgery to account for a suppressed contralateral adrenal gland[56]. Interestingly, a minority of people can develop prolonged zona glomerulosa insufficiency causing hyperkalemia after adrenalectomy. Reported by Fischer et al[57], this outcome had an incidence of 5% of adrenalectomized PA patients in their cohort and required long-term fludrocortisone treatment post-operatively.
Resolution of hypertension in primary hyperaldosteronism is etiology-specific. For cases not appropriate for surgical resection, blood pressure control is best achieved by mineralocorticoid antagonists, as previously discussed. Conversely, for localized APAs adrenalectomy results in improvement in blood pressure control in over 90% of patients, and complete resolution, as defined by BP < 140/90 mmHg without the need for antihypertensive medications, in 30%-60%[6,58]. Patients that are not cured generally experience lower mean blood pressures and take fewer antihypertensive medications after surgery[59]. Persistent hypertension after adrenalectomy may result from misdiagnosis of unilateral aldosterone hypersecretion, or more likely, coexistent essential hypertension with underlying end organ damage. Chronic aldosterone excess has been shown to increase arterial stiffness, and may contribute to enduring hypertension in these patients[60]. Blood pressure typically normalizes or shows maximal improvement in one to six months after adrenalectomy, though it can continue to decrease for up to one year following surgery[56].
Multiple studies have looked at outcomes of adrenalectomy for APA to characterize predictive factors for resolution of hypertension. Factors that have been correlated with favorable results include younger age, female sex, lower BMI, fewer pre-operative antihypertensive medications, shorter duration of hypertension preoperatively, fewer first-degree family members with hypertension, better renal function as evidenced by higher glomerular filtration rate, lower creatinine and less proteinuria, lower serum aldosterone and higher urine aldosterone, histopathologic features, and smaller tumor size[58,61-64]. Recently, in a large series, Zhang et al[65] showed by multivariate regression that shorter duration of hypertension and lower serum aldosterone level were predictive of resolution of hypertension after adrenalectomy. Furthermore, several studies have linked the TT genotype of CYP11B2 gene encoding aldosterone synthase to successful outcomes after adrenalectomy for PA[66-68].
To better predict which of these features result in resolution of hypertension after adrenalectomy in patients with APA, Zarnegar et al[55] proposed the Aldosteronoma Resolution Score (ARS) which takes into account four readily available pre-operative clinical parameters including BMI ≤ 25 kg/m2, female sex, duration of preoperative hypertension ≤ 6 years, and number of preoperative antihypertensive medications ≤ 2. Each parameter receives a score of 1, with the exception of number of preoperative medications, which is scored by 2 points due to its relative significance in the prediction model. A score of 0-1 predicts a low likelihood of resolution, while patients with ARS 4-5 have a high likelihood of resolution of hypertension after adrenalectomy. In the study, 27.6% of patients with ARS 0-1 were cured, whereas 75% with ARS 4-5 had complete resolution of hypertension. Using an external cohort, the authors also demonstrated external validity of the model. Utsumi et al[61] further validated the accuracy of the ARS model using a Japanese population, confirming the utility of the ARS as a clinical tool for counseling patients on expected surgical outcomes.
While surgery abolishes the source of excess aldosterone secretion and significantly improves or resolves biochemical disturbances and blood pressure control, the long-lasting effects of exposure on the vasculature, heart, brain and kidney have yet to be completely delineated[63]. Nonetheless, several studies have shown that the progression of at least some of these effects are slowed or even reversed by adrenalectomy. Strauch et al[60] showed that resection of APA reduced arterial stiffness parameters compared to medical management. Rossi et al[15] showed regression of left ventricular hypertrophy in patients with primary hyperaldosteronism after appropriate medical or surgical intervention compared to optimally treated patients with primary hypertension, while Lin et al[69] showed adrenalectomy reversed myocardial fibrosis in these patients. Renal function has also been shown to improve after resection with resolution of microalbuminuria in APA patients compared to those with essential hypertension owing to the resolution of relative glomerular hyperfiltration in PA from the volume-expanding and hypertensive effects of the hormone[50,70].
Primary hyperaldosteronism is a common and treatable cause of secondary hypertension. Aldosterone excess has been linked to systemic disturbances in the cardiovascular, renal, and vascular systems, in addition to causing hypokalemia and hypertension. Multiple studies have shown worse morbidity with higher rates of myocardial infarction, stroke and renal dysfunction compared to patients with essential hypertension. Depending on the subtype, medical or surgical treatment is effective at halting or even reversing some, if not all, of these effects. Diagnosis and subtype differentiation relies on ARR, possible confirmatory testing, and localization studies with CT and adrenal venous sampling. Unilateral adrenalectomy for patients with APA successfully reverses biochemical disturbances, resolves or significantly improves hypertension, and halts progression of systemic perturbations. Though a variety of parameters have been found to be associated with resolution of hypertension after resection of APA, the ARS is currently the most accurate prediction model for resolution. Adrenalectomy for APA is a safe procedure that should be performed for appropriate candidates to improve long-term outcomes.
P- Reviewers: Okumura K, Petretta M S- Editor: Gou SX L- Editor: A E- Editor: Wu HL
| 1. | World Health Organization. A global brief on hypertension: Silent killer, global public health crisis (2013). Available from: http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/. |
| 2. | World Health Organization. World Health Statistic 2013. Vasa (2013). Available from: http://medcontent.metapress.com/index/A65RM03P4874243N.pdf. |
| 3. | Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;1-8. [PubMed] |
| 4. | US Census Bureau. State and County QuickFacts (2013). Available from: http://quickfacts.census.gov/qfd/states/00000.html. |
| 5. | Schwartz GL, Turner ST. Screening for primary aldosteronism in essential hypertension: diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem. 2005;51:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66:607-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 433] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 7. | CONN JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45:3-17. [PubMed] |
| 8. | Al Fehaily M, Duh QY. Clinical manifestation of aldosteronoma. Surg Clin North Am. 2004;84:887-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Moraitis A, Stratakis C. Adrenocortical causes of hypertension. Int J Hypertens. 2011;2011:624691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Chao CT, Wu VC, Kuo CC, Lin YH, Chang CC, Chueh SJ, Wu KD, Pimenta E, Stowasser M. Diagnosis and management of primary aldosteronism: an updated review. Ann Med. 2013;45:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Schirpenbach C, Seiler L, Maser-Gluth C, Rüdiger F, Nickel C, Beuschlein F, Reincke M. Confirmatory testing in normokalaemic primary aldosteronism: the value of the saline infusion test and urinary aldosterone metabolites. Eur J Endocrinol. 2006;154:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 650] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 13. | Rocha R, Stier CT. Pathophysiological effects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab. 2001;12:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Tanabe A, Naruse M, Naruse K, Hase M, Yoshimoto T, Tanaka M, Seki T, Demura R, Demura H. Left ventricular hypertrophy is more prominent in patients with primary aldosteronism than in patients with other types of secondary hypertension. Hypertens Res. 1997;20:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 16. | Young WF. Minireview: primary aldosteronism--changing concepts in diagnosis and treatment. Endocrinology. 2003;144:2208-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Stowasser M, Ahmed AH, Pimenta E, Taylor PJ, Gordon RD. Factors affecting the aldosterone/renin ratio. Horm Metab Res. 2012;44:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Tomaschitz A, Pilz S. Aldosterone to renin ratio--a reliable screening tool for primary aldosteronism? Horm Metab Res. 2010;42:382-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Fischer E, Beuschlein F, Bidlingmaier M, Reincke M. Commentary on the Endocrine Society Practice Guidelines: Consequences of adjustment of antihypertensive medication in screening of primary aldosteronism. Rev Endocr Metab Disord. 2011;12:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Ducher M, Mounier-Véhier C, Baguet JP, Tartière JM, Sosner P, Régnier-Le Coz S, Perez L, Fourcade J, Jabourek O, Lejeune S. Aldosterone-to-renin ratio for diagnosing aldosterone-producing adenoma: a multicentre study. Arch Cardiovasc Dis. 2012;105:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Yin G, Zhang S, Yan L, Wu M, Xu M, Li F, Cheng H. One-hour upright posture is an ideal position for serum aldosterone concentration and plasma renin activity measuring on primary aldosteronism screening. Exp Clin Endocrinol Diabetes. 2012;120:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Montori VM. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266-3281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1056] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 23. | Mulatero P, Monticone S, Bertello C, Mengozzi G, Tizzani D, Iannaccone A, Veglio F. Confirmatory tests in the diagnosis of primary aldosteronism. Horm Metab Res. 2010;42:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 505] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 25. | Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR, Deinum J. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 26. | Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, Tanabe A. Guidelines for the diagnosis and treatment of primary aldosteronism--the Japan Endocrine Society 2009. Endocr J. 2011;58:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 434] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 27. | Rossi GP, Barisa M, Allolio B, Auchus RJ, Amar L, Cohen D, Degenhart C, Deinum J, Fischer E, Gordon R. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012;97:1606-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 266] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 28. | Mathur A, Kemp CD, Dutta U, Baid S, Ayala A, Chang RE, Steinberg SM, Papademetriou V, Lange E, Libutti SK. Consequences of adrenal venous sampling in primary hyperaldosteronism and predictors of unilateral adrenal disease. J Am Coll Surg. 2010;211:384-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Siracuse JJ, Gill HL, Epelboym I, Clarke NC, Kabutey NK, Kim IK, Lee JA, Morrissey NJ. The Vascular Surgeon’s Experience with Adrenal Venous Sampling for the Diagnosis of Primary Hyperaldosteronism. Ann Vasc Surg. 2013;Dec 16; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Zarnegar R, Bloom AI, Lee J, Kerlan RK, Wilson MW, Laberge JM, Gordon RL, Kebebew E, Clark OH, Duh QY. Is adrenal venous sampling necessary in all patients with hyperaldosteronism before adrenalectomy? J Vasc Interv Radiol. 2008;19:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Tan YY, Ogilvie JB, Triponez F, Caron NR, Kebebew EK, Clark OH, Duh QY. Selective use of adrenal venous sampling in the lateralization of aldosterone-producing adenomas. World J Surg. 2006;30:879-885; discussion 886-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, Satoh F, Young WF. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 419] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 33. | Handler J. Overlapping spironolactone dosing in primary aldosteronism and resistant essential hypertension. J Clin Hypertens (Greenwich). 2012;14:732-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Jeunemaitre X, Chatellier G, Kreft-Jais C, Charru A, DeVries C, Plouin PF, Corvol P, Menard J. Efficacy and tolerance of spironolactone in essential hypertension. Am J Cardiol. 1987;60:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 187] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Karagiannis A, Tziomalos K, Papageorgiou A, Kakafika AI, Pagourelias ED, Anagnostis P, Athyros VG, Mikhailidis DP. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother. 2008;9:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Parthasarathy HK, Ménard J, White WB, Young WF, Williams GH, Williams B, Ruilope LM, McInnes GT, Connell JM, MacDonald TM. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011;29:980-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 37. | Karagiannis A. Treatment of primary aldosteronism: Where are we now? Rev Endocr Metab Disord. 2011;12:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Rossi GP. Diagnosis and treatment of primary aldosteronism. Rev Endocr Metab Disord. 2011;12:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Gagner M, Lacroix A, Prinz RA, Bolté E, Albala D, Potvin C, Hamet P, Kuchel O, Quérin S, Pomp A. Early experience with laparoscopic approach for adrenalectomy. Surgery. 1993;114:1120-1124; discussion 1124-1125. [PubMed] |
| 40. | Berber E, Tellioglu G, Harvey A, Mitchell J, Milas M, Siperstein A. Comparison of laparoscopic transabdominal lateral versus posterior retroperitoneal adrenalectomy. Surgery. 2009;146:621-625; discussion 625-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Suzuki K, Kageyama S, Hirano Y, Ushiyama T, Rajamahanty S, Fujita K. Comparison of 3 surgical approaches to laparoscopic adrenalectomy: a nonrandomized, background matched analysis. J Urol. 2001;166:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Lee CR, Walz MK, Park S, Park JH, Jeong JS, Lee SH, Kang SW, Jeong JJ, Nam KH, Chung WY. A comparative study of the transperitoneal and posterior retroperitoneal approaches for laparoscopic adrenalectomy for adrenal tumors. Ann Surg Oncol. 2012;19:2629-2634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Nigri G, Rosman AS, Petrucciani N, Fancellu A, Pisano M, Zorcolo L, Ramacciato G, Melis M. Meta-analysis of trials comparing laparoscopic transperitoneal and retroperitoneal adrenalectomy. Surgery. 2013;153:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Constantinides VA, Christakis I, Touska P, Palazzo FF. Systematic review and meta-analysis of retroperitoneoscopic versus laparoscopic adrenalectomy. Br J Surg. 2012;99:1639-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 45. | Brandao LF, Autorino R, Laydner H, Haber GP, Ouzaid I, De Sio M, Perdonà S, Stein RJ, Porpiglia F, Kaouk JH. Robotic Versus Laparoscopic Adrenalectomy: A Systematic Review and Meta-analysis. Eur Urol. 2014;65:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 46. | Connell JM, MacKenzie SM, Freel EM, Fraser R, Davies E. A lifetime of aldosterone excess: long-term consequences of altered regulation of aldosterone production for cardiovascular function. Endocr Rev. 2008;29:133-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Rossi GP, Sechi LA, Giacchetti G, Ronconi V, Strazzullo P, Funder JW. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrinol Metab. 2008;19:88-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 48. | Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 49. | Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 410] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 50. | Sechi LA, Colussi G, Di Fabio A, Catena C. Cardiovascular and renal damage in primary aldosteronism: outcomes after treatment. Am J Hypertens. 2010;23:1253-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 356] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 52. | Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1069] [Cited by in RCA: 1104] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 53. | Ribstein J, Du Cailar G, Fesler P, Mimran A. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol. 2005;16:1320-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 54. | Quillo AR, Grant CS, Thompson GB, Farley DR, Richards ML, Young WF. Primary aldosteronism: results of adrenalectomy for nonsingle adenoma. J Am Coll Surg. 2011;213:106-112; discussion 112-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Zarnegar R, Young WF, Lee J, Sweet MP, Kebebew E, Farley DR, Thompson GB, Grant CS, Clark OH, Duh QY. The aldosteronoma resolution score: predicting complete resolution of hypertension after adrenalectomy for aldosteronoma. Ann Surg. 2008;247:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 56. | Carey RM. Primary aldosteronism. J Surg Oncol. 2012;106:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Fischer E, Hanslik G, Pallauf A, Degenhart C, Linsenmaier U, Beuschlein F, Bidlingmaier M, Mussack T, Ladurner R, Hallfeldt K. Prolonged zona glomerulosa insufficiency causing hyperkalemia in primary aldosteronism after adrenalectomy. J Clin Endocrinol Metab. 2012;97:3965-3973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Sawka AM, Young WF, Thompson GB, Grant CS, Farley DR, Leibson C, van Heerden JA. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med. 2001;135:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 213] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 59. | van der Linden P, Steichen O, Zinzindohoué F, Plouin PF. Blood pressure and medication changes following adrenalectomy for unilateral primary aldosteronism: a follow-up study. J Hypertens. 2012;30:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Strauch B, Petrák O, Zelinka T, Wichterle D, Holaj R, Kasalický M, Safarík L, Rosa J, Widimský J. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens. 2008;21:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 61. | Utsumi T, Kawamura K, Imamoto T, Kamiya N, Komiya A, Suzuki S, Nagano H, Tanaka T, Nihei N, Naya Y. High predictive accuracy of Aldosteronoma Resolution Score in Japanese patients with aldosterone-producing adenoma. Surgery. 2012;151:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Kim RM, Lee J, Soh EY. Predictors of resolution of hypertension after adrenalectomy in patients with aldosterone-producing adenoma. J Korean Med Sci. 2010;25:1041-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Carter Y, Roy M, Sippel RS, Chen H. Persistent hypertension after adrenalectomy for an aldosterone-producing adenoma: weight as a critical prognostic factor for aldosterone’s lasting effect on the cardiac and vascular systems. J Surg Res. 2012;177:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Waldmann J, Maurer L, Holler J, Kann PH, Ramaswamy A, Bartsch DK, Langer P. Outcome of surgery for primary hyperaldosteronism. World J Surg. 2011;35:2422-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Zhang X, Zhu Z, Xu T, Shen Z. Factors affecting complete hypertension cure after adrenalectomy for aldosterone-producing adenoma: outcomes in a large series. Urol Int. 2013;90:430-434. [PubMed] |
| 66. | Wang B, Zhang G, Ouyang J, Deng X, Shi T, Ma X, Li H, Ju Z, Wang C, Wu Z. Association of DNA polymorphisms within the CYP11B2/CYP11B1 locus and postoperative hypertension risk in the patients with aldosterone-producing adenomas. Urology. 2010;76:1018.e1-1018.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 67. | Wang W, Hu W, Zhang X, Wang B, Bin C, Huang H. Predictors of successful outcome after adrenalectomy for primary aldosteronism. Int Surg. 2012;97:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Wang W, Hu WL, Zhang LC, Xiao YS, Liu J, Bin C. Polymorphic variation of CYP11B2 predicts postoperative resolution of hypertension in patients undergoing adrenalectomy for aldosterone-producing adenomas. Int J Urol. 2012;19:813-820; author reply 820-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Lin YH, Wu XM, Lee HH, Lee JK, Liu YC, Chang HW, Lin CY, Wu VC, Chueh SC, Lin LC. Adrenalectomy reverses myocardial fibrosis in patients with primary aldosteronism. J Hypertens. 2012;30:1606-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 70. | Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, Catena C. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295:2638-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 276] [Article Influence: 14.5] [Reference Citation Analysis (0)] |