Published online Nov 26, 2013. doi: 10.4330/wjc.v5.i11.410
Revised: September 20, 2013
Accepted: October 11, 2013
Published online: November 26, 2013
Symptomatic bradycardia is effectively treated with the implantation of a cardiac pacemaker. Although a highly successful therapy, during recent years there has been a focus on the negative effects associated with long-term pacing of the apex of the right ventricle (RV). It has been shown in both experimental and clinical studies that RV pacing leads to ventricular dyssynchrony, similar to that of left bundle branch block, with subsequent detrimental effects on cardiac structure and function, and in some cases adverse clinical outcomes such as atrial fibrillation, heart failure and death. There is substantial evidence that patients with reduced left ventricular function (LVEF) are at particular high risk of suffering the detrimental clinical effects of long-term RV pacing. The evidence is, however, incomplete, coming largely from subanalyses of pacemaker and implantable cardiac defibrillator studies. In this group of patients with reduced LVEF and an expected high amount of RV pacing, biventricular pacing (cardiac resynchronization therapy) devices can prevent the negative effects of RV pacing and reduce ventricular dyssynchrony. Therefore, cardiac resynchronization therapy has emerged as an attractive option with promising results and more clinical studies are underway. Furthermore, specific pacemaker algorithms, which minimize RV pacing, can also reduce the negative effects of RV stimulation on cardiac function and may prevent clinical deterioration.
Core tip: A high amount of long-term right ventricular (RV) pacing produces ventricular dyssynchrony and clinical deterioration in patients with reduced left ventricular ejection fraction (LVEF). In this patient group, cardiac resynchronization therapy has been shown to improve clinical outcomes and should be considered before a conventional pacemaker. In subjects with normal LVEF, the deleterious effects of RV pacing is less clear; however, specific pacemaker algorithms that minimize RV pacing may improve clinical outcomes in selected patients. Future studies will help to better identify those at risk of suffering the negative effects of RV pacing and define the correct use of preventive therapeutic strategies.
- Citation: Akerström F, Arias MA, Pachón M, Jiménez-López J, Puchol A, Juliá-Calvo J. The importance of avoiding unnecessary right ventricular pacing in clinical practice. World J Cardiol 2013; 5(11): 410-419
- URL: https://www.wjgnet.com/1949-8462/full/v5/i11/410.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i11.410
Cardiac pacing has greatly improved the prognosis of patients with symptomatic bradycardia, approximating that of the general population. However, animal and human studies have shown that RV pacing leads to abnormal electrical and mechanical activation patterns (dyssynchrony), which leads to impaired hemodynamic parameters and myocardial remodeling[1-5]. Large pacemaker and implantable cardiac defibrillator (ICD) trials have reported an association between long-term RV pacing and deterioration of cardiac structure and function, as well as increased risk of heart failure (HF), atrial fibrillation (AF) and death[1,6,7]. This has subsequently caused concerns about the potential deleterious clinical effect of long-term right ventricular (RV) pacing. As a result, several therapeutic strategies, such as alternative RV pacing sites, cardiac resynchronization therapy (CRT) and alternative pacemaker programming options/algorithms that minimize RV pacing have emerged. This review will outline the available evidence concerning the negative effects of long-term RV pacing and comment on how to minimize RV pacing, with a focus on CRT and specific pacemaker algorithms.
The large bulk of information regarding the negative effects of RV pacing in patients without baseline HF comes from large pacemaker randomized clinical trials (RCT) of elderly patients with mainly Sick sinus syndrome (SSS) that were designed to assess the difference between atrial (AAI or DDD) and ventricular-based pacing strategies[8-14]. In the single center Danish trial, 225 patients with SSS were randomized to either single chamber atrial pacing (AAI) or single chamber ventricular pacing (VVI)[8]. After a mean of 5.5 years of follow-up, a significant increase in total and cardiovascular mortality, HF and AF in the ventricle-based pacing group was reported. The authors also found that the VVI pacing led to increased dilatation of the left atrial diameter and reduced left ventricular (LV) fractional shortening. An assumption that conserving atrioventricular (AV) synchrony is beneficial was made and because of that, several RCTs that compared dual chamber (DDD) vs single chamber (VVI) pacing in elderly patients with SSS only[10,12], SSS and AV block[9,11], and AV block alone[14] were carried out. To the surprise of many, DDD pacing was not associated with a decrease in mortality or hospitalization for HF, although a reduction in AF and minor improvements in quality of life were observed in the same group. These results were later confirmed in a meta-analysis[15] that included the Danish trial[8] and 4 of the recently mentioned RCTs that compared DDD vs VVI pacing[9-11,14] (Table 1). With atrial-based pacing, there was no significant difference in mortality (HR = 0.95, 95%CI: 0.87-1.03) or HF (HR = 0.89, 95%CI: 0.77-1.03), but a significant reduction in AF (HR = 0.81, 95%CI: 0.72-0.89) was observed[15].
| Ref. | Patients | Follow-up | Pacing/ICD | Study groups | Endpoints | Results |
| (n) | (yr) | indication | ||||
| Danish study[8] (1997) | 225 | 5.5 | SSS | AAI vs VVI | All-cause mortality, CV mortality, AF, stroke, HF, and AV block | Significant reduction in CV mortality, AF, stroke and HF in the AAI group |
| PASE[11] (1998) | 407 | 1.5 | SSS and AVB | DDDR vs VVIR | Quality of life, all-cause mortality1, HF1, and AF1 | No overall difference in quality of life albeit moderate improvement in patients with SSS but not AVB in the DDDR group No difference in mortality, HF or AF |
| CTOPP[9] (2000) | 2568 | 6.4 | SSS and AVB | DDD/AAI vs VVI(R) | Stroke, CV mortality, all-cause mortality1, AF1, and HF1 | No difference in stroke, CV mortality, all-cause mortality or HF Significant reduction in AF in the DDD/AAI group. |
| MOST[10] (2002) | 2010 | 2.8 | SSS | DDDR vs VVIR | All-cause mortality, stroke, AF1, HF1, QoL1, pacemaker syndrome1 | No difference in all-cause mortality, stroke Significant reduction in AF, HF, and QoL in the DDDR group 18.3% cross-over due to pacemaker syndrome in the VVIR group |
| UK-PACE[14] (2005) | 2021 | 3 | AVB | DDD(R) vs VVI(R) | All-cause mortality, AF1, HF1, stroke1 | No difference in any of the endpoints |
| DANPACE[13] (2011) | 1415 | 5.4 | SSS | AAIR vs DDDR | All-cause mortality, AF1, HF1, stroke1, need for pacemaker reoperation1 | No difference in all-cause mortality, chronic AF, HF or stroke Increased risk of paroxysmal AF and need for pacemaker reoperation (development of AVB) in the AAIR group |
| DAVID[7] (2002) | 506 | 0.8 | Primary and secondary prevention ICD | VVI 40 vs DDDR 70 ICD | Composite of hospitalization for HF and mortality | Prematurely interrupted due to increased occurrences of the composite endpoint in the DDDR 70 group |
| MADIT II substudy[17] (2005) | 1232 | 1.7 | Primary prevention ICD | 0%-50% vs 51%-100% VP | Composite of HF and mortality | Nearly two-fold increase in hospitalization for HF in the 51%-100% VP group |
One of the previously mentioned studies is The Mode Selection Trial in Sinus Node Dysfunction (MOST), which was a multicenter randomized study that randomized a total of 2010 patients with SSS to either VVIR or DDDR pacing[10]. Unexpectedly, after 33 mo of follow-up, a small but significant increased incidence of AF and hospitalization for HF in the DDD pacing group was reported, with no difference in all cause mortality between the two groups. When analyzing 1339 patients from the same study, the DDDR group received significantly more RV pacing than the VVIR group (90% vs 58%, respectively) and interestingly, the amount of RV pacing was a strong predictor for AF [HR = 1.36 (95%CI: 1.09-1.69) for each 25% increase in cumulative RV pacing] and HF hospitalization [HR = 2.99 (95%CI: 1.15-7.75) for > 40% of cumulative RV pacing][6]. On the contrary, the recent Danish Multicenter Randomized Trial on Single Lead Atrial Pacing vs Dual Chamber Pacing in Sick Sinus Syndrome (DANPACE) trial which included 1415 patients reported no difference in mortality, HF or chronic AF between DDDR or AAIR pacing[13] or the amount of RV pacing[16] after a mean follow-up of 5.4 years. Furthermore, there was a significant increase in paroxysmal AF and need for pacemaker reoperation, mainly due to the development of AV conduction disease in the AAIR group[13].
The conflicting results of the DANPACE trial[13] and the study limitations of the subanalyses from the older pacemaker trials, like patient heterogenicity (a minority presented clinical HF) and no echocardiographic evaluation, make it difficult to estimate to what extent long-term RV pacing causes clinical deterioration in patients without baseline HF. Although with the data available, it seems likely that most patients with normal LV function tolerate some degree of RV pacing without developing HF during long-term follow up.
The Dual Chamber and VVI Implantable (DAVID) trial[7] and a subanalysis of the Multicenter Automatic Defibrillator Trial II (MADIT II)[17] have provided strong evidence for the negative effects of RV pacing in patients with reduced baseline LVEF (Table 1). The DAVID trial was designed to study whether DDDR pacing with a lower rate limit at 70/min (DDDR 70) would decrease total mortality and hospitalization for HF when compared against VVI backup pacing with a lower rate of 40/min (VVI 40) through increased cardiac output and allowing higher doses of β-blocker therapy[7]. A total of 506 patients with standard indication for ICD implantation as secondary prevention but without indications for antibradycardia pacing were included in the trial, with an LVEF of 28% ± 8%. After a median follow-up of 8.4 mo, the study was prematurely interrupted due to more occurrences of the composite endpoint in the DDDR 70 group, largely driven by hospitalization for HF (1 year survival free of the composite endpoint: 73.3% for DDDR 70% and 83.9% for VVI 40; P = 0.02). Like in the MOST trial[10], a subanalysis of the DAVID trial[18] reported a continuous relationship between the percentage of RV pacing and the primary endpoint, with the most significant divergence of outcomes occurring with RV pacing > 40%. The results were further supported by the DAVID II trial[19], which randomized 600 patients with baseline characteristics similar to the DAVID trial to receive ICD implantation with either AAI pacing with a lower rate of 70/min or VVI 40. No difference in mortality or hospitalization for HF was observed after a mean follow-up of 2.7 years. Additional evidence comes from a subanalysis of the MADIT II trial[17], which randomized 1232 patients with previous myocardial infarction and LVEF < 30% to ICD plus optimal medical therapy vs medical therapy alone[20]. A significant 31% reduction in mortality risk was observed in the ICD arm but there was a worrisome trend towards more hospitalizations for HF in the ICD group and the subanalysis reported a nearly two-fold increased risk of hospitalization for HF in those who received > 50% of cumulative pacing. A recent report of the 8 years of follow-up of the patients with > 50% in the MADIT II trials reported mortality rates similar to the optimal medical therapy group, with the ICD group with a low percentage of RV pacing presenting a continued significant mortality benefit[21]. The MADIT II trial[20] results therefore illustrate how the clinical expression of the detrimental effects of RV pacing is the result of years of a high amount of RV pacing.
The purpose of RV non-apical (RVNA) pacing is to take advantage of the specialized conduction system and thereby reduce ventricular dyssynchrony. Three main anatomical sites have been evaluated: right ventricular outflow tract (RVOT), intraventricular septum (IVS) and the His bundle. Overall, evidence from several small studies suggests that dyssynchrony is reduced and that LVEF is improved with RVOT[22], IVS[23] and His bundle[24] pacing, although negative results have also been reported[25]. Nevertheless, there is conflicting evidence regarding the clinical benefit of RVNA pacing in terms of exercise capacity or quality of life scores[23,25,26]. Furthermore, only one study evaluated whether RVNA pacing would result in prolonged survival benefit and failed to find such an association, although this endpoint was not powered properly[27]. Furthermore, a recent meta-analysis reported improved LVEF with RVNA pacing but no demonstrable clinical benefit when compared with RV pacing[28]. Large RCTs with statistical power to evaluate clinical endpoints are needed in order to establish whether RVNA pacing is an effective alternative to conventional RV apical pacing.
There are several pacemaker algorithms that permit prolonged AV intervals, all potentially capable of reducing RV pacing, and they can be divided into two large groups: (1) algorithms which periodically prolong the AV interval to search for, and if present, allow intrinsic AV conduction (AV hysteresis); and (2) algorithms that operate in a primary atrial pacing mode, with mode switch to secondary mode ventricular pacing (DDD) in case of significant loss of AV conduction[29-31] (Table 2). The most studied algorithm is probably the Managed Ventricular Pacing™ (MVP) (Medtronic, Minneapolis, MN, United States) that operates in primary atrial based mode labeled (AAI[R]+) with switch to secondary DDD[R] mode in the case of loss of AV conduction occurring in 2 out of 4 atrial-atrial intervals[30]. In a short-term study without clinical endpoints with patients with SSS and various degrees of AV block, the MVP algorithm was reported to be significantly more effective in reducing the amount of RV pacing when compared to one AV hysteresis algorithm (66.1% vs 54.3% had < 40% of RV pacing, respectively)[29].
| Reverse Mode Switch/RYTHMIQ™ (Boston Scientific, St. Paul, MN, United States) |
| Atrial based pacing in AAI(R) with VVI backup (LRL minus 15/min) with the two modes operate independently from one another. If complete AVB occurs, ventricular paces will be delivered at backup VVI rate, asynchronous to the AAI rate. If 3 slow ventricular beats are detected in a window of 11 beats, AV conduction is considered blocked and switch to DDD (R) takes place. The algorithm will switch back to AAI if intact AV conduction is recuperated |
| Managed Ventricular Pacing™ (Medtronic, Minneapolis, MN, United States) |
| Atrial based pacing (labeled as AAI(R)+) with switch to DDD(R) if AV block is detected, defined as 2/4 absent ventricular event. The algorithm checks for AV conduction at regular intervals and if present it will switch back to AAI(R)+ |
| Ventricular Intrinsic Preference™ (St. Jude Medical, Sylmar, CA, United States) |
| Intrinsic AV conduction is assessed by increasing AV delay at regular intervals (programmable AV extension of up to 200 ms; maximum AV delay 350 ms). If present, the longer AV delay will be maintained until a programmable number of cycles of absent ventricular sensed events (i.e., continuous need for ventricular pacing), thus deactivating the algorithm |
| AV hysteresis (Biotronik, Berlin, Germany) |
| Similar to Ventricular Intrinsic Preference™ (St. Jude) |
| AAISafeR™ and AAISafeR2™ (Sorin Group, Mirandola, Italy) |
| Atrial based pacing in AAI (R). Abnormal AV intervals (> 350 ms if atrial sensed; > 450 ms if atrial paced) are monitored. Switch to DDD in response to any of the following: |
| > 6 abnormal AV intervals (“first degree AVB”) |
| > 3/12 nonconducted atrial events (“second degree AVB”) |
| > 2 consecutive nonconducted atrial event (“advanced AVB”) |
| Ventricular pauses of 2–4 s (programmable) |
In terms of the potential clinical benefits associated with algorithms that minimize RV pacing, this has been evaluated by a few RCTs (Table 3). The Inhibition of Unnecessary RV Pacing With AVSH in ICDs (INTRINSIC RV) Study[32] was a multicenter non-inferiority trial which included 1530 patients with conventional indication for ICD implantation to dual chamber pacing (DDDR mode with lower rate of 60) with AV Search Hysteresis™ (DDDR 60 AVSH) (Boston Scientific, St. Paul, MN, USA) or backup VVI 40 pacing. However, due to the worrisome results of the DAVID trial[7], eventually only 988 patients with < 20% RV pacing at 1 wk with DDDR 60 AVSH were randomized to the two programming modes. After a mean follow-up of 10.4 mo, non-inferiority of the primary endpoint, hospitalization for HF or total mortality had been met with a trend towards superiority for the primary endpoint in the DDDR 60 AVSH group. The DDDR AVSH 60 and VVI 40 groups presented with a mean RV pacing percentage of 10% and 3%, respectively. The results of the INTRISC RV study are reassuring since they suggests that in ICD recipients with a need for dual chamber pacing (e.g., SSS and various degrees of AV block), the deleterious effects of long-term RV pacing as observed in the DAVID trial[7] can be avoided by the use of the AV hysteresis algorithm. However, since only those with < 20% of RV pacing were included in the trial, one should only consider the pacing algorithm in this patient profile and not in patients with high grade AV block who would expect to receive a significant amount of RV pacing (> 20%) despite the use of the algorithm. The Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) trial[33] assessed whether dual chamber pacing, using the Search AV+™ or MVP algorithms (Medtronic), decreases time to development of persistent AF compared with conventional dual chamber pacing (AV interval 120-180 ms) in patients with SSS and normal LVEF. After a follow-up of a mean of 1.7 years, significantly less patients in the minimal pacing group developed persistent AF (7.9% vs 12.7%, respectively, P = 0.004). However, no difference in the secondary endpoints, hospitalization for HF or mortality was found. As expected, the median percentage of atrial pacing was similar between the two groups but the amount of RV pacing in the conventional group was markedly increased when compared to the minimal pacing group (99.0% vs 9.1%). For the first time, a prospective association between a reduction in RV pacing and clinical benefit (freedom from AF) was reported. The results therefore support the use of algorithms that minimize RV pacing in patients with SSS.
| Study | Design | Pacing | Patients | Follow-up | Outcomes |
| indication | (n) | (mo) | |||
| Sweeney et al[30] | Randomized, crossover MVP vs DDD(R) | SSS | 181 | 1 | Amount of pacing: MVP™: 4.1%; DDD(R): 73.8% |
| Murakami et al[29] | Randomized, crossover MVP vs Search AV+ | SSS and AVB | 127 | 1 | Amount of pacing: MVP: 66.1%; Search AV+: 54.3% (patients with %RVP < 40) MVP: 57.5%; Search AV+: 38.6% (patients with %RVP < 10) |
| Olshansky et al[32] | RCT DDD(R) AVSH 60/min vs VVI 40/min (non-inferiority) | ICD1 | 1530 | 10.4 | Trend towards a lower rate of death and hospitalization for HF in the DDD(R) AVSH group |
| Sweeney et al[33] | RCT Search AV+/MVP vs DDD(R) | SSS | 1065 | 12 | Amount of pacing: DDD(R): 99%; Search AV+/MVP: 9.1% Reduction in time to development of AF (primary endpoint) in the search AV+/MVP group No difference in hospitalization for HF or death (secondary endpoints) |
| Sweeney et al[36] | RCT MVP 60/min vs VVI 40/min (non-inferiority) | ICD1 | 1030 | 29 | Prematurely interrupted due slightly more deaths and hospitalization for HF in MVP group |
Although the results are promising, the algorithms that minimize RV pacing may not be suitable in some patients. No large long-term trials with clinical endpoints have evaluated these algorithms in patients with high-degree AV block (although there is evidence that supports their short-term safety and effectiveness in reducing RV pacing)[34]. Furthermore, allowing severely prolonged AV intervals may lead to compromised cardiac output resulting from inefficient atrial systole and various degrees of diastolic mitral regurgitation[35]. The Managed Ventricular Pacing Versus VVI 40 Pacing Trial[36] compared dual chamber pacing with the MVP algorithm (with a lower rate of 60) and backup VVI 40 pacing in ICD patients. The trial was unexpectedly terminated early since non-inferiority for the primary combined endpoint of HF events or total mortality could not be demonstrated, with a trend towards more primary endpoint events in the MVP. Interestingly, the subgroup analysis found that the increase in HF and mortality was largely contributed to by patients with a PR interval of ≥ 230 ms. Moreover, a subanalysis of the INTRINSIC RV study[37] reported on a J-shaped relationship between amount of RV pacing and the clinical event rate, with the best outcome for those with RV pacing between 10% and 19%. It therefore seems that a certain amount of RV pacing in those with impaired baseline AV conduction is necessary, although the equilibrium between low amounts RV pacing and preserved AV synchrony is not fully known. However, patients with normal or near normal AV conduction and the need for antibradycardia pacing are likely to benefit from the use of algorithms that minimize RV pacing.
Finally, sometimes pacemaker algorithms, like the ones previously discussed, may not work as expected. We recently evaluated the performance of the reverse mode switch™ (RMS) algorithm (Boston Scientific) which offers, like the MVP algorithm, primary atrial pacing AAI(R) mode with switch to DDD(R) secondary mode in the case of AV conduction loss, in a small retrospective study of 21 patients[38]. A large majority (84%) of the RMS episodes analyzed revealed an inappropriate switch to DDD(R) mode, mainly triggered by premature ventricular contractions (PVC). Therefore, our results suggest that patients with the RMS algorithm and high amounts of PVCs are paradoxically subject to an increased risk of unnecessary RV pacing through inappropriate RMS episodes. The results are also transferable to the newer but similar algorithm RYTHMIQ™ (Boston Scientific), given that the only difference between the two algorithms is the availability of the atrial tachycardia response feature in AAI(R) mode in RYTHMIQ.
It is well established that CRT improves ventricular dyssynchrony, LVEF, hospitalization for HF and mortality in patients with HF, prolonged QRS interval and NYHA class II-IV and it has become a part of standard HF treatment[39,40]. Until recently, there was little data on the benefit of CRT in patients with a conventional indication for antibradycardia pacing; however, the results from the Biventricular Versus Right Ventricular Pacing in Patients with AV block (BLOCK HF) study[41] were recently published. It constitutes the first large scale RCT that assesses the clinical benefits of CRT compared to RV pacing in patients with LV systolic dysfunction (LVEF ≤ 50%) and AV conduction loss with a standard pacemaker indication but without conventional indication for CRT. A total of 691 patients, with mean QRS of 125 ms and 121 ms and mean LVEF 43% ± 7% and 33% ± 8% (CRT pacing and CRT-ICD groups, respectively) were randomized to RV pacing and CRT. The patients presented with first (19%), second (33%) and third degree (48%) AV block. After a mean of 37 mo of follow-up, CRT was associated with a 26% risk reduction in the primary composite endpoint of all-cause mortality, HF-related urgent care and LV end-systolic index [HR = 0.74 (95% credible interval 0.60-0.90)] and a 27% risk reduction in all-cause mortality and HF-related urgent care [HR = 0.73 (95% credible interval 0.57-0.92)]. The findings from the BLOCK study therefore confirm the results from previous small studies[42] that CRT in patients with a pacemaker indication for AV block and a high degree of expected RV pacing and LV systolic dysfunction improves LV function and clinical outcomes. Furthermore, there is also data suggesting that patients with reduced LVEF and a reported high amount of long-term RV pacing may benefit from a device upgrade to CRT. For example, in a retrospective study, Fröhlich et al[43] reported inverse LV remodeling (LVEF and LV end-systolic and end-diastolic diameters) and improved NYHA functional class in patients with chronic RV pacing and reduced LVEF who received a CRT upgrade. A recent small RCT that included 50 patients with LV systolic dysfunction listed for routine pacemaker generator replacement with > 80% RV pacing in the preceding 12 mo found that an CRT upgrade was associated with improved LVEF, reduced N-terminal pro-B-type natriuretic peptide levels, exercise capacity and quality of life[44].
There is less evidence in favor of CRT in patients with normal LVEF. The Pacing to Avoid Cardiac Enlargement (PACE) study[45], which was a multicenter, double blind trial, randomized 177 patients with SSS or AV advanced block to receive either RV pacing (DDDR mode) or CRT. After 1 year of follow-up, the authors reported maintained LVEF and LV end-systolic volume (primary endpoints) in the CRT group but a reduction in these parameters in the RV pacing group. However, CRT did not improve the secondary clinical endpoints: 6 min walking test, hospitalization for HF or quality of life. It should be noted that no increased procedure-related complications were reported in the discussed studies, indicating that CRT is also a safe alternative to conventional RV pacing.
In AF patients with rapid ventricular rates who undergo catheter ablation of the AV node to create complete AV block due to irresponsiveness to pharmacological treatment, there is a high risk of suffering the detrimental effects associated with prolonged high density RV pacing, such as LV dyssynchrony, reduced LVEF and worsened HF symptoms[46]. Five small short-term RCTs studied the potential benefit of CRT compared to RV pacing in patients with AF and AV node ablation[47-51] and found improved LVEF and in some instances a reduction in hospitalization for HF. Thus, there is evidence that this group of patients would also benefit from CRT. Nevertheless, in asymptomatic individuals with AV node ablation for AF and normal LV function there is currently no evidence to support CRT. A list of some of the major clinical studies that compared CRT vs RV pacing is shown in Table 4.
| Study | Design | Patient | Patients | Follow-up | Baseline LVEF | LVEF in RV | LVEF in CRT | Clinical benefit from CRT |
| characteristics | (n) | (mo) | pacing | |||||
| Martinelli et al[42] | RCT multicenter | AVB | 60 | 5 (crossover) | 30.1% ± 9.2% | 22.5% ± 8.1% | 29.3% ± 6.9%a | Improved NYHA class and QoL |
| Yu et al[45] | RCT multicenter | AVB and SSS | 177 | 12 | 61.6% ± 6.6% | 54.8% ± 9.1% | 62.2% ± 7%b | No difference in hospitalization for HF, exercise capacity or QoL |
| Curtis et al[41] | RCT multicenter | AVB | 691 | 37 | 43% ± 7% (CRT-P) 33% ± 8% (DRT-D) | - | - | Reduction in composite endpoint (mortality, HF urgent care and LVESI) |
| Brignole et al[47] | RCT multicenter | AVN ablation | 186 | 20 | 38% ± 14% | Increasing from baseline + 4.7% | Increasing from baseline +6.6% (NS) | Reduction in composite endpoint (death from HF, hospitalization for HF or worsened HF) |
| Doshi et al[49] | RCT multicenter | AVN ablation | 184 | 6 | 46% ± 16% | 41.1% ± 13% | 46% ± 13%a | Improved exercise capacity |
| No difference in QoL | ||||||||
| Orlov et al[51] | RCT multicenter | AVN ablation | 127 | 6 | 56.1% ± 9.4% (CRT group) 57.2% ± 7.5% (RVP group) | 54.6% ± 11.5% | 59.3% ± 7.7%a | No difference in NYHA class, exercise capacity or QoL |
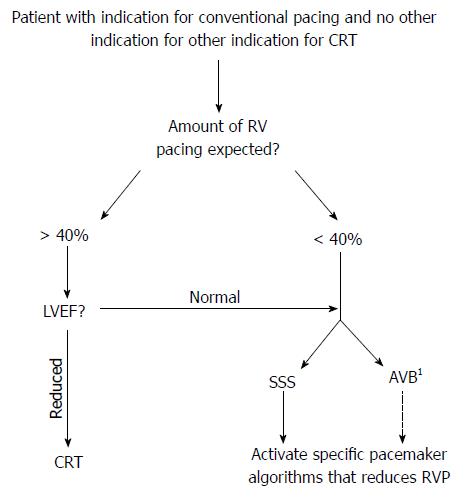
Data from subanalyses of ICD trials, including patients with reduced LVEF, suggests that > 40%-50% of RV pacing is associated with adverse clinical outcomes[17,18]. In addition, patients with reduced LVEF are at a significantly higher risk of suffering the negative clinical effects of RV pacing when compared to those with a normal cardiac function and most patients with normal LVEF appear to tolerate some degree of chronic RV pacing[16,52]. It is therefore useful to stratify the patient with an indication for permanent cardiac pacing according to LVEF (reduced or conserved) and the likelihood of a high amount of RV pacing (high or low) (Figure 1). However, it may sometimes be difficult to estimate the latter although some more clear-cut scenarios also exist, such as patients with complete AV block (high risk) and patients with SSS and intact AV conduction (low risk).
Considering the available evidence, in a patient with a normal LVEF, a conventional pacemaker is currently the best option and unnecessary RV pacing should be avoided through appropriate device programming. This includes the correct selection of the pacing mode and also the lower rate limits (e.g., VVI mode at 40 ppm in a patient with infrequent paroxysmal AV block), AV interval (e.g., long AV intervals or AV hysteresis if there is no significant AV conduction loss) and the use of special algorithms aimed at minimizing RV pacing (if available). As discussed, several algorithms that all reduce the amount of RV pacing exists, although the MVP algorithm is the most studied with results indicating improvement in LV mechanics and also some clinical benefit[33,53]. However, there is currently insufficient evidence on the use of the MVP algorithm in patients with high grade AV block and in addition it may have a neutral or even negative effect when the PR interval is prolonged (> 230 ms)[36,54]. We therefore suggest that the MVP algorithm should only be used in those with SSS with no significant AV conduction disease (narrow QRS and PR interval < 230 ms).
If a patient presents with a reduced LVEF, with the results from the BLOCK HF study[41], there is now strong evidence that CRT should be chosen over a conventional pacemaker in order to improve both LV reverse remodeling as well as clinical outcomes[41,42]. The recently published European guidelines on cardiac pacing and CRT thus recommend de novo CRT in patients with HF, reduced LVEF (≤ 50%), bradycardia indication for pacing and an expected high percentage of RV pacing[39]. Furthermore, patients with reduced LVEF and planned AV node ablation for AF are likely to benefit from CRT[55]. Also, an important group of patients with indication for antibradycardia pacing also have a conventional indication for CRT and should obviously be offered this therapy[40]. Finally, there is presently not enough evidence to support the use of alternative RV pacing sites, such as RVOT, IVS and His bundle[28].
Despite the publication of a significant amount of evidence that has made us aware of the adverse pathophysiological mechanisms and clinical effects of prolonged RV pacing, as well as on the use of different strategies to overcome them, several questions remain unresolved. There is currently a lack of specifically designed studies that evaluate the potentially negative effect of long-term RV pacing in patients with normal LVEF. Most of the available information comes from old pacemaker trials aimed at evaluating atrial vs ventricular based pacing strategies in which echocardiography evaluation of LV function or percent RV stimulation was not always reported[8-11,14]. The conflicting results of the recent DANPACE trial[13], in which no association between the amount of RV pacing and clinical outcome was observed in patients with normal LVEF, further complicates the matter. Therefore, the extent of the negative effects of RV pacing in patients with normal LVEF and whether this is clinically relevant is at present debatable and future studies in this area are subsequently warranted. Other areas of future research include the indications and potential benefits of therapeutic strategies aimed at minimizing RV pacing such as specific algorithms, alternative pacing sites and CRT; however, several large scale trials are ongoing and the results will provide guidance for future clinical practice. The Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BIOPACE) study[56] is an international large randomized prospective mortality-driven trial that is comparing CRT vs conventional RV pacing in patients without a standard indication for CRT. Since patients with severely depressed to normal LVEF are being included, the results will not only help in defining the role of CRT in a wide range of patient characteristics but also provide information on the detrimental effects of RV pacing in those with normal LVEF. Finally, future large RCTs with long-term follow-up and clinical endpoints are currently evaluating the MVP algorithm and should provide important new information on its potential benefits[57,58].
In a significant number of patients, chronic RV pacing leads to negative effects such as reduced LV function and adverse cardiac remodeling, as well as increased incidence of HF, AF and death. Those with reduced LVEF and long-term high amount of RV pacing are at particular risk and there is now solid evidence that CRT improves LV function and clinical outcomes in this group. However, patients with normal LVEF seem to tolerate some degree of long-term RV pacing and thus the clinical relevance of the detrimental effects of RV pacing is less certain in these individuals. Appropriate pacemaker programming and the use of different pacemaker algorithms represent important methods to avoid unnecessary RV pacing in this patient group.
P- Reviewers: Chawla M, Escobar C, De Maria E, Providência R, Trohman RG, Zielinski T S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Wang CH
| 1. | Delgado V, Tops LF, Trines SA, Zeppenfeld K, Marsan NA, Bertini M, Holman ER, Schalij MJ, Bax JJ. Acute effects of right ventricular apical pacing on left ventricular synchrony and mechanics. Circ Arrhythm Electrophysiol. 2009;2:135-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol. 1990;259:H300-H308. [PubMed] [Cited in This Article: ] |
| 3. | Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol. 1999;33:1735-1742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 450] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Prinzen FW, Peschar M. Relation between the pacing induced sequence of activation and left ventricular pump function in animals. Pacing Clin Electrophysiol. 2002;25:484-498. [PubMed] [Cited in This Article: ] |
| 5. | Vassallo JA, Cassidy DM, Miller JM, Buxton AE, Marchlinski FE, Josephson ME. Left ventricular endocardial activation during right ventricular pacing: effect of underlying heart disease. J Am Coll Cardiol. 1986;7:1228-1233. [PubMed] [Cited in This Article: ] |
| 6. | Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932-2937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1195] [Cited by in F6Publishing: 1131] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 7. | Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115-3123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1485] [Cited by in F6Publishing: 1422] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 8. | Andersen HR, Nielsen JC, Thomsen PE, Thuesen L, Mortensen PT, Vesterlund T, Pedersen AK. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997;350:1210-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 511] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 9. | Connolly SJ, Kerr CR, Gent M, Roberts RS, Yusuf S, Gillis AM, Sami MH, Talajic M, Tang AS, Klein GJ. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med. 2000;342:1385-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 439] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 10. | Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, Marinchak RA, Flaker G, Schron E, Orav EJ. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002;346:1854-1862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 692] [Cited by in F6Publishing: 624] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 11. | Lamas GA, Orav EJ, Stambler BS, Ellenbogen KA, Sgarbossa EB, Huang SK, Marinchak RA, Estes NA, Mitchell GF, Lieberman EH. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacemaker Selection in the Elderly Investigators. N Engl J Med. 1998;338:1097-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 431] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 12. | Nielsen JC, Kristensen L, Andersen HR, Mortensen PT, Pedersen OL, Pedersen AK. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol. 2003;42:614-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 382] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 13. | Nielsen JC, Thomsen PE, Højberg S, Møller M, Vesterlund T, Dalsgaard D, Mortensen LS, Nielsen T, Asklund M, Friis EV. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011;32:686-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 14. | Toff WD, Camm AJ, Skehan JD. Single-chamber versus dual-chamber pacing for high-grade atrioventricular block. N Engl J Med. 2005;353:145-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 228] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Healey JS, Toff WD, Lamas GA, Andersen HR, Thorpe KE, Ellenbogen KA, Lee KL, Skene AM, Schron EB, Skehan JD. Cardiovascular outcomes with atrial-based pacing compared with ventricular pacing: meta-analysis of randomized trials, using individual patient data. Circulation. 2006;114:11-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Riahi S, Nielsen JC, Hjortshøj S, Thomsen PE, Højberg S, Møller M, Dalsgaard D, Nielsen T, Asklund M, Friis EV. Heart failure in patients with sick sinus syndrome treated with single lead atrial or dual-chamber pacing: no association with pacing mode or right ventricular pacing site. Europace. 2012;14:1475-1482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Steinberg JS, Fischer A, Wang P, Schuger C, Daubert J, McNitt S, Andrews M, Brown M, Hall WJ, Zareba W. The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J Cardiovasc Electrophysiol. 2005;16:359-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 18. | Sharma AD, Rizo-Patron C, Hallstrom AP, O’Neill GP, Rothbart S, Martins JB, Roelke M, Steinberg JS, Greene HL. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm. 2005;2:830-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Wilkoff BL, Kudenchuk PJ, Buxton AE, Sharma A, Cook JR, Bhandari AK, Biehl M, Tomassoni G, Leonen A, Klevan LR. The DAVID (Dual Chamber and VVI Implantable Defibrillator) II trial. J Am Coll Cardiol. 2009;53:872-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5067] [Cited by in F6Publishing: 4676] [Article Influence: 212.5] [Reference Citation Analysis (0)] |
| 21. | Barsheshet A, Moss AJ, McNitt S, Jons C, Glikson M, Klein HU, Huang DT, Steinberg JS, Brown MW, Zareba W. Long-term implications of cumulative right ventricular pacing among patients with an implantable cardioverter-defibrillator. Heart Rhythm. 2011;8:212-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Leong DP, Mitchell AM, Salna I, Brooks AG, Sharma G, Lim HS, Alasady M, Barlow M, Leitch J, Sanders P. Long-term mechanical consequences of permanent right ventricular pacing: effect of pacing site. J Cardiovasc Electrophysiol. 2010;21:1120-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Cano O, Osca J, Sancho-Tello MJ, Sánchez JM, Ortiz V, Castro JE, Salvador A, Olagüe J. Comparison of effectiveness of right ventricular septal pacing versus right ventricular apical pacing. Am J Cardiol. 2010;105:1426-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Catanzariti D, Maines M, Cemin C, Broso G, Marotta T, Vergara G. Permanent direct his bundle pacing does not induce ventricular dyssynchrony unlike conventional right ventricular apical pacing. An intrapatient acute comparison study. J Interv Card Electrophysiol. 2006;16:81-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Stambler BS, Ellenbogen K, Zhang X, Porter TR, Xie F, Malik R, Small R, Burke M, Kaplan A, Nair L. Right ventricular outflow versus apical pacing in pacemaker patients with congestive heart failure and atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:1180-1186. [PubMed] [Cited in This Article: ] |
| 26. | Occhetta E, Bortnik M, Magnani A, Francalacci G, Piccinino C, Plebani L, Marino P. Prevention of ventricular desynchronization by permanent para-Hisian pacing after atrioventricular node ablation in chronic atrial fibrillation: a crossover, blinded, randomized study versus apical right ventricular pacing. J Am Coll Cardiol. 2006;47:1938-1945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Dabrowska-Kugacka A, Lewicka-Nowak E, Tybura S, Wilczek R, Staniewicz J, Zagozdzon P, Faran A, Kozłowski D, Raczak G, Swiatecka G. Survival analysis in patients with preserved left ventricular function and standard indications for permanent cardiac pacing randomized to right ventricular apical or septal outflow tract pacing. Circ J. 2009;73:1812-1819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Shimony A, Eisenberg MJ, Filion KB, Amit G. Beneficial effects of right ventricular non-apical vs. apical pacing: a systematic review and meta-analysis of randomized-controlled trials. Europace. 2012;14:81-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Murakami Y, Tsuboi N, Inden Y, Yoshida Y, Murohara T, Ihara Z, Takami M. Difference in percentage of ventricular pacing between two algorithms for minimizing ventricular pacing: results of the IDEAL RVP (Identify the Best Algorithm for Reducing Unnecessary Right Ventricular Pacing) study. Europace. 2010;12:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Sweeney MO, Ellenbogen KA, Casavant D, Betzold R, Sheldon T, Tang F, Mueller M, Lingle J. Multicenter, prospective, randomized safety and efficacy study of a new atrial-based managed ventricular pacing mode (MVP) in dual chamber ICDs. J Cardiovasc Electrophysiol. 2005;16:811-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Sperzel J, Jung M, Gerog M, Schmitt J, Neumann T, Zaltsberg S, Pitschner H, Hamm CW. First clinical experience with a new algorithm to avoid unnecessary right ventricular pacing in patients with preserved intrinsic conduction. ICPES. Rome: World Congress, December 2007; . [Cited in This Article: ] |
| 32. | Olshansky B, Day JD, Moore S, Gering L, Rosenbaum M, McGuire M, Brown S, Lerew DR. Is dual-chamber programming inferior to single-chamber programming in an implantable cardioverter-defibrillator Results of the INTRINSIC RV (Inhibition of Unnecessary RV Pacing With AVSH in ICDs) study. Circulation. 2007;115:9-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick D, Sheldon T, Lamas GA. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357:1000-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 371] [Cited by in F6Publishing: 330] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 34. | Milasinovic G, Tscheliessnigg K, Boehmer A, Vancura V, Schuchert A, Brandt J, Wiggenhorn C, Hofman M, Sperzel J. Percent ventricular pacing with managed ventricular pacing mode in standard pacemaker population. Europace. 2008;10:151-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Ishikawa T, Kimura K, Miyazaki N, Tochikubo O, Usui T, Kashiwagi M, Ishii M. Diastolic mitral regurgitation in patients with first-degree atrioventricular block. Pacing Clin Electrophysiol. 1992;15:1927-1931. [PubMed] [Cited in This Article: ] |
| 36. | Sweeney MO, Ellenbogen KA, Tang AS, Whellan D, Mortensen PT, Giraldi F, Sandler DA, Sherfesee L, Sheldon T. Atrial pacing or ventricular backup-only pacing in implantable cardioverter-defibrillator patients. Heart Rhythm. 2010;7:1552-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Olshansky B, Day JD, Lerew DR, Brown S, Stolen KQ. Eliminating right ventricular pacing may not be best for patients requiring implantable cardioverter-defibrillators. Heart Rhythm. 2007;4:886-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Akerström F, Arias MA, Pachón M, Puchol A, Jiménez-López J, Rodríguez-Padial L. The reverse mode switch algorithm: how well does it work. Heart Rhythm. 2013;10:1146-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281-2329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1437] [Cited by in F6Publishing: 1438] [Article Influence: 130.7] [Reference Citation Analysis (0)] |
| 40. | Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NA, Ferguson TB, Hammill SC, Karasik PE, Link MS. 2012 ACCF/AHA/HRS Focused Update of the 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm. 2012;9:1737-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, Shinn T, Sutton MS. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 520] [Cited by in F6Publishing: 538] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 42. | Martinelli Filho M, de Siqueira SF, Costa R, Greco OT, Moreira LF, D’avila A, Heist EK. Conventional versus biventricular pacing in heart failure and bradyarrhythmia: the COMBAT study. J Card Fail. 2010;16:293-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Fröhlich G, Steffel J, Hürlimann D, Enseleit F, Lüscher TF, Ruschitzka F, Abraham WT, Holzmeister J. Upgrading to resynchronization therapy after chronic right ventricular pacing improves left ventricular remodelling. Eur Heart J. 2010;31:1477-1485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Gierula J, Cubbon RM, Jamil HA, Byrom R, Baxter PD, Pavitt S, Gilthorpe MS, Hewison J, Kearney MT, Witte KK. Cardiac resynchronization therapy in pacemaker-dependent patients with left ventricular dysfunction. Europace. 2013;15:1609-1614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Yu CM, Chan JY, Zhang Q, Omar R, Yip GW, Hussin A, Fang F, Lam KH, Chan HC, Fung JW. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009;361:2123-2134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 309] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 46. | Tops LF, Schalij MJ, Holman ER, van Erven L, van der Wall EE, Bax JJ. Right ventricular pacing can induce ventricular dyssynchrony in patients with atrial fibrillation after atrioventricular node ablation. J Am Coll Cardiol. 2006;48:1642-1648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 47. | Brignole M, Botto G, Mont L, Iacopino S, De Marchi G, Oddone D, Luzi M, Tolosana JM, Navazio A, Menozzi C. Cardiac resynchronization therapy in patients undergoing atrioventricular junction ablation for permanent atrial fibrillation: a randomized trial. Eur Heart J. 2011;32:2420-2429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 48. | Brignole M, Gammage M, Puggioni E, Alboni P, Raviele A, Sutton R, Vardas P, Bongiorni MG, Bergfeldt L, Menozzi C. Comparative assessment of right, left, and biventricular pacing in patients with permanent atrial fibrillation. Eur Heart J. 2005;26:712-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 49. | Doshi RN, Daoud EG, Fellows C, Turk K, Duran A, Hamdan MH, Pires LA. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J Cardiovasc Electrophysiol. 2005;16:1160-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 423] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 50. | Leclercq C, Walker S, Linde C, Clementy J, Marshall AJ, Ritter P, Djiane P, Mabo P, Levy T, Gadler F. Comparative effects of permanent biventricular and right-univentricular pacing in heart failure patients with chronic atrial fibrillation. Eur Heart J. 2002;23:1780-1787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 332] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 51. | Orlov MV, Gardin JM, Slawsky M, Bess RL, Cohen G, Bailey W, Plumb V, Flathmann H, de Metz K. Biventricular pacing improves cardiac function and prevents further left atrial remodeling in patients with symptomatic atrial fibrillation after atrioventricular node ablation. Am Heart J. 2010;159:264-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Sweeney MO, Hellkamp AS. Heart failure during cardiac pacing. Circulation. 2006;113:2082-2088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 53. | Sweeney MO, Shea JB, Fox V, Adler S, Nelson L, Mullen TJ, Belk P, Casavant D, Sheldon T. Randomized pilot study of a new atrial-based minimal ventricular pacing mode in dual-chamber implantable cardioverter-defibrillators. Heart Rhythm. 2004;1:160-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Sweeney MO, Ellenbogen KA, Tang AS, Johnson J, Belk P, Sheldon T. Severe atrioventricular decoupling, uncoupling, and ventriculoatrial coupling during enhanced atrial pacing: incidence, mechanisms, and implications for minimizing right ventricular pacing in ICD patients. J Cardiovasc Electrophysiol. 2008;19:1175-1180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Stavrakis S, Garabelli P, Reynolds DW. Cardiac resynchronization therapy after atrioventricular junction ablation for symptomatic atrial fibrillation: a meta-analysis. Europace. 2012;14:1490-1497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Funck RC, Blanc JJ, Mueller HH, Schade-Brittinger C, Bailleul C, Maisch B. Biventricular stimulation to prevent cardiac desynchronization: rationale, design, and endpoints of the ‘Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BioPace)’ study. Europace. 2006;8:629-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Quesada A, Botto G, Erdogan A, Kozak M, Lercher P, Nielsen JC, Piot O, Ricci R, Weiss C, Becker D. Managed ventricular pacing vs. conventional dual-chamber pacing for elective replacements: the PreFER MVP study: clinical background, rationale, and design. Europace. 2008;10:321-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Funck RC, Boriani G, Manolis AS, Püererfellner H, Mont L, Tukkie R, Pisapia A, Israel CW, Grovale N, Grammatico A. The MINERVA study design and rationale: a controlled randomized trial to assess the clinical benefit of minimizing ventricular pacing in pacemaker patients with atrial tachyarrhythmias. Am Heart J. 2008;156:445-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |