Revised: December 24, 2012
Accepted: January 5, 2013
Published online: January 26, 2013
Processing time: 149 Days and 7.1 Hours
AIM: To investigate endothelium-dependent and -independent coronary microvascular functions in patients with vasospastic angina (VSA).
METHODS: Thirty-six patients with VSA (30 men and 6 women; mean age, 58 years) were enrolled in this study. VSA was defined as ≥ 90% narrowing of the epicardial coronary arteries on angiography performed during a spasm provocation test, presence of chest pain, and/or ST-segment deviation on an electrocardiogram (ECG). Patients (n = 36) with negative spasm provocation test results and those matched for age and sex were enrolled as a control group (nonVSA group). Low-dose acetylcholine (ACh; 3 μg/min) was infused into the left coronary ostium for 2 min during the spasm provocation test. Following the spasm provocation test, nitroglycerin (0.2 mg) was administered intracoronally. Coronary blood flow (was calculated from quantitative angiography and Doppler flow velocity measurements, and the coronary flow reserve was calculated as the ratio of coronary flow velocity after injection of adenosine triphosphate (20 μg) to the baseline value. Changes in the coronary artery diameter in response to ACh and nitroglycerin infusion were expressed as percentage changes from baseline measurements.
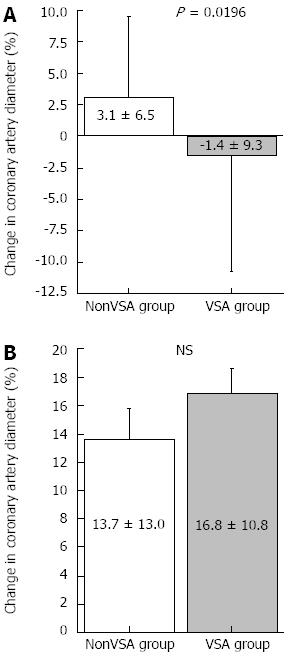
RESULTS: Body mass index was significantly lower in the VSA group than in the nonVSA group. The frequency of conventional coronary risk factors and the rate of statin use were similar between the 2 groups. The left ventricular ejection fraction as evaluated by echocardiography was similar between the 2 groups. The duration of angina was 9 ± 2 mo. The results of blood chemistry analysis were similar between the 2 groups. Low-dose ACh did not cause coronary spasms. The change in coronary artery diameter in response to ACh was lower in the VSA group (-1.4% ± 9.3%) than in the nonVSA group (3.1% ± 6.5%, P < 0.05), whereas nitroglycerin-induced coronary artery dilatation and coronary blood flow increase in response to ACh or coronary flow reserve did not differ significantly between the 2 groups.
CONCLUSION: These findings suggest that microvascular coronary function may be preserved despite endothelial dysfunction of the epicardial coronary arteries in patients with VSA.
- Citation: Teragawa H, Mitsuba N, Ishibashi K, Nishioka K, Kurisu S, Kihara Y. Evaluation of coronary microvascular function in patients with vasospastic angina. World J Cardiol 2013; 5(1): 1-7
- URL: https://www.wjgnet.com/1949-8462/full/v5/i1/1.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i1.1
Vasospastic angina (VSA) is characterized by coronary spasms, which occur because of a dynamic, transient decrease in the luminal diameter of epicardial coronary arteries due to increased vasomotor tone, ultimately leading to myocardial ischemia[1,2]. Abnormal vascular functions of the epicardial coronary arteries, including endothelial dysfunction[3,4] and vascular smooth muscle dysfunction[5-7], play pivotal roles in VSA pathogenesis. However, there are few studies that have investigated coronary microvascular function in patients with VSA[8-12] Therefore, to confirm the presence of coronary microvascular dysfunction in patients with VSA, we investigated their endothelium-dependent and -independent coronary microvascular functions and compared the results with those of patients without VSA.
Thirty-six patients with VSA (VSA group; 30 males and 6 females; mean age, 58 years) diagnosed by a positive spasm provocation test and another 36 patients with negative results of the spasm provocation test performed for the evaluation of chest symptoms (nonVSA group) were enrolled in this study. The 2 groups were well matched with respect to age and sex. Patients with organic coronary stenosis (> 50%), history of myocardial infarction, cardiomyopathy, heart failure, or any other serious medical condition were excluded from the study. Written informed consent was obtained from all patients prior to the study. The protocol was approved by the Ethics Committee of our institution.
All antianginal agents were discontinued at least 48 h prior to catheterization with the exception of sublingual nitroglycerin (NTG), which was withheld for 1 h prior to catheterization. Diagnostic left heart catheterization and coronary angiography were performed using a standard percutaneous brachial approach. A 6F guide catheter was introduced into the left main coronary artery. A 0.0014 Doppler flow guidewire (Volcano FloWire; Volcano Therapeutics Inc., Rancho Cordova, CA, United States) was advanced through the guide catheter into the proximal segment of the left anterior descending coronary artery (LAD). The wire tip was positioned in a straight segment of the vessel to obtain a reliable flow velocity signal.
After baseline control conditions were established, incremental doses (3 and 30 μg/min) of acetylcholine (ACh) were infused into the left coronary artery for 2 min, with 5-min intervals between consecutive doses. If a coronary spasm was not induced by ACh, incremental doses of methylergometrine maleate (EM) were infused into the left coronary artery (10, 20, and 30 μg/min) for 1 min, with 1-min intervals between consecutive doses. If coronary spasms were not induced by this infusion also, incremental doses (15 and 25 μg/min) of EM were infused into the right coronary artery following the same protocol as that followed for the left coronary artery. When coronary spasms were induced or EM infusion was discontinued, because coronary spasms were not provoked, NTG (200 μg) was administered by intracoronary injection. Intracoronary ACh and EM were administered using an infusion pump (TE-311; Terumo, Tokyo, Japan) at a rate of 1 mL/min. Coronary angiography was performed immediately after the appearance of chest symptoms and/or ST segment changes after each dose of ACh administration, after the last dose of EM when neither chest symptoms nor ST segment changes were induced, or 2 min after NTG injection. Finally, adenosine triphosphate (20 μg) was infused into the left coronary artery. Coronary blood flow (CBF) velocity was monitored continuously using a 12-MHz pulsed Doppler velocimeter (FloMap; Volcano Therapeutics Inc.). Arterial pressure, heart rate, and electrocardiography (ECG) readings were monitored continuously and recorded using a multichannel recorder (Polygraph 1600; Nihon Electric Corporation, Tokyo, Japan).
A method for measuring the coronary artery diameter has been described previously[13-16]. The coronary segment 2 mm distal to the Doppler wire tip, which was not the spastic segment was selected for quantitative analysis. For each patient, luminal diameters of the selected LAD segments were measured by a single investigator blinded to the clinical data, using an end-diastolic frame by a computer-assisted coronary angiographic analysis system (CAAS II/QUANTCOR; Siemens, Berlin, Germany). Measurements were performed 3 times, and the average value was used for analysis. Changes in coronary artery diameter in response to ACh and NTG infusion were expressed as percentage changes from baseline angiographic measurements obtained before infusion. Intraobserver and interobserver variability of this method were previously shown to be excellent[17].
Coronary flow reserve (CFR) was calculated as the product of CBF velocity and vessel diameter using the following formula: π× average peak velocity × 0.125 × diameter2. For CBF calculations, the internal diameter of the vessel at the location of the flow measurements (2 mm distal to the wire tip) was measured using the method described above. CFR was calculated as the ratio of CBF velocity after adenosine triphosphate infusion to the baseline velocity.
VSA was defined as ≥ 90% narrowing of the epicardial coronary arteries on angiography performed during the spasm provocation test, presence of characteristic chest pain, and/or ST-segment deviation on ECG[18]. The LAD trunk was divided into proximal, middle, and distal segments of equal lengths. The location of the spastic segment is expressed with reference to these 3 segments. When coronary spasms occurred diffusely from the proximal to the distal segment, the location was defined as proximal, middle, and distal. A diffuse spasm was defined as that when the length of the spastic segment was ≥ 20 mm, and a focal spasm was defined as that when the length of the spastic segment was < 20 mm. A totally occluded spastic segment was also considered to represent a diffuse spasm. In the present study, coronary spasms occurred in all LADs and also in the left circumflex coronary artery in some VSA patients (multivessel spasm). The duration of angina was obtained from the patients’ medical examinations performed via interviews.
As described previously[14,17,19-21], in the present study, we adopted the percent changes in epicardial coronary diameter in response to ACh and NTG infusions as the endothelium-dependent and -independent functions of the coronary artery at the level of conduit vessels, and adopted the percent change in CBF in response to ACh infusion and CFR as the endothelium-dependent and -independent functions of the coronary artery at the level of resistance vessels.
Fasting blood samples were obtained on the same day of coronary angiography. The patients were questioned about their smoking status and classified as a current smoker, past smoker (who had stopped smoking for at least 1 mo), or nonsmoker. Blood pressure was measured, and hypertension was defined as present if systolic blood pressure was ≥ 140 mmHg, diastolic blood pressure was ≥ 90 mmHg, and/or the patient was on antihypertensive drugs. Blood chemistry parameters, including levels of total cholesterol, triglycerides, high-density lipoprotein cholesterol, fasting blood sugar, insulin, hemoglobin A1C, and creatinine were also measured. Low-density lipoprotein cholesterol was calculated using the Friedewald equation[22]. Hyperlipidemia was defined as present if low-density lipoprotein cholesterol was ≥ 120 mg/dL and/or on the patient was on medication for the same. Diabetes mellitus was defined as present if fasting blood sugar was ≥ 126 mg/dL, hemoglobin A1C was ≥ 6.5%, and/or the patient was on medication for the same.
All data are expressed as mean ± SD. Baseline characteristics of the 2 groups were compared using the Student’s unpaired t-test or χ2 analysis as appropriate. The Pearson’s correlation coefficient was used to investigate the relationship between coronary microvascular parameters and clinical parameters. A P value of < 0.05 was considered statistically significant.
Patient characteristics are presented in Table 1. Body mass index (BMI) was significantly lower in the VSA group than in the nonVSA group. The frequency of conventional coronary risk factors and the rate of statin use were similar between the 2 groups. The left ventricular ejection fraction as evaluated by echocardiography was similar between the 2 groups. The duration of angina was 9 ± 2 mo. The results of blood chemistry analysis were similar between the 2 groups (Table 2).
| NonVSA group | VSA group | P value | |
| (n = 36) | (n = 36) | ||
| Age (yr) | 60 ± 9 | 59 ± 9 | NS |
| Male/female | 31/5 | 30/6 | NS |
| Body mass index (kg/m2) | 25.2 ± 3.2 | 23.2 ± 2.6 | 0.0045 |
| Coronary risk facto | |||
| Smoking | 25 (69) | 24 (67) | NS |
| Current/passt smoker | 11/13 | 12/13 | NS |
| Hypertension | 21 (58) | 19 (53) | NS |
| Dyslipidemia | 11 (31) | 11 (31) | NS |
| Diabetes mellitus | 9 (25) | 4 (11) | NS |
| Taking statins (%) | 4 (11) | 3 (9) | NS |
| LVEF on echocardiography (%) | 71 ± 8 | 68 ± 7 | NS |
| NonVSA Group | VSA Group | P value | |
| Total cholesterol (mg/dL) | 196 ± 38 | 205 ± 27 | NS |
| Triglyceride (mg/dL) | 158 ± 63 | 142 ± 57 | NS |
| HDL-cholesterol (mg/dL) | 52 ± 15 | 55 ± 13 | NS |
| LDL-cholesterol (mg/dL) | 113 ± 30 | 123 ± 24 | NS |
| Fasting blood sugar (mg/dL) | 103 ± 24 | 97 ± 13 | NS |
| Hemoglobin A1C (%) | 5.6 ± 0.8 | 5.4 ± 0.6 | NS |
| C-reactive protein (mg/L) | 1.8 ± 2.9 | 1.6 ± 3.6 | NS |
Although coronary spasm was not induced by ACh infusion at 3 μg/min, it was induced by ACh infusion at 30 μg/min in 14 patients, EM infusion at 20 μg/min in 1 patient, and EM infusion at 30 μg/min in 21 patients. Coronary spasm occurred in the proximal segment in 1 patient; proximal and middle segments in 1 patient; middle segment in 14 patients; middle and distal segments in 8 patients; distal segment in 6 patients; and proximal, middle, and distal segments in 6 patients. Therefore, coronary spasm occurred in the distal segments in 20 patients (56%). A focal spasm was identified in 13 patients (36%), while a diffuse spasm was identified in 23 patients (64%). Multivessel coronary spasm occurred in 6 patients (17%).
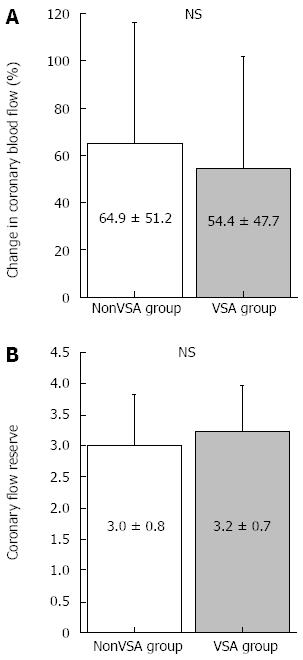
Low-dose ACh infusion (3 μg/min) did not cause coronary spasms; therefore, we adopted the coronary artery response to low-dose ACh as the endothelial-dependent coronary artery parameter. The results of coronary vasomotion in response to each drug are shown in Table 3 and Figures 1 and 2. Heart rate, mean blood pressure, coronary artery diameter, and CBF at baseline did not differ between groups. The change in coronary artery diameter in response to ACh infusion at 3 μg/min was lesser in the VSA group than in the nonVSA group, although NTG-induced coronary artery dilatation was similar between the 2 groups (Table 3 and Figure 1). CFR and CBF increase in response to low-dose ACh infusion did not differ between groups (Table 3 and Figure 2). Neither CFR nor the increase in CBF induced by low-dose ACh was correlated with several clinical factors, including age, duration of angina, smoking status, and presence of diffuse and distal spasms (Table 4).
| NonVSA Group | VSA Group | P value | |
| Heart rate at baseline (/min) | 67 ± 13 | 67 ± 9 | NS |
| Mean arterial pressure (mmHg) | 107 ± 11 | 103 ± 16 | NS |
| Coronary artery diameter (mm) | |||
| Baseline | 3.14 ± 0.48 | 2.95 ± 0.55 | NS |
| Acetylcholine 3 μg/min | 3.24 ± 0.55 | 2.90 ± 0.59 | 0.013 |
| Nitroglycerin infusion | 3.57 ± 0.65 | 3.43 ± 0.55 | NS |
| Coronary blood flow (mL/min) | |||
| Baseline | 94 ± 48 | 84 ± 69 | NS |
| Acetylcholine 3 μg/min | 153 ± 15 | 130 ± 15 | NS |
| Average peak velocity (cm/s) | |||
| Baseline | 21 ± 8 | 21 ± 12 | NS |
| After ATP infusion | 63 ± 25 | 64 ± 24 | NS |
| Parameters | ACh-induced increase in CBF | P value | CFR | P value |
| Age (yr) | r = 0.188 | NS | r = 0.204 | NS |
| Disease period (mo) | r = -0.016 | NS | r = -0.260 | NS |
| Current smoking | ||||
| (+, n = 12) | 54.5 ± 14.0 | NS | 3.1 ± 0.6 | NS |
| (-, n = 24) | 54.4 ± 9.9 | 3.3 ± 0.8 | ||
| Diffuse spasm | ||||
| (+, n = 23) | 60.8 ± 49.9 | NS | 3.2 ± 0.6 | NS |
| (-, n = 13) | 43.2 ± 43.3 | 3.3 ± 0.9 | ||
| Distal spasm | ||||
| (+, n = 20) | 57.0 ± 49.3 | NS | 3.2 ± 0.7 | NS |
| (-, n = 16) | 51.3 ± 47.2 | 3.3 ± 0.8 | ||
| Multi-vessel spasm | ||||
| (+, n = 7) | 41.8 ± 26.4 | NS | 3.1 ± 0.7 | NS |
| (-, n = 29) | 57.5 ± 51.5 | 3.3 ± 0.7 |
In the present study, we compared endothelium-independent and -dependent coronary microvascular functions between patients with VSA characterized by coronary spasms in LAD and age-matched and sex-matched patients who tested negative in the coronary spasm provocation test. ACh-induced changes in the epicardial coronary arteries were impaired in the VSA group; however, other vascular functions, such as NTG-induced epicardial coronary artery dilatation, CFR, and ACh-induced increase in CBF, were similar between the 2 groups. Coronary endothelial dysfunction at the level of conduit vessels, but not at the level of resistance vessels, may contribute to VSA pathogenesis.
The vascular endothelium is not only a simple passive barrier between the circulating blood and surrounding tissues but also a multifunctional organ, the integrity of which is essential to normal vascular physiology[23]. It releases various vasodilators, including nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factor, as well as vasoconstrictors. NO plays an important role in the regulation of vascular tone, inhibition of platelet aggregation, and suppression of vascular smooth muscle cell proliferation[24,25]. ACh causes vasodilation by releasing NO from the endothelium in healthy humans, whereas it causes vasoconstriction in patients with coronary atherosclerosis[23,26]. The coronary arteries in patients with VSA are highly sensitive to the vasoconstrictive effect of intracoronary ACh infusion, thereby resulting in spasms[27-29]. Thus, intracoronary ACh injection is used as a provocative test for coronary spasm[27-29]. Therefore, in the present study, we assessed coronary endothelial function using low-dose ACh infusion (3 μg/min), which did not cause significant coronary spasms.
Regarding coronary vascular function at the level of conduit vessels, it is accepted that abnormal vascular function of the epicardial coronary arteries is present in VSA patients[3-7], although it has not been clarified whether coronary endothelial dysfunction only, coronary smooth muscle dysfunction only, or both contribute to VSA pathogenesis[3,5]. In the present study, low-dose ACh infusion caused significant vasoconstriction in patients with VSA compared with those without, whereas NTG administration did not cause significant differences between groups, suggesting that endothelial dysfunction of the epicardial coronary artery was present in patients with VSA.
Regarding coronary microvascular function in patients with VSA, several studies have investigated the coronary microvascular endothelium -independent function using CFR measurements[9-12]. According to these reports[9-12], several clinical factors, including patient age, disease period, smoking status, and presence of diffuse and distal spasms were associated with decreased CFR. In the present study, CFR in the VSA group was not decreased compared with that in the nonVSA group. In addition, CFR in the VSA group was not associated with any clinical parameter suggested by previous studies[9-12]. Our subjects were patients with VSA characterized by coronary spasms in LADs. However, coronary spasms occur in other coronary vessels as well[30,31], and it sometimes occurs prominently in the right coronary artery. Therefore, the assessment of coronary microvascular function only in LADs may be insufficient to determine coronary microvascular function in patients with VSA. Furthermore, the nonVSA group in which patients underwent coronary angiography and spasm provocation tests for the evaluation of chest symptom s may have included patients with microvascular angina. It is well known that coronary microvascular function is impaired in such patients[32]. However, if such patients were included in the nonVSA group, CFR in this group would have already been low. Differences in these patient characteristics as well as differences in methodologies and materials, such as the stress agents, their doses, the administration sites (intravenous or intracoronary), and segments in which Doppler flow guidewires are placed, may have led to the differing results.
On the other hand, only few studies have investigated the coronary microvascular endothelium-dependent function. Okumura et al[8] showed that CBF significantly increased during coronary spasms in LAD, indicating that the microvascular endothelium-dependent function was preserved. Our results, assessed under the nonspastic status, showed that microvascular endothelial function was not impaired in the VSA group compared with that in the nonVSA group. As mentioned above, it was possible that the microvascular endothelial function was already impaired in the nonVSA group if patients with microvascular angina were included. However, our results showed that coronary microvascular endothelial dysfunction was not involved in the pathogenesis of VSA.
Our results suggest two clinical implications. First, endothelial dysfunction of the epicardial coronary artery, even at the nonspastic segment, was present in patients with VSA. Although endothelial dysfunction may occur throughout the vasculature, the degree of endothelial dysfunction may not always be consistent. Regarding the relationship between the presence of a myocardial bridge and VSA[33], it is possible that local external force may cause the difference in the development of endothelial dysfunction even in the same coronary artery. Therefore, the differences in the degree or development of endothelial dysfunction of the epicardial coronary artery may cause the heterogeneity of occurrence of a coronary spasm. Second, coronary microvascular function may be preserved in patients with VSA to countermeasure myocardial ischemia due to vasospasm of the epicardial coronary artery.
There were several limitations to the present study. First, as mentioned above, coronary spasms do not always occur in LAD; therefore, our results may not have accounted for all VSA patients. Second, patients in the nonVSA group with chest symptoms, angiographically normal coronary arteries, and a negative spasm provocation test may be heterogenous; in addition, patients with microvascular angina may have been included in this group. Therefore, it was possible that the nonVSA group was not a pure control. Finally, BMI was not similar between the 2 groups. ACh doses, which were not adjusted according to BMI in the present study, may have contributed to ACh-induced coronary vascular responses. However, coronary artery diameter and CBF at baseline was similar between groups, and we believe that differences in BMI did not affect the coronary vascular responses.
In conclusion, the present study showed that coronary microvascular function, including endothelium-dependent and -independent functions, may be preserved despite coronary endothelial dysfunction at the level of conduit vessels. The latter may contribute to VSA pathogenesis.
We would like to thank Dr. Junko Soga, Dr. Yuichi Fujii, Dr. Noritaka Fujimura, Dr. Shinsuke Mikami, and Dr. Tatsuya Maruhashi for their help with coronary catheterization. We would also like to thank Ms. Michiko Aoyama, Yuka Shiroshita, and Ryoko Tachiyama for their secretarial assistance.
Vasospastic angina (VSA) is characterized by coronary spasms, which occur because of a dynamic, transient reduction in the luminal diameter of epicardial coronary arteries, leading to myocardial ischemia. It has been reported that abnormal vascular functions of the epicardial coronary arteries were involved in the pathogenesis of VSA. However, coronary microvascular function in patients with VSA remains to be elucidated.
The pathogenesis of VSA remains to be unclear. Therefore, to assess coronary vascular response, especially at the level of resistance vessels, may, in part, contribute to the pathogenesis of VSA.
Several studies investigating coronary vascular function in patients with VSA have been reported and their results have identified coronary vascular dysfunction at the level of the conduit vessels. However, it has not been clarified whether coronary endothelial dysfunction only, coronary smooth muscle dysfunction only, both contribute to VSA pathogenesis. Furthermore, only a few studies have investigated coronary microvascular functions in patients with VSA. In the present study, the authors assessed coronary vascular functions using quantitative coronary angiography and Doppler velocity measurements in VSA patients whose coronary spasm occurred in the left anterior descending coronary artery, and compared them with those in nonVSA patients with negative spasm prevocational test. The results showed that the change in coronary artery diameter in response to a low dose of acetylcholine (ACh) was lower in the VSA group, whereas nitroglycerin-induced coronary artery dilatation and coronary blood flow increase in response to ACh or coronary flow reserve did not differ significantly between the 2 groups. These findings suggest that microvascular coronary function may be preserved despite endothelial dysfunction of the epicardial coronary arteries in patients with VSA.
The results suggest two clinical implications. First, endothelial dysfunction of the epicardial coronary artery was present in patients with VSA. Second, coronary microvascular functions including endothelium-dependent and may be preserved in patients with VSA. Such coronary vascular response may highlight the pathogenesis of VSA. In addition, the latter finding may countermeasure myocardial ischemia due to vasospasm of the epicardial coronary artery.
Regarding coronary vascular functions, there are two components: at the level of conduit vessels (epicardial coronary artery) and at the level of resistance vessels (microvascular coronary artery). In addition, regarding the factors of coronary artery vasodilation, there are two factors: endothelium-dependent and -independent ones. In the present study, using quantitative coronary angiography and Doppler velocity measurements, the authors defined the percent changes in epicardial coronary diameter in response to ACh and NTG infusions as the endothelium-dependent and -independent functions of the coronary artery at the level of conduit vessels, and defined the percent change in coronary blood flow in response to ACh infusion and coronary flow reserve as the endothelium-dependent and -independent functions of the coronary artery at the level of resistance vessels.
The results presented in this paper are good, with the references are properly Quoted and work is new. The paper is very well organized and the results presented in this paper are justified, and the paper may be accepted in the format in which it is submitted, with the references listed.
P- Reviewers Hardt S, Fineschi V, Chawla M S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Yasue H, Kugiyama K. Coronary spasm: clinical features and pathogenesis. Intern Med. 1997;36:760-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Hung MJ. Current advances in the understanding of coronary vasospasm. World J Cardiol. 2010;2:34-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Kugiyama K, Ohgushi M, Motoyama T, Sugiyama S, Ogawa H, Yoshimura M, Inobe Y, Hirashima O, Kawano H, Soejima H. Nitric oxide-mediated flow-dependent dilation is impaired in coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 1997;30:920-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Fukuda Y, Teragawa H, Matsuda K, Yamagata T, Matsuura H, Chayama K. Tetrahydrobiopterin improves coronary endothelial function, but does not prevent coronary spasm in patients with vasospastic angina. Circ J. 2002;66:58-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Egashira K, Inou T, Yamada A, Hirooka Y, Takeshita A. Preserved endothelium-dependent vasodilation at the vasospastic site in patients with variant angina. J Clin Invest. 1992;89:1047-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Shimokawa H, Seto M, Katsumata N, Amano M, Kozai T, Yamawaki T, Kuwata K, Kandabashi T, Egashira K, Ikegaki I. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc Res. 1999;43:1029-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 256] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 8. | Okumura K, Yasue H, Matsuyama K, Ogawa H, Kugiyama K, Sakaino N, Yamabe H, Morita E. A study on coronary hemodynamics during acetylcholine-induced coronary spasm in patients with variant angina: endothelium-dependent dilation in the resistance vessels. J Am Coll Cardiol. 1992;19:1426-1434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Akasaka T, Yoshida K, Hozumi T, Takagi T, Kawamoto T, Kaji S, Morioka S, Yoshikawa J. Comparison of coronary flow reserve between focal and diffuse vasoconstriction induced by ergonovine in patients with vasospastic angina. Am J Cardiol. 1997;80:705-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 10. | Anzai H, Saijo T, Nakajima R, Tezuka N, Takagi T, Tsunoda T, Kobayashi N, Nakamura S, Yamaguchi T. [Evaluation of coronary flow reserve in patients with vasospastic angina]. J Cardiol. 2000;36:17-27. [PubMed] [Cited in This Article: ] |
| 11. | Sueda S, Kohno H, Fukuda H, Uraoka T. Coronary flow reserve in patients with vasospastic angina: correlation between coronary flow reserve and age or duration of angina. Coron Artery Dis. 2003;14:423-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 12. | Ashikaga T, Nishizaki M, Fujii H, Niki S, Maeda S, Yamawake N, Kishi Y, Isobe M. Examination of the microcirculation damage in smokers versus nonsmokers with vasospastic angina pectoris. Am J Cardiol. 2007;100:962-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Matsuda K, Teragawa H, Fukuda Y, Ueda K, Higashi Y, Sakai K, Miura F, Hirao H, Yamagata T, Yoshizumi M. Response of the left anterior descending coronary artery to acetylcholine in patients with chest pain and angiographically normal coronary arteries. Am J Cardiol. 2003;92:1394-1398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Teragawa H, Fukuda Y, Matsuda K, Ueda K, Higashi Y, Oshima T, Yoshizumi M, Chayama K. Relation between C reactive protein concentrations and coronary microvascular endothelial function. Heart. 2004;90:750-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Teragawa H, Ueda K, Matsuda K, Kimura M, Higashi Y, Oshima T, Yoshizumi M, Chayama K. Relationship between endothelial function in the coronary and brachial arteries. Clin Cardiol. 2005;28:460-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Teragawa H, Mitsuba N, Nishioka K, Ueda K, Kono S, Higashi Y, Chayama K, Kihara Y. Impaired coronary microvascular endothelial function in men with metabolic syndrome. World J Cardiol. 2010;2:205-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Teragawa H, Kato M, Yamagata T, Matsuura H, Kajiyama G. Magnesium causes nitric oxide independent coronary artery vasodilation in humans. Heart. 2001;86:212-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ J. 2010;74:1745-1762. [PubMed] [Cited in This Article: ] |
| 19. | Shiode N, Morishima N, Nakayama K, Yamagata T, Matsuura H, Kajiyama G. Flow-mediated vasodilation of human epicardial coronary arteries: effect of inhibition of nitric oxide synthesis. J Am Coll Cardiol. 1996;27:304-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Shiode N, Nakayama K, Morishima N, Yamagata T, Matsuura H, Kajiyama G. Nitric oxide production by coronary conductance and resistance vessels in hypercholesterolemia patients. Am Heart J. 1996;131:1051-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Kato M, Shiode N, Yamagata T, Matsuura H, Kajiyama G. Bradykinin induced dilatation of human epicardial and resistance coronary arteries in vivo: effect of inhibition of nitric oxide synthesis. Heart. 1997;78:493-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502. [PubMed] [Cited in This Article: ] |
| 23. | Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22 Suppl 4:S1-14. [PubMed] [Cited in This Article: ] |
| 24. | Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1105] [Cited by in F6Publishing: 1103] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 25. | Vanhoutte PM. Endothelium and control of vascular function. State of the Art lecture. Hypertension. 1989;13:658-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 472] [Cited by in F6Publishing: 454] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 26. | Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8681] [Cited by in F6Publishing: 8024] [Article Influence: 182.4] [Reference Citation Analysis (1)] |
| 27. | Yasue H, Horio Y, Nakamura N, Fujii H, Imoto N, Sonoda R, Kugiyama K, Obata K, Morikami Y, Kimura T. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986;74:955-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 383] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Okumura K, Yasue H, Horio Y, Takaoka K, Matsuyama K, Kugiyama K, Fujii H, Morikami Y. Multivessel coronary spasm in patients with variant angina: a study with intracoronary injection of acetylcholine. Circulation. 1988;77:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 172] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 30. | Sueda S, Kohno H, Fukuda H, Ochi N, Kawada H, Hayashi Y, Uraoka T. Induction of coronary artery spasm by two pharmacological agents: comparison between intracoronary injection of acetylcholine and ergonovine. Coron Artery Dis. 2003;14:451-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 31. | Takagi Y, Yasuda S, Takahashi J, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y. Clinical implications of provocation tests for coronary artery spasm: safety, arrhythmic complications, and prognostic impact: Multicentre Registry Study of the Japanese Coronary Spasm Association. Eur Heart J. 2013;34:258-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 32. | Kaski JC. Overview of gender aspects of cardiac syndrome X. Cardiovasc Res. 2002;53:620-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Teragawa H, Fukuda Y, Matsuda K, Hirao H, Higashi Y, Yamagata T, Oshima T, Matsuura H, Chayama K. Myocardial bridging increases the risk of coronary spasm. Clin Cardiol. 2003;26:377-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |